Abstract
Background
The National Health Service (NHS) is faced with increasing cost pressures that make the efficient use of resources paramount. Irritable bowel syndrome (IBS) places a large burden on the NHS as it has been estimated that at least 12% of the UK population is affected. However, poor clinical coding makes accurate assessment of this burden challenging.
Objective
To calculate primary-care prescribing and both hospital outpatient and admission costs associated with the management of IBS in England.
Design and main outcome measures
Hospital Episode Statistics data for 2012–2013 for all clinical commissioning groups in England were analysed to calculate the tariff cost of IBS. Prescribing analysis and cost tabulation (PACT) data for this period were also analysed.
Results
In 2012–2013, there were 1 219 961 outpatient attendances in gastroenterology and colorectal surgery specialties. Despite this, only 1982 patients were recorded with IBS-specific codes, with a total estimated tariff cost of £812 336. In addition, 28 849 patients were recorded with IBS-related symptom codes at a cost of £11 002 874. In 2011–2012, there were 658 698 diagnostic lower gastrointestinal endoscopies at a tariff cost of £16 967 670 4. Of these, 323 752 (49%) had no further follow-up in secondary care over the subsequent 12 months. PACT data indicated that £44 977 959 and £25 582 752, respectively, were spent on selected laxatives and antispasmodics commonly used to treat IBS in primary care.
Conclusions
Better diagnosing, through improved clinical coding and standardisation of diagnostic criteria, is required to more accurately assess the true burden and allow optimal management of IBS.
Keywords: IRRITABLE BOWEL SYNDROME, COST-EFFECTIVENESS, HEALTH SERVICE RESEARCH
Introduction
Functional gastrointestinal disorders, including functional dyspepsia and irritable bowel syndrome (IBS), are common and account for 40–60% of referrals to gastroenterology outpatient clinics.1 IBS is a chronic and relapsing disorder. Characteristic symptoms include disordered defaecation (constipation or diarrhoea), abdominal distension and abdominal pain and cramping, relieved by defaecation.2 Although IBS does not cause pathological damage to the bowel wall, for some, it can cause pain and significantly affect their quality of life.3
Current evidence suggests an average prevalence of IBS in Europe of 11.5%.4 In the UK specifically, the prevalence of IBS was found to be 4.8% formally diagnosed and 7.2% not formally diagnosed. This equates to a total of 12% of the UK population.4 However, this is likely to be an underestimate, since many people never consult their general practitioner. More women are affected than men, with an OR of 1.67.5
The diagnosis of IBS can be challenging, as there is no biological disease marker. In an effort to standardise diagnosis, symptom-dependent criteria have been developed.
The Manning criteria were originally developed in 1978,6 followed by the Rome criteria in 1992,7 which have been periodically revised (Rome III criteria).2 The Manning criteria include relief of pain with bowel movements, while the Rome criteria define IBS as recurrent abdominal pain associated with altered defaecation. Both Manning and Rome criteria have been criticised for their lack of accuracy and specificity, which can be better obtained by the physician from a thorough history.8 9
Four subtypes of IBS have been described, each dependent on prominent stool pattern:2 IBS with constipation (IBS-C), IBS with diarrhoea, mixed IBS (IBS-M) and unsubtyped IBS (in which stool pattern does not match those previously described). This stratification of IBS allows for more effective management of patient symptoms.
Current guidance is that a diagnosis of IBS should be suspected in patients with abdominal pain relieved by defaecation or associated with abnormal stool, with or without abnormal frequency, and accompanied by two of the following: altered stool passage, abdominal bloating, symptoms worsened by eating and mucus per rectum.2 Basic blood tests are performed on patients who fulfil these criteria. If these are unremarkable, a diagnosis of IBS can be made.10
Both National Institute of Health and Care Excellence (NICE)11 and British Society of Gastroenterology10 guidelines maintain that management of IBS should be within the setting of primary care. However, a significant proportion of those patients who are diagnosed are still referred to secondary care. The main reasons for this are diagnostic uncertainty and the persistence of symptoms. Even once a diagnosis of IBS is made in primary care, despite current guidelines, around half of these patients are referred for endoscopic evaluation.12 Patients with IBS-C identified in primary care rarely have this diagnosis changed following referral and investigation in secondary care.13
Clearly the burden of IBS is high in terms of consultations in both primary and secondary care, and the impact on endoscopy services and prescribing costs. However, poor clinical coding, with no specific codes for the various subtypes of IBS, makes accurate assessment of this burden challenging.4
Impairments in health status for those with IBS have been well documented. In addition, several studies have quantified the burden of IBS on healthcare resources. Hungin and colleagues showed that 7% of European patients with IBS were hospitalised for their condition over a period of 12 months, and 5% had been hospitalised more than once.4 Another study found that 40% of patients with IBS had visited their physician at least once for their symptoms in the past 3 months, and 12% had visited their physician several times.14 Indirect costs through lost work productivity have also been documented. In a systematic review of data from the USA and the UK for 1990–2004, mean work loss attributed to IBS ranged from 8.5 to 21.6 days per year.15
It can be concluded from the literature that the burden of IBS on services is high. The objective of this study was to calculate primary-care prescribing and both hospital outpatient and admission costs associated with the management of IBS in England.
