Abstract
Background
Patients with bronchiectasis often suffer from concurrent comorbidities but their nature, prevalence and impact on disease severity and outcome is poorly understood. We aimed to evaluate comorbidities in bronchiectasis patients and determine their prognostic value on disease severity and mortality.
Methods
An observational cohort analysis of 986 bronchiectasis patients across four European centres was performed for score derivation. Comorbidity diagnoses were based on standardised definitions obtained on full review of hard copy and electronic records, prescriptions and investigator definitions. Weibull parametric survival analysis was used to model the prediction of 5-year mortality to construct the Bronchiectasis Aetiology Comorbidity Index (BACI). We tested the BACI as a predictor of outcomes and explored whether the BACI added further prognostic information when used alongside the Bronchiectasis Severity Index (BSI).
Findings
Median number of comorbidities per patient was 4 (IQR 2-6), range 0-20. Thirteen comorbidities independently predicting mortality were integrated into the BACI. The overall hazard ratio for death conferred by a one point increase in the BACI was 1.18 (1.14-1.23), p<0.0001. The BACI predicted 5-year mortality, hospitalisations, exacerbations and health-related quality of life across all BSI risk strata (p<0.0001). When used in conjunction with the BSI, the combined model was superior to either model alone. The BACI was validated in two independent international cohorts.
Interpretation
Multimorbidity is frequent in bronchiectasis and can negatively influence survival. The BACI complements the BSI in assessing mortality and disease outcomes in patients with bronchiectasis.
Funding
1. European Bronchiectasis Network (EMBARC).2. Health Research Board Ireland.
Keywords: Comorbidity, bronchiectasis, severity, mortality
Introduction
Bronchiectasis is a chronic airway disease showing an increasing prevalence over the past decade with an associated growing morbidity and mortality worldwide.1 As a complex multi-component disease, bronchiectasis is characterised by chronic systemic inflammation that frequently co-exists with comorbidities, which may be causative, synergistic, or coincidental, depending on the manner in which they interact.2
In addition to known aetiologies of bronchiectasis, several other diseases may occur at any stage of bronchiectasis and are likely major contributors to increased hospitalisations, healthcare utilisation and socioeconomic costs.3 These include cardiovascular disorders, gastro-oesophageal reflux disease (GORD), psychological illnesses, pulmonary hypertension, cognitive impairment, and lung, oesophageal and hematological malignancies.4–12 A few studies have explored bronchiectasis-related comorbidities and suggest that, compared with age and sex-matched controls, some comorbidities are more likely to coexist with bronchiectasis4–12 with a relevant impact on different outcomes, such as exercise capacity,4 exacerbation frequency,6,8 lung function,7,11 health-related quality of life,6,8,9,11 healthcare utilisation,8 and mortality.10,12 Few studies have systematically evaluated the prevalence and role of comorbidities in bronchiectasis; several were performed in single centres with small patient numbers4–6,10,11 or utilised retrospective databases or cross-sectional designs7–9,12 limiting the applicability of their findings. However, none have systematically evaluated how comorbidities impact on prognosis in a prospective study.
It is suggested that individual comorbidities and aetiologies of bronchiectasis, such as chronic obstructive pulmonary disease (COPD) and rheumatoid arthritis (RA), confer an increased severity and mortality compared to other aetiologies despite targeted treatment of underlying conditions.13,14 Recent literature has also shown that in approximately 30-40% of patients with bronchiectasis, the primary cause of death is attributed to non-respiratory disease.15 However, current guidelines fail to provide clear recommendations on how comorbidities should be identified, assessed and treated within the context of bronchiectasis. Neither of the two prognostic scoring indices recently developed to estimate mortality in patients with bronchiectasis (the bronchiectasis severity index – BSI- and the FACED score-see online supplement for calculation of scores) were planned to systematically evaluate the prevalence and role of comorbidities.16,17
In view of this lack of data, we designed a study which aimed to determine not only the prevalence of individual comorbidities in bronchiectasis patients but also the strength of association between the number and nature of comorbidities and risk of death over time. We further aimed to develop a disease-specific comorbidity index and explore if this could provide additional prognostic information to that provided by the BSI.
Our hypothesis was that multiple comorbidities would be a common finding across national cohorts, that these would contribute significantly to mortality and that it was practicable to apply a standardised assessment to assess the role of comorbidities in the mortality of patients with bronchiectasis.
Methods
Data collection
This study included data from four databases of prospectively enrolled outpatients with bronchiectasis in Dundee (UK), Galway (Ireland), Leuven (Belgium) and Monza (Italy). Consecutive patients aged ≥18 years were enrolled on the basis of a radiological diagnosis made on high-resolution computed tomography (HRCT) and a clinical history consistent with bronchiectasis. Patients with cystic fibrosis or traction bronchiectasis due to pulmonary fibrosis were excluded in all four cohorts. Data from each cohort was collected independently following individual ethics approval and collated for statistical analysis. Standardised assessment and diagnostic work-up according to the 2010 British Thoracic Society (BTS) guidelines was performed at each site as detailed in the online supplement. Enrolment into the study required that all variables required to calculate clinical prediction scores and the key relevant outcomes of mortality, hospitalisations and exacerbations on follow-up were available. Exacerbations and hospitalisations were defined according to BTS guidelines, and mortality, evaluated at the end of the 5-year follow-up period, was confirmed in 100% of participants.18 This cohort is entirely independent from the cohorts used to derive the BSI or FACED scores.16,17
Comorbidities
All comorbidity diagnoses were systematically recorded according to standardised definitions and were retrospectively obtained on full review of hard copy or electronic records, patients’ prescriptions and review of confirmatory tests where available. The 19 conditions included in the Charlson Comorbidity Index (CCI) were included in data collection plus any other identified comorbidity.19 Conditions that had completely resolved, e.g. pneumonia, were excluded. Objective confirmation of diagnoses was sought in each case where possible. Self-reported diagnoses consisted of GORD, depression and anxiety as per standard practice internationally. Further details of comorbidity assessment are available in the online supplement.