Methods
Data collection and synthesis
The data used in this study were obtained from the AXON Database. AXON is a health data warehouse that provides interrogative analysis and health intelligence on Hospital Episode Statistics (HES). Each HES record has a Healthcare Resource Group (HRG) code that is linked to the national tariff.
HES data from all the clinical commissioning groups in England for the year 2012–2013 (12 months from April 2012 to March 2013) were extracted from the AXON Database to calculate the financial cost of IBS. This was based on the numbers of patients diagnosed with IBS, or symptoms suggestive of IBS, together with the associated costs of diagnosis, management and treatment, and in which treatment specialty, for example, gastroenterology and colorectal surgery units.
Five International Classification of Diseases - 10 (ICD-10) diagnosis codes related to a specific diagnosis of IBS with or without diarrhoea, and symptoms suggestive of IBS were included in this analysis to identify and characterise patients with IBS (table 1). Patients diagnosed with ICD-10 codes for organic gastrointestinal diseases (see online supplementary table S1) were excluded from the analysis.
Table 1.
ICD-10 diagnosis codes for IBS and symptoms suggestive of IBS
| IBS specific | Symptoms suggestive of IBS |
|---|---|
| K58.0 Irritable bowel syndrome with diarrhoea | K59.0 Constipation |
| K58.9 Irritable bowel syndrome without diarrhoea | R19.4 Change in bowel habit |
| R10.4 Abdominal pain |
IBS, irritable bowel syndrome.
Patients with suspected IBS are often referred to gastroenterology and colorectal surgery departments for a diagnostic endoscopy. Data from all hospital provider trusts, including specialty gastroenterology and colorectal surgery departments, that performed endoscopies from 2010 to 2012 were obtained using the Office of Population Censuses and Surveys (OPCS) Classification of Interventions and Procedures V.4 (OPCS-4) codes within the HES data set. The OPCS-4 codes used to identify diagnostic endoscopies are shown in online supplementary table S2.
The costs of antispasmodics and laxatives commonly used to treat IBS in 2012–2013 were obtained from the prescription analysis and cost tabulation (PACT) national data set.
Results
Outpatient attendances and tariff costs
The number of all outpatient attendances in the gastroenterology and colorectal surgery specialty units during 2012–2013 was 1 219 961 (approximately 7.5% of the total of 16 327 150 outpatient attendances in all hospital departments); this compared with 1 057 897 in 2010, 1 133 085 in 2011 and 1 217 993 in 2012, representing a 15.3% increase over this 4-year period.
The total cost of all outpatient attendances in the gastroenterology and colorectal surgery units for patients with a primary diagnosis of IBS was £812 336, of which £244 812 and £567 523 related to costs of patients with (n=564) and without (n=1418) diarrhoea, respectively (table 2). A further 28 849 outpatients were diagnosed with IBS-related symptoms (table 2) at a tariff cost of £11 002 874.
Table 2.
Numbers of outpatients with primary diagnostic ICD-10 codes for IBS and related symptoms in specialty gastroenterology and colorectal surgery units with associated costs
| ICD-10 code | N | Per cent | Cost | Per cent of overall cost |
|---|---|---|---|---|
| IBS specific | ||||
| K58.0 | 564 | 1.8 | £244 812 | 2.1 |
| K58.9 | 1418 | 4.6 | £567 523 | 4.8 |
| Total | 1982 | 6.4 | £812 335 | 6.9 |
| IBS-related symptoms | ||||
| K59.0 | 5200 | 16.9 | £2 027 693 | 17.2 |
| R19.4 | 9284 | 30.1 | £3 276 896 | 27.7 |
| R10.4 | 14 365 | 46.6 | £5 698 284 | 48.2 |
| Total | 28 849 | 93.6 | £11 002 874 | 93.1 |
| Overall total | 30 831 | 11 815 209 | ||
IBS, irritable bowel syndrome.
Overall, the total tariff cost of treating outpatients in England with IBS or related symptoms in 2012–2013 amounted to £11 815 209, of which only 6.9% of the costs were for IBS-specific diagnoses (table 2), and almost half (48.2%) was borne by patients with abdominal pain.
Hospital admissions and associated costs
The total number of patients admitted to gastroenterology and colorectal surgery departments during 2012–2013 was 116 307, of which 918 patients were primarily coded with IBS with diarrhoea and 2599 as IBS without diarrhoea, at tariff costs of £824 897 and £1 959 408, respectively (table 3). A further 112 790 patients were primarily coded with symptoms of constipation (20.2%), change in bowel habit (13.7%) or abdominal pain (63.0%), at a total cost of £92 907 764. Overall, the total tariff cost of treating hospitalised patients with IBS or related symptoms in England in 2012–2013 amounted to £95 692 068, the majority of which were for abdominal pain (62.3%) and constipation (25.6%, table 3).
Table 3.
Numbers of inpatients with primary diagnostic ICD-10 codes for IBS and related symptoms in specialty gastroenterology and colorectal surgery units with associated costs
| ICD-10 code | N | Per cent | Cost | Per cent of overall cost |
|---|---|---|---|---|
| IBS specific | ||||
| IBS with diarrhoea (K58.0) | 918 | 0.8 | £824 897 | 0.9 |
| IBS without diarrhoea (K58.9) | 2599 | 2.2 | £1 959 408 | 2.0 |
| Total | 3517 | 3.0 | £2 784 305 | 2.9 |
| IBS-related symptoms | ||||
| Constipation (K59.0) | 23 529 | 20.2 | £24 526 655 | 25.6 |
| Change in bowel habit (R19.4) | 15 986 | 13.7 | £8 756 006 | 9.2 |
| Abdominal pain (R10.4) | 73 275 | 63.0 | £59 625 103 | 62.3 |
| Total | 112 790 | 97.0 | £92 907 764 | 97.1 |
| Overall total | 116 307 | 95 692 068 | ||
IBS, irritable bowel syndrome.
Diagnostic endoscopies
Between 2011 and 2013, there was a 13.5% increase in inpatient and outpatient lower gastrointestinal endoscopies, from 600 432 to 681 541. During 2011–2012, 96.2% of the endoscopies (658 698) were performed either as day cases or as outpatients, at an overall tariff cost of £169 676 704 (average of £258 per endoscopy). Overall, 54% of these patients were women and 46% were men; the ages ranged from 47 to 79 years (median 57 years). The majority of endoscopies (76.5%) were performed as day case procedures. A total of 323 752 patients (49%) had no further contact as an inpatient or outpatient in the 12 months following the endoscopy.
Prescribing patterns and costs
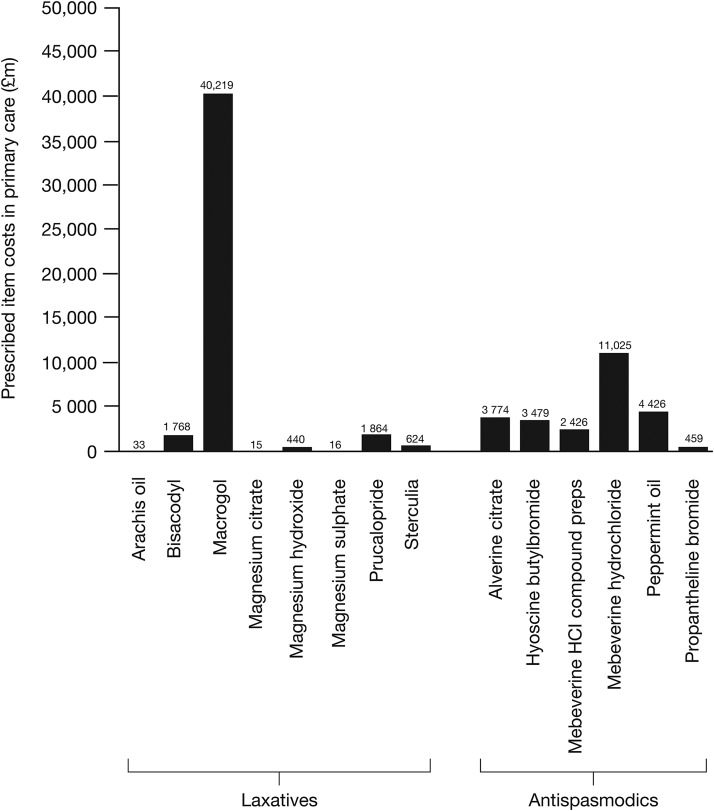
National Health Service (NHS) prescribing (PACT) data indicated that, nationally, the costs of laxatives and antispasmodics that are commonly prescribed by general practitioners to treat IBS were £44 977 959 and £25 582 752, respectively (figure 1). Of those treatments analysed, the laxative with the highest individual cost was macrogol (polyethylene glycol), at a cost of £40 219 270, while the antispasmodic with the highest individual cost was mebeverine, at a cost of £11 024 948 (figure 1).