Statistical Analysis and Derivation of Clinical Prediction Tool
Continuous data are presented as mean and standard deviation (SD) or median and interquartile range (IQR) where appropriate, and categorical data as frequencies and percentages. The Mann Whitney U and chi-squared test were used for comparison of numerical and categorical data, respectively. For comparisons of more than two groups, one-way analysis of variance or the Kruskal-Wallis test were used as appropriate. Weibull parametric survival analysis was used to model the prediction of 5-year mortality. Three candidate comorbidity scores were considered and compared to the CCI, BSI and FACED scores: (a) The Comorbidity count - a simple sum of the number of comorbidities per individual patient; (b) The Bronchiectasis Comorbidity Index (BCI) - a weighted comorbidity score without those conditions regarded as potential underlying aetiologies of bronchiectasis; (c) The Bronchiectasis Aetiology Comorbidity Index (BACI) - a weighted comorbidity score including conditions regarded as underlying aetiologies.
Based on Peduzzi’s modelling, a maximum of 13 variables could be incorporated into the multivariable models in order to comply with statistical norms based on the number of outcomes in our cohort.20 Comorbidities with <1% prevalence or those with significant collinearity were excluded. Variables were included in the model using a backward stepwise approach requiring a p<0.2 for retention in the model. All models were adjusted for age and gender. These variables were then formed into prediction tools using the rounded averaged β-coefficient to award “points” for each variable as previously described.16 The sum of the points intends to capture the individual or combination of diseases affecting each patient. The performance of the resulting models for mortality was assessed using the area under the receiver operator characteristic curve (AUC) with the exception of the UK validation cohort which had a much longer median follow up of 19 years, whereby Kaplan-Meier analysis was performed to avoid favoring fixed effects at the expense of modifiable risk factors that may increase short-term risk but not necessarily guarantee long-term risk. We subsequently tested the predictive ability of the optimal model to determine future disease outcomes using Spearman’s rho correlation and explored if it could add further prognostic information when used alongside the BSI and FACED mortality index. Some endpoints, such as quality of life, were only available in 2 cohorts (Dundee and Monza). Such analyses were only conducted in patients with available data – no imputation or other methods of handling missing data were used. For all analyses, p< 0.05 was considered statistically significant. All analyses were performed using SPSS Version 21 (SPSS, Chicago, IL, USA) for Windows platform and Graph Pad Prism Version 5 (Graph Pad Software, Inc. San Diego, CA, USA). The reporting of this study conforms to STROBE and TRIPOD recommendations.21 22
Validation cohorts
The derived index was subsequently validated in two independent cohorts. One was a historical cohort of patients recruited for the validation of the SGRQ in bronchiectasis.15 This cohort was selected as a prospective study with the longest duration of follow-up available in the literature to date, where data on comorbidities was systematically collected. The other validation cohort consisted of prospectively recruited bronchiectasis patients in Serbia in Eastern Europe, enabling further assessment of the generalisability of the score.
Role of the funding source
The funding source had no role in study design, data collection, analysis or interpretation or in the writing of the report. The corresponding and lead senior authors had full access to all of the data and the final responsibility to submit for publication."
Results
Characteristics of the cohort and comorbidities
The demographic and baseline characteristics of the 986 patients included in the analysis are summarised in Table 1 and are consistent with other published series in terms of older age, female predominance and bacterial colonisation rates. The cohort consisted primarily of Caucasian females with a median FEV1% predicted of 75% (54-95) and a median FEV1/FVC% predicted of 70% (59-79) demonstrating moderate airflow limitation. The median BSI was 6 (4-10) and all BSI tertiles (mild, moderate and severe) were evenly represented. Mortality, n (%) at 1, 2, 3 and 5 years of follow-up were 37 (3.7), 47 (4.8), 85 (8.6) and 122 (12.4), respectively. No patients received lung transplantation during follow-up.
Table 1.
Derivation Cohort Patient Characteristics
| Patient characteristics | Derivation Cohort (n=986) |
| Demographic variables | |
| Age, Years, Median (IQR) | 67 (57-74) |
| Female, n (%) | 589 (59.7) |
| Body Mass Index, Median (IQR) | 24.6 (21.2-27.8) |
| Smokers / Ex-smokers, n (%) | 379 (38.4) |
| Clinical status | |
| MRC, Median (IQR) | 2 (1-3) |
| Exacerbations in the previous year, Median (IQR) | 2 (1-3) |
| At least one hospitalisation in the previous year, n (%) | 224 (22.7) |
| Lung function | |
| FEV1 % predicted, Median (IQR) | 75 (54-95) |
| Radiology status | |
| Reiff score, Median (IQR) | 4 (2-6) |
| Microbiological status | |
| Pseudomonas colonisation, n (%) | 122 (12.4) |
| Other colonisation, n (%) | 229 (25.3) |
| Disease severity | |
| BSI score, Median (IQR) | 6 (4-10) |
| BSI 0-4 (mild), n (%) | 312 (31.6) |
| BSI 5-8 (moderate), n (%) | 351 (35.6) |
| BSI ≥ 9 (severe), n (%) | 323 (32.8) |
| Comorbidities | |
| No. of comorbidities, Median (IQR) | 4 (2-6) |
| Range | 0-20 |
Definition of abbreviations: MRC: Medical Research Council dyspnoea score, FEV1%: forced expiratory volume in 1 second % predicted; BSI: Bronchiectasis Severity Index.