Figure 1.
Cost of individual prescriptions for gastrointestinal disorders in England between 1 April 2012 and 31 March 2013.
Discussion
These analyses demonstrated the significant burden of IBS on the English NHS in terms of both strain on specialist services and monetary cost. Outpatient attendances to gastroenterology and colorectal surgery specialties for patients with IBS or IBS-related symptoms are increasing, accounting for approximately 7.5% of total outpatient attendances across all specialties. There was an increase in 15.3% from 2010 to 2013, with a total attributable cost of almost £12 million for 2012–2013, at an average cost per patient of £383.20. These figures may still be an underestimate as patients coming from primary care and into secondary care often are not given a final diagnosis of IBS until a number of investigations are carried out to exclude potential pathology, both as a result of physician hesitation and often anxiety from patients. These may also be some of the reasons behind the increased referrals to specialist care for functional bowel disorders despite guidance from NICE that these conditions should be managed in primary care. Some primary care physicians may not be confident in using (or unaware of) the Rome III criteria to help them in diagnosing IBS without the need for referral. Difficult to manage patients may require input from secondary care with regard to less used medical therapies and specialist dietician input, not available to the primary care physician. Some patients with high anxiety levels may request specialist referral themselves.
One study from 2002 (data from 1997) calculated the direct cost per patient of IBS in England to be £316.20, 25.3% of which was attributed to outpatient visits (£80).16 Taking into account inflation from 1997 to 2012, this relates to a current equitable cost of £123.30. Comparing this figure with our calculated figure of £383.20, it may be assumed that the cost of outpatient attendances for patients diagnosed with IBS has increased approximately threefold. There were also 116 307 admissions to specialist gastroenterology and colorectal surgery departments for patients diagnosed with IBS with or without diarrhoea in 2012–2013, at an average cost per patient of £822.75.
Data from three US studies (1997–2003) found that inpatient costs equated to £432.12, £835.81 and £1224.72 (adjusted for inflation to 2012).17–19 More recently, a US study (2010 data) calculated inpatient and outpatient annual costs associated with IBS-C to be equivalent to £96.01 and £612.04, respectively, with overall costs of £857.20 per patient per year for IBS-C-related medical and prescription costs.20 When all medical and prescription costs for patients with IBS-C were included, this rose to annual costs equivalent to £1565.23, £3855.64 and £7179.96 in 2010, respectively.
Furthermore, Guerin et al21 showed that during a 12-month treatment period since index prescription (1997–2010), adjusted incremental costs for patients with IBS-C with treatment failure versus patients without were equivalent to £2795.06 in 2010.
Against current guidance, patients are still being referred for specialist assessment and endoscopy even after a diagnosis of IBS is made in primary care. This study found that 49% of patients in 2011–2012 who had undergone an outpatient endoscopy had no further contact either as an inpatient or outpatient in the subsequent 12 months, suggesting that their symptoms were functional in nature. Clearly, this does not take into account those patients who will also not have any further follow-up for the subsequent 12 months for other reasons (eg, screening colonoscopies, polyp surveillance and IBD surveillance). However, this is in line with findings from the British Society of Gastroenterology, which quotes a figure of 50% of patients with IBS being referred from primary care for endoscopy.12
Several studies have assessed the burden and health impact of IBS across Western Europe and the USA; however, less effort has gone into focusing on the impact of different subtypes of IBS. Clinical coding exists only for IBS with diarrhoea and IBS without diarrhoea (codes K58.0 and K58.9, respectively). Identification of IBS-C is more of a challenge; ICD-10 code K58.9 (IBS without diarrhoea) is the most closely aligned clinical code. Given that IBS is poorly coded currently, and that IBS-C does not have a clinical code, it is likely that a proportion of patients who are yet to be definitively coded as having IBS may be given a more symptom-oriented coding diagnosis.
A group in the USA and Spain that assessed the burden of IBS-C in three European countries reported prevalences of 0.55%, 1.44% and 1.35% in France, Italy and the UK, respectively.22 There are few comparable data in the literature; however, a recent report from Lin et al13 (2013) evaluated the population prevalence of differing IBS subtypes within the UK and reported a total population prevalence of 6%, with the highest being IBS-M (2.7%). The prevalence of IBS-C was reported to be 0.7% (lower than the previous study of 1.35%).13 Other groups have also reported inconsistencies in estimates of IBS-C.
Without a definitive code for IBS-C, it is not clear how much of the true burden of IBS-C is being captured with ICD-10 code K58.9 (IBS without diarrhoea). This study showed that healthcare costs for both inpatients and outpatients with constipation, change in bowel habit or abdominal pain are close to £1.2 billion for 2012–2013, or an average cost of £733.63. Most patients are diagnosed with IBS-related symptoms and not a diagnosis of IBS specifically. In view of this, costs attributable to IBS-specific coding may appear to be significantly lower than costs attributed to IBS-related symptoms.
There is little information in the literature with which to compare these findings: one study in the USA investigated direct costs of patients with chronic constipation only. Nguyen et al23 included non-gastrointestinal outpatient visits as well as gastrointestinal outpatient visits, but not inpatient care, and reported a cost of $7510, equating to £5098 (adjusted for inflation). They did, however, find throughout all their reviews of IBS and/or chronic constipation that estimates of economic burden of disease varied widely by a factor of 5 for direct costs and by a factor of 10 for indirect costs.23
This wide range of cost estimates for patients with both IBS and chronic constipation will be affected by not only the lack of diagnostic consensus (Manning vs Rome criteria) across studies but also poor coding, especially in the case of identifying patients with IBS-C. It may be expected that a proportion of the patients coded K59.0, R19.4 and R10.4 (constipation, change in bowel habit and abdominal pain, respectively) may actually have IBS-C.
There are a number of limitations to this study. Data errors are inevitable but the size of the study should limit this impact. While the accuracy of routine coding from which HES is generated has been questioned,24 other studies have supported the quality of HES data and have validated it as accurate.25 Multiple codings for a single hospital episode may have overestimated costs, which is more likely to be the case with the symptoms suggestive of IBS codes. Our study also did not specifically exclude other possible medical and surgical conditions presenting with abdominal pain which (coding R10.4)
With regard to cost calculations, there may be the additional confounding factor of multiple reviews. However, both this analysis and that of the study in comparison calculated mean costs. Taking into account inflation, as well as an increase in IBS diagnoses as treatment options increase, this may well be a true significant rise in cost.
Using all of the above data, commissioners and clinicians may recognise that the true burden of IBS is much higher than the actual recorded cases. Standardisation of diagnostic criteria and definitive coding for all subtypes is needed to more accurately assess the true burden and allow optimal management of the spectrum of IBS.
Supplementary Material
Footnotes
Contributors: All authors were involved in the critical revision of the manuscript for important intellectual content. The introduction and context for discussion were provided by Anet Soubieres. All authors contributed to the development of the manuscript, approved the final content and agreed to proceed with submission for publication.
Funding: This study was funded by Almirall. Harvey Walsh Ltd provided aggregated HES data reports from AXON via a commercial reuse licence from the Health and Social Care Information Centre. Medical writing assistance for the Methods and Results sections of the paper was provided by K Ian Johnson, on behalf of Complete Medical Communications and Complete Clarity, funded by Almirall UK.