A total of 81 comorbidities were reported in this cohort. The median (IQR) number of comorbidities was 4 (2-6) per subject for the whole cohort with a range of 0-20; males had significantly more co-morbidities than females, median 4 (2-6) for males and 3 (2-5) for females, p=0.005. . The median number of comorbidities was higher for non-survivors compared with survivors [6 (4-9) vs 3 (2-5) respectively, p<0.0001. A significant association was also observed between the median number of comorbidities and the BSI score (low risk: 3; intermediate risk: 3; high risk: 4; p<0.0001).
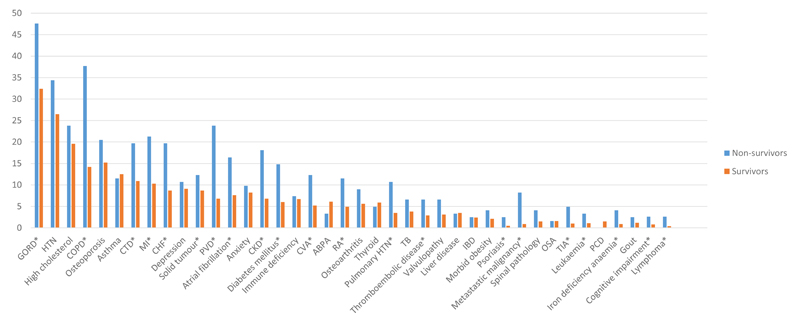
The distribution of the most prevalent (>1%) and significant comorbidities is shown in Figure 1. There is a heavy tailed distribution, ranging from 34% to less than 1%. 26 comorbidities had a significantly higher prevalence in non-survivors compared with survivors (the majority are shown here by the presence of asterisks in Figure 1 with full details in Table S1 in the online supplement).
Figure 1.
Comorbidites in order of overall prevalence among survivor and non-survivor bronchiectasis patients. The figure also includes those comorbidities with a significantly higher prevalence in non-survivors compared with survivors regardless of their absolute prevalence (asterisk). Definitions of abbreviations: GORD: Gastro-oesophageal reflux disease; HTN: hypertension; COPD: Chronic obstructive pulmonary disease; CTD: Connective tissue disease; MI: myocardial infarction; CHF: Chronic heart failure; PVD: Peripheral vascular disease; CKD: Chronic kidney disease; CVA: Cerebrovascular attack; RA: Rheumatoid arthritis; ABPA: Allergic bronchopulmonary aspergillosis; TB: Tuberculosis; OSA: Obstructive sleep apnea.
Comorbidity scores
The Comorbidity Count
In its simplest form, the comorbidity count, i.e. the sum of the number of comorbidities per patient, was significantly associated with mortality, with a hazard ratio (HR) of 1.17, 95% CI 1.12-1.23 on univariate analysis, suggesting that an increase of 1 comorbidity in the count equates to a 17% increase in mortality. When adjusted for BSI, the HR (95% CI) was still significant at 1.13 (1.08-1.18).
The Bronchiectasis Aetiology Comorbidity Index (BACI)
The comorbidities included in the BACI are shown in Table 2. COPD, connective tissue disease, inflammatory bowel disease and asthma were all included in the final model predicting mortality and are recognised aetiologies of bronchiectasis that may be associated with poorer outcomes. Overall, the HR (95% CI) for death conferred by a one-point increase in the BACI score was 1.18 (1.14-1.23), p<0.0001. Interestingly, this is higher than the adjusted HR for the BSI of 1.10 (1.06-1.14), p<0.0001 suggesting that the BACI has independent prognostic value comparable to the BSI.
Table 2.
Derivation of the Bronchiectasis Aetiology Comorbidity Index (BACI) and Point Allocation
| Comorbidity | Hazard Ratio | 95% CI | P value | Points |
| Metastatic malignancy | 6.69 | 3.53-12.68 | <0.0001 | 12 |
| Haematological malignancy | 2.85 | 1.17-6.97 | 0.02 | 6 |
| COPD | 2.22 | 1.53-3.23 | <0.0001 | 5 |
| Cognitive impairment | 2.21 | 0.82-6.01 | 0.12 | 5 |
| Inflammatory bowel disease | 2.01 | 0.75-5.40 | 0.17 | 4 |
| Liver disease | 1.94 | 0.80-4.72 | 0.14 | 4 |
| Connective tissue disease | 1.78 | 1.19-2.68 | 0.005 | 3 |
| Iron deficiency anaemia | 1.78 | 0.80-2.68 | 0.16 | 3 |
| Diabetes | 1.76 | 1.10-2.80 | 0.02 | 3 |
| Asthma | 1.65 | 1.00-2.73 | 0.050 | 3 |
| Pulmonary hypertension | 1.58 | 0.88-2.84 | 0.12 | 3 |
| Peripheral vascular disease | 1.50 | 1.00-2.25 | 0.052 | 2 |
| Ischaemic heart disease | 1.31 | 0.91-1.89 | 0.14 | 2 |
Definition of abbreviations: COPD: Chronic Obstructive Pulmonary Disease.
The Bronchiectasis Comorbidity Index (BCI)
As a sensitivity analysis, we evaluated a model excluding the above conditions thought to be associated with bronchiectasis, producing similar results (Table S2 in online supplement). The HR for the BCI was comparable at 1.17 (1.12-1.23), confirming the importance of comorbidities in bronchiectasis prognosis.
Comparison of comorbidity scores to predict 5-year mortality
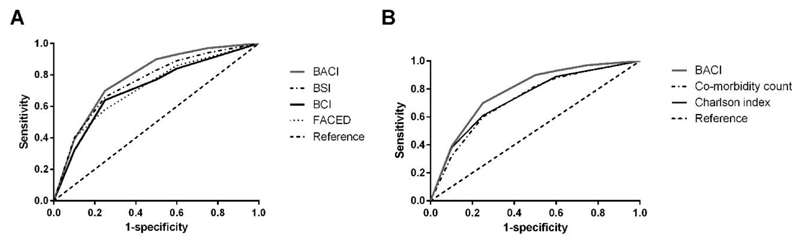
Comparative AUC (95% CI) scores for the BACI and BCI with the widely validated BSI can be seen in figure 2(a). The BACI has the highest overall predictive ability in this cohort to predict 5-year survival with an AUC score of 0.79 (0.75-0.83) versus 0.74 (0.69-0.78) for the BCI, 0.78 (0.73-0.84) for the BSI and 0.71 (0.66-0.75) for the FACED score, respectively. The CC and CCI (figure 2(b)) showed AUC scores of 0.72 (0.67-0.76) and 0.74 (0.69-0.78) respectively, which were inferior to the BACI (p=0.0001 on comparing AUC values), suggesting that a specific comorbidity index for bronchiectasis is appropriate.
Figure 2.
(a) The performance of the Bronchiectasis Aetiology Comorbidity Index (BACI) in relation to the Bronchiectasis Comorbidity Index (BCI) without aetiologies and the widely validated Bronchiectasis Severity Index (BSI) using area under the receiver operator characteristic curve (AUC) scores. (b) The performance of the BACI in relation to the Comorbidity Count and the widely validated Charlson Comorbidity Index (CCI) using area under the receiver operator characteristic curve (AUC) scores.
The BACI performed consistently better than all scores in predicting 2, 3 and 5-year mortality in this cohort, with AUC scores of 0.75, 0.76 and 0.79 respectively, indicating that the score works similarly for annual prediction as for longer term prediction.
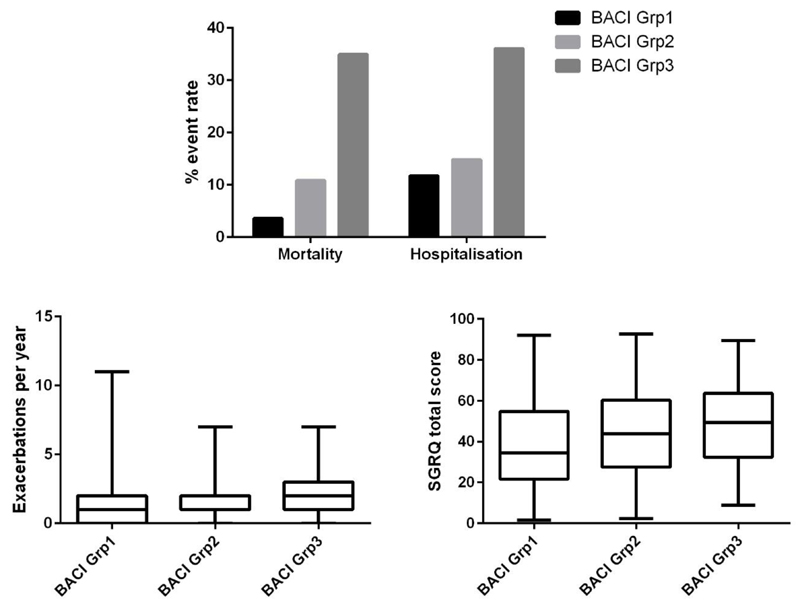
The AUC was used to identify the level of the BACI with the greatest predictive value for death in patients with bronchiectasis. Patients were classified into tertiles designated no high-risk comorbidities (for patients with a score of zero, n=402), intermediate risk comorbidities (for patients with ≥1 and <6 points, n=398) and high-risk comorbidities (for patients with a score ≥ 6 points, n=186). The relationship between these risk groups and mortality and morbidity are shown in Figure 3.
Figure 3.
The performance of the Bronchiectasis Aetiology Comorbidity Index (BACI) in predicting mortality, hospitalisations, exacerbation frequency and quality of life across all risk strata. All between group comparisons for mortality and hospitalisations were statistically significant at p<0.001. Between group comparisons for exacerbations were statistically significant at p=0.03. Correlation between the BACI and St George’s Respiratory Questionnaire (SGRQ) assessing quality of life was statistically significant at p<0.01.
The sensitivity and specificity values for the BACI, BCI and the BSI are shown in table 3.
Table 3.
Sensitivity and specificity values for derived clinical prediction tools
| Organisms | PLR | NLR | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| BACI | ||||||
| Grp 2 and 3 vs Grp 1 | 1.61 (1.47-1.75) | 0.26 (0.16-0.42) | 88.5 (81.5-93.6) | 44.9 (41.6-48.3) | 18.5 (15.4-21.9) | 96.5 (94.2-98.1) |
| Grp 3 vs Grp 1 and 2 | 3.80 (3.01-4.81) | 0.54 (0.45-0.66) | 53.3 (44.0-62.4) | 86.0 (83.5-88.2) | 34.9 (28.1-42.3) | 92.9 (90.9-94.6)) |
| BCI | ||||||
| Grp 2 and 3 vs Grp 1 | 1.57 (1.42-1.74) | 0.35 (0.23-0.53) | 83.6 (75.8-89.7) | 46.9 (43.5-50.3) | 18.2 (15.1-21.6) | 95.3 (92.8-97.1) |
| Grp 3 vs Grp 1 and 2 | 4.68 (3.27-6.69) | 0.73 (0.65-0.83) | 32.0 (23.8-41.0) | 93.2 (91.3-94.8) | 39.8 (30.0-50.2) | 90.7 (88.6-92.5) |
| BSI | ||||||
| Mod/severe vs mild | 1.37 (1.26-1.48) | 0.31 (0.18-0.52) | 89.3 (82.5-94.2) | 34.6 (31.4-37.9) | 16.2 (13.5-19.2) | 95.8 (92.9-97.8) |
| Severe vs Mod/mild | 2.18 (1.83-2.59) | 0.53 (0.42-0.67) | 62.3 (53.1) | 71.4 (68.3-74.4) | 23.5 (19.0-28.5) | 93.1 (90.9-94.9) |
Definition of abbreviations: BACI: Bronchiectasis Aetiology and Comorbidity Index; BCI Bronchiectasis Comorbidity Index; BSI: Bronchiectasis Severity Index
The BACI, BSI and mortality
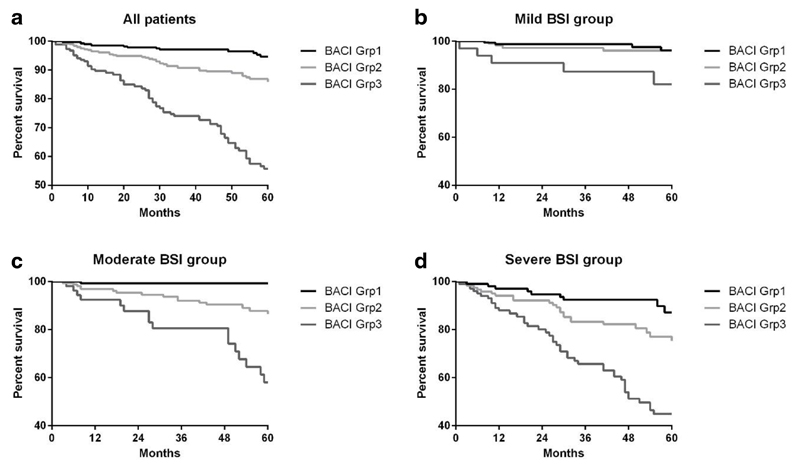
Comparable with previous studies, the BSI was a significant predictor of death in patients with bronchiectasis. To demonstrate the predictive contribution of the BACI to the BSI, Kaplan-Meier survival curves of the BACI groups stratified according to BSI severity are shown in Figure 4. All between group comparisons were statistically significant at p<0.001.
Figure 4.
Kaplan-Meier survival curves representing survival probability at 5 years. (a) Kaplan-Meier survival curve according to the Bronchiectasis Severity Index (BSI) with mild (0-4 points), moderate (5-8 points) and severe (≥ 9 points). To demonstrate the predictive contribution of the Bronchiectasis Aetiology Comorbidity Index (BACI) to the BSI, the survival curves were represented for each BSI tertile (b) mild disease, (c) moderate disease, and (d) severe disease. BACI group 1 = score of O, BACI group 2 = score <6, BACI group 3 = score ≥ 6. Survival is significantly lower in BACI groups 2 and 3 compared to group 1 which represents no high risk comorbidities. The gap between groups becomes much more evident as disease severity increases.
A prediction model incorporating both the BSI and the BACI was superior to either model alone for the prediction of 5-year mortality in this cohort with an AUC (95% CI) of 0.83 (0.79-0.87).
The BACI and other disease outcomes
Significant correlations of the BACI with a number of baseline demographic variables and important clinical outcomes were noted. The BACI correlated with both the BSI and FACED disease severity scores as well as lung function, radiological scores, dyspnoea scores, prior exacerbations and hospitalisations. Of note, it also predicts subsequent exacerbations and hospitalisations on follow-up and is independently correlated with Pseudomonas aeruginosa colonisation, offering further predictive potential in the clinical setting and suggesting that comorbidities directly influence pulmonary outcomes (Table 4).
Table 4.
Correlation of the BACI with clinical scores and severity indices
| Patient characteristics (n=986) | Spearman’s Rho | P value |
| BSI | 0.23 | <0.0001 |
| FACED | 0.24 | <0.0001 |
| Age | 0.20 | <0.0001 |
| Male gender | 0.20 | <0.0001 |
| Smoking history | 0.33 | <0.0001 |
| Reiff radiological score | 0.08 | 0.008 |
| MRC dyspnoea score | 0.31 | <0.0001 |
| LTOT | 0.23 | <0.0001 |
| Prior exacerbations | 0.12 | 0.0002 |
| Prior hospitalisations | 0.13 | <0.0001 |
| FEV1 % | -0.26 | <0.0001 |
| Pseudomonas colonisation | 0.078 | 0.01 |
| Exacerbations on follow-up | 0.11 | 0.0006 |
| Hospitalisations on follow-up | 0.22 | <0.0001 |
Definition of abbreviations: BSI: Bronchiectasis Severity Index, FACED: Acronym for a 5-component disease severity score; MRC: Medical Research Council, LTOT: long term oxygen therapy, FEV1%: Forced expiratory volume in 1 second % predicted.
Independent validation cohorts
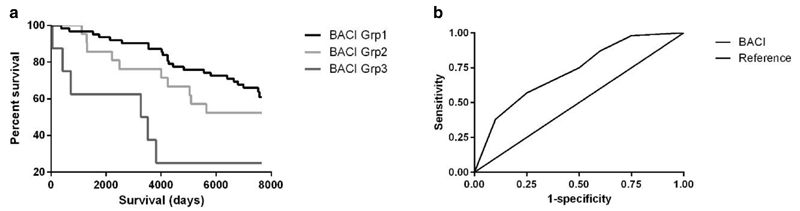
The Serbian validation cohort consisted of 113 patients, mean age 62 years (13) at diagnosis, 70% female. 5-year mortality was 17.7%. The AUC for predicting 5 year mortality in the Serbian cohort was 0.74 (95% CI 0.63-0.86). The UK validation cohort included 88 patients, mean age (SD) 51 years (12.1) at enrolment, with 57% female. Mortality at 20 years was 40.9%. The BACI was significantly associated with mortality at 20 years, p=0.004 (Kaplan-Meier), (figure 5).
Figure 5.
Validation of the Bronchiectasis Aetiology Comorbidity Index (BACI) in two independent cohorts: (a) Kaplan-Meier survival curves representing survival probability in BACI groups at 20 years in UK population, p=0.004; (b) Area under the receiver operator characteristic curve (AUC) score of BACI score in the Serbian cohort.
Discussion
The present study is the first multicentre international observational study to systematically describe the prevalence and associations of comorbidities on mortality in patients with bronchiectasis. A new disease-specific comorbidity risk index (the BACI) was derived to help predict which patients with bronchiectasis are at increased risk of death independently of their baseline physiological state. The BACI accurately stratified the risk of mortality and hospitalisations whilst demonstrating that comorbidities contribute to exacerbation frequency and impaired quality of life. The BACI may be a useful clinical predictive tool, when used independently, or in conjunction with the BSI, to risk-stratify patients and assist clinical decision making and personalised medicines approaches in bronchiectasis.
This is one of the largest cohort studies performed to date in bronchiectasis and is in keeping with other derivation studies in bronchiectasis and other comorbidity derivation tools. In bronchiectasis, the BSI and FACED consisted of 608 and 397 patients respectively in their derivation cohorts.16,17 The Charlson Comorbidity Index, which is perhaps one of the most widely utilised comorbidity assessment tools worldwide, consisted of 604 patients in their derivation cohort.19 Therefore, our sample size of 986 is more than adequate to derive this score.
Most respiratory diseases have disease-specific assessment tools, designed to identify patients at high risk of complications who may benefit from early treatment intensification. There is accumulating evidence that patients with bronchiectasis, similar to COPD, are prone to develop other important diseases, over and above what can be expected in an age and sex-matched general population, including cardiovascular disease, pulmonary hypertension and lung cancer, among others.4,10,12 With bronchiectasis, there is a “double hit” as many patients may already have an underlying aetiology that led to the development of bronchiectasis, potentially increasing the likelihood of developing further complications. For example, patients with rheumatoid arthritis and bronchiectasis may be receiving immunosuppressive treatments that increase the likelihood of complications, or patients with COPD-associated bronchiectasis may be at increased risk of lung cancer due to synergistic effects of airway inflammation and smoking.23
Systemic inflammation has been proposed as a potential explanation of the mechanistic pathway relating bronchiectasis with its comorbidities, in part due to the ageing process, which is strongly associated with an increased likelihood of developing multiple chronic conditions.24 The association between biomarkers of systemic inflammation and outcomes in bronchiectasis, including comorbidities, has not been well documented. In COPD, studies have demonstrated that elevated baseline inflammatory markers are associated with an increased risk of myocardial infarction, diabetes mellitus, lung cancer and pneumonia with the “inflamed comorbids” having the lowest survival in COPD populations.25 Addressing this knowledge gap may allow us to identify pathway-specific treatment targets that could be beneficial in the treatment of multi-diseased bronchiectasis patients. Statins and macrolides have both been shown in randomised controlled trials to modify disease prognosis and improve clinical outcomes in bronchiectasis, owing to their anti-inflammatory effects; the development of new selective anti-inflammatory agents may hold promise for the future.26,27
A total of 81 different comorbidities were identified during the 5-year follow-up of these patients. As expected, not all comorbidities were equally prevalent and there were highly varying strengths of association with mortality. Healthcare providers are often limited in their assessment of patients due to time constraints and high patient numbers, therefore guidance that could identify comorbidities at highest risk of worse outcomes could optimise patient care. Our results show that, of the 81 comorbidities identified, 26 differed significantly between survivors and non-survivors. This is far higher than the 15 identified in the derivation of the CO-morbidity TEst (COTE) in COPD.28 The 13 comorbidities associated with the highest risk of death on multivariate analysis were incorporated into the BACI. Similar to those in COPD, these could constitute a core of “red flag” comorbidities that healthcare providers should pay increased attention to in guiding a targeted personalised screening and treatment approach in patients with bronchiectasis.28 Some, such as cardiovascular disease, pulmonary hypertension, cognitive impairment, and lung, oesophageal and hematological malignancies, are highly consistent with the little information relating to comorbidities in bronchiectasis that is currently available.4–7,10–12 However, the increased risk of death conferred by iron deficiency anemia, diabetes mellitus and peripheral vascular disease in this population is less well described. These findings therefore raise the possibility of a shared common biological pathway among these diseases, which requires further exploration.
Although hypertension, high cholesterol and osteoporosis were in the top five most prevalent comorbidities, the direct risk of mortality conferred by these conditions was not significant. Whether this is because they are all treatable or they are risk factors for other potentially more harmful diseases, such as myocardial infarction, is unclear. However, selected solid tumors, such as lung and oesophageal cancer, conferred a significant increased risk of death with prevalence rates of 5% vs. 1% and 3.5% vs. 0.5% in non-survivors versus survivors (p=0.004 and p=0.01), respectively (Figure S2 in online supplement for breakdown of prevalence of solid tumors). Hematological malignancies, including lymphoma and leukemia, were also associated with a significantly increased mortality risk in this patient population. These findings have previously been demonstrated in a nationwide cohort study of >53,000 bronchiectasis patients in Taiwan compared to >215,000 age and sex-matched controls whereby a 2.5 fold increased risk of lung cancer and a 2-fold increased risk of oesophageal and hematological malignancies was demonstrated.12
A novel finding in this study was the relatively high prevalence of peripheral vascular disease (9%) and its strong independent association with risk of death, the mechanism of which remains unclear. Diabetes and iron deficiency anemia have both been described in COPD, the former possibly linked to overuse of inhaled corticosteroids in this patient population but more likely, both support the systemic inflammation hypothesis due to repeated infection, inflammation and chronic immune activation.25,29 Correction of anaemia could improve symptoms of fatigue and dyspnoea, thereby improving patients’ QoL and exercise capacity, reducing hospitalisations and improving overall survival. Anxiety and depression have been reported to be highly prevalent among bronchiectasis patients correlating with quality of life measures.8,9 In COPD, anxiety is an independent risk factor for mortality but no association of depression or anxiety with mortality was identified in this patient cohort.28
This study confirms that patients with bronchiectasis are frequently afflicted by comorbidities which may drive disease, many of which confer an independent risk of death and may be missed unless specifically searched for. Although the data may be somewhat intuitive, our finding that COPD, inflammatory bowel disease, connective tissue diseases and asthma are associated with a higher mortality risk may inform decisions about which patients with bronchiectasis should be followed up more closely. Health care providers caring for these patients should routinely screen for the comorbidities outlined in the BACI because there may be effective interventions or changes in management that could reduce the risk of death. Further follow up studies are needed as with the development of any score to substantiate its utility, and determine how this score may impact clinical practice. It would also be interesting to further explore the relationship between high BACI scores and lung or systemic inflammation in light of the association between higher exacerbations and Pseudomonas colonisation in co-morbid patients.
The BACI is a quantitative risk stratification aetiology and comorbidity index for clinicians and researchers to quantify and prioritise comorbidities in bronchiectasis. Our data demonstrate that measurements of comorbidities as captured by the BACI improve the prognostic accuracy for mortality, particularly when used in conjunction with the BSI.19 The BACI captures diseases not included in the CCI and carries independent prognostic value relating to future disease outcomes such as future exacerbations, hospitalisations and Pseudomonas colonisation. Combining the BACI and BSI equips healthcare workers and researchers to better stratify patients and provides a platform for comparative effectiveness research.
This study has several limitations: firstly, there is the potential for missed or as yet, undiagnosed comorbidities. For example, we experienced a somewhat lower prevalence of depression and anxiety in our cohort compared with studies that utilised the Hospital Anxiety and Depression Score to assess psychological wellbeing. However, in clinical practice, depression and anxiety are diagnosed upon history-taking and therefore this should not influence the results of the study. Similarly, there is no objective assessment for GORD, as we rely heavily on questionnaires for diagnosis in the clinical setting, often only resorting to the gold-standard 24h pH-impedance studies in refractory cases due to cost constraints. We may also have underestimated the prevalence of other conditions, such as pulmonary hypertension, in patients who had not had an adequate work-up for the same but who may in fact, still have co-existing disease. There may have been variation between diagnostic criteria used in diagnosing comorbidities due to changes in guidelines throughout the study time period and variation in clinical practice between primary and secondary care and different healthcare institutions.
Secondly, although a small number of patients in our derivation cohort had undergone transplant assessment, none of the patients in our derivation or validation cohorts had received a transplant therefore we are unable to comment on the utility of the score in this patient population.30 Comorbidity assessment is routine in the assessment of lung transplant candidates in order to determine suitability. The BACI score may highlight comorbidities that could negate transplant, e.g. in the case of metastatic malignancy, or perhaps delay transplant, e.g. with cases of iron deficiency anaemia where additional treatment may be needed beforehand. However, the BACI would not be considered in isolation in the assessment of transplant suitability and, as with any clinical prediction tool, it needs to be considered in the context of all other available information.
Thirdly, with regards to our validation cohorts, we were unable to account for potential recruitment bias in the younger less co-morbid patients recruited to the original Brompton cohort of 19 years follow up compared to the Serbian cohort. Nevertheless, it is reassuring to see that the BACI works well in different cohorts among different healthcare systems.
Finally, our derived score is relatively complex, awarding different points for each comorbidity. To aid calculation of the score, an online calculator is accessible at http://www.bronchiectasisseverity.com. This assigns a total on inputting the relevant data in sequential order and can therefore be completed in a very short space of time in the clinical setting. The BACI requires validation in developed countries such as the US and developing countries to further substantiate its utility, and further studies determining how this score may impact clinical practice are now needed. In support of our findings, however, our large representative derivation cohort was made up of almost 1000 patients of varying severity across different healthcare systems in four European countries, with external validation in two independent cohorts, one with 19-years follow-up and one from Eastern Europe, which should make these results generalisable to many bronchiectasis clinics worldwide.
Conclusions
Comorbidities in bronchiectasis are common and significantly contribute to disease burden and mortality. Surprising links with certain comorbidities may provide new insights into the underlying pathogenesis of this disease. We have derived a disease-specific bronchiectasis aetiology and comorbidity assessment tool for predicting future risk of mortality in bronchiectasis. Greater focus is needed to identify, assess and manage comorbidities in bronchiectasis in both clinical and research settings. Future interventions and treatment approaches should consider multiple comorbidities in these patients in order to maximise outcome and reduce the illness burden associated with this disease.
Supplementary Material
Research in context.
Evidence before this study
There is limited literature available on the prevalence and impact of comorbidities on mortality and other disease-related outcomes in bronchiectasis.
Added value of this study
In this study, we have confirmed that patients with bronchiectasis are frequently afflicted by comorbidities which may drive disease, many of which confer an independent risk of death and may be missed unless specifically searched for. We have developed a quantitative risk stratification tool, the Bronchiectasis Aetiology and Comorbidity Index (BACI) from a large multicentre derivation cohort of 986 bronchiectasis patients with validation in two independent cohorts with a median of 5 and 19 years of follow-up. Our data demonstrate that measurements of comorbidities as captured by the BACI improve the prognostic accuracy for mortality, when used independently, or in conjunction with the Bronchiectasis Severity Index (BSI), to identify patients at risk of future mortality, hospitalisations and exacerbations across different healthcare systems. The BACI also carries independent prognostic value relating to future disease outcomes including future exacerbations, hospitalisation for severe exacerbations and Pseudomonas aeruginosa infection. The BACI performs significantly better than any comorbidity scores currently available including the Charlson Comorbidity index and a simple comorbidity count suggesting that a disease-specific comorbidity score is useful in this patient population.
Implications of all the available evidence
The BACI may be a useful clinical predictive tool, when used independently, or in conjunction with the BSI, to risk-stratify patients and assist clinical decision making and personalised medicines approaches in bronchiectasis. The identification of “risky” comorbidities that may lead to stricter follow up of these patients may provide a practical viewpoint for clinicians beyond the BACI score calculation. Future interventions and treatment approaches should consider multiple comorbidities in these patients in order to maximise outcome and reduce the illness burden associated with this disease.
140 character summary: The Bronchiectasis Aetiology and Comorbidity Index (BACI) identifies patients at risk of future mortality, hospitalisations, exacerbations and other outcomes.
Acknowledgments
Funding: MJM acknowledges fellowship support from the European Respiratory Society/European Lung Foundation and Health Research Board, Ireland. JDC acknowledges fellowship support from the Medical Research Council and the Wellcome Trust. This study was supported by the European Bronchiectasis Network (EMBARC) - a European Respiratory Society Clinical Research Collaboration (www.bronchiectasis.eu) which has received funding from the European Respiratory Society and Bayer HealthCare. The funding agencies had no role in the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of interest: All authors declare no conflicts of interest in relation to the present study.
References
- 1.Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leroy L, Bayliss E, Domino M, et al. The Agency for Healthcare Research and Quality Multiple Chronic Conditions Research Network: overview of research contributions and future priorities. Med Care. 2014;52 Suppl 3:S15–22. doi: 10.1097/MLR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 3.Joish VN, Spilsbury-Cantalupo M, Operschall E, et al. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl Health Econ Health Policy. 2013;11:299–304. doi: 10.1007/s40258-013-0027-z. [DOI] [PubMed] [Google Scholar]
- 4.Gale NS, Bolton CE, Duckers JM, et al. Systemic comorbidities in bronchiectasis. Chron Respir Dis. 2012;9:231–238. doi: 10.1177/1479972312459973. [DOI] [PubMed] [Google Scholar]
- 5.Lee AL, Button BM, Denehy L, et al. Proximal and distal gastro-oesophageal reflux in chronic obstructive pulmonary disease and bronchiectasis. Respirology. 2014;19:211–217. doi: 10.1111/resp.12182. [DOI] [PubMed] [Google Scholar]
- 6.Mandal P, Morice AH, Chalmers JD, et al. Symptoms of airway reflux predict exacerbations and quality of life in bronchiectasis. Respir Med. 2013;107:1008–1013. doi: 10.1016/j.rmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell MJ, Ahmed M, Das J, et al. Hiatal hernias are correlated with increased severity of non-cystic fibrosis bronchiectasis. Respirology. 2015;20:749–757. doi: 10.1111/resp.12522. [DOI] [PubMed] [Google Scholar]
- 8.Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res. 2013;22:597–605. doi: 10.1007/s11136-012-0188-5. [DOI] [PubMed] [Google Scholar]
- 9.Giron Moreno RM, Fernandes Vasconcelos G, Cisneros C, et al. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Arch Bronconeumol. 2013;49:415–420. doi: 10.1016/j.arbres.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj A, Wells AU, Meister MG, et al. Pulmonary hypertension in patients with bronchiectasis: prognostic significance of CT signs. AJR Am J Roentgenol. 2011;196:1300–1304. doi: 10.2214/AJR.10.5221. [DOI] [PubMed] [Google Scholar]
- 11.Gulhan PY, Bulcun E, Gulhan M, et al. Low Cognitive Ability in Subjects with Bronchiectasis. Respir Care. 2015 doi: 10.4187/respcare.03905. [DOI] [PubMed] [Google