Competing interests: MR is an employee of Almirall UK.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jones J, Boorman J, Cann P, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut 2000;47(Suppl 2):ii1–19. doi:10.1136/gut.47.suppl_2.ii1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. doi:10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Burnett CK, Cook EW III, et al. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci 1996;41:2248–53. doi:10.1007/BF02071408 [DOI] [PubMed] [Google Scholar]
- 4.Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40 000 subjects. Aliment Pharmacol Ther 2003;17:643–50. doi:10.1046/j.1365-2036.2003.01456.x [DOI] [PubMed] [Google Scholar]
- 5.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21. doi:10.1016/j.cgh.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 6.Manning AP, Thompson WG, Heaton KW, et al. Towards positive diagnosis of the irritable bowel. Br Med J 1978;2:653–4. doi:10.1136/bmj.2.6138.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WG, Creed F, Drossman DA, et al. Functional bowel disease and functional abdominal pain. Gastroenterol Int 1992;5:75–91. [Google Scholar]
- 8.Talley NJ, Phillips SF, Melton LJ, et al. Diagnostic value of the Manning criteria in irritable bowel syndrome. Gut 1990;31:77–81. doi:10.1136/gut.31.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M. Do the symptom-based, Rome Criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? The con argument. Clin Gastroenterol Hepatol 2009;8:129 doi:10.1016/j.cgh.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770–98. doi:10.1136/gut.2007.119446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence. Clinical guideline 61: irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. 2008. [Google Scholar]
- 12.British Society of Gastroenterology. Chronic management: IBS/Functional symptoms. 2014. http://www.bsg.org.uk/clinical/commissioning-report/ibs/functional-symptoms.html (accessed Apr 2014).
- 13.Lin S, Mooney PD, Kurien M, et al. Prevalence, investigational pathways and diagnostic outcomes in differing irritable bowel syndrome subtypes. Eur J Gastroenterol Hepatol 2014;26:1176–80. doi:10.1097/MEG.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 14.Gulewitsch MD, Enck P, Hautzinger M, et al. Irritable bowel syndrome symptoms among German students: prevalence, characteristics, and associations to somatic complaints, sleep, quality of life, and childhood abdominal pain. Eur J Gastroenterol Hepatol 2011;23:311–16. doi:10.1097/MEG.0b013e3283457b1e [DOI] [PubMed] [Google Scholar]
- 15.Maxion-Bergemann S, Thielecke F, Abel F, et al. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics 2006;24:21–37. doi:10.2165/00019053-200624010-00002 [DOI] [PubMed] [Google Scholar]
- 16.Akehurst RL, Brazier JE, Mathers N, et al. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics 2002;20:455–62. doi:10.2165/00019053-200220070-00003 [DOI] [PubMed] [Google Scholar]
- 17.Leong SA, Barghout V, Birnbaum HG, et al. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch Intern Med 2003;163:929–35. doi:10.1001/archinte.163.8.929 [DOI] [PubMed] [Google Scholar]
- 18.Creed F, Ratcliffe J, Fernandez L, et al. Health-related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann Intern Med 2001;134:860–8. doi:10.7326/0003-4819-134-9_Part_2-200105011-00010 [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Brook R, Nichol B. Similarities between constipation with and without irritable bowel syndrome in a California Medicaid (Medi-Cal) population: costs trends by category in the 12 months after diagnosis from 1997–2002 [abstract]. Gastroenterology 2008;134:PA468. [Google Scholar]
- 20.Doshi JA, Cai Q, Buono JL, et al. Economic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured population. J Manag Care Pharm 2014;20:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerin A, Carson RT, Lewis B, et al. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J Med Econ 2014;17:577–86. doi:10.3111/13696998.2014.919926 [DOI] [PubMed] [Google Scholar]
- 22.Dibonaventura MD, Prior M, Prieto P, et al. Burden of constipation-predominant irritable bowel syndrome (IBS-C) in France, Italy, and the United Kingdom. Clin Exp Gastroenterol 2012;5:203–12. doi:10.2147/CEG.S35568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen T, Palsson O, Von Korff M. Health care costs and utilization in patients with chronic constipation [abstract]. Gastroenterology 2014;134:PA280. [Google Scholar]
- 24.Campbell SE, Campbell MK, Grimshaw JM, et al. A systematic review of discharge coding accuracy. J Public Health Med 2001;23:205–11. doi:10.1093/pubmed/23.3.205 [DOI] [PubMed] [Google Scholar]
- 25.Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health (Oxf) 2012;34:138–48. doi:10.1093/pubmed/fdr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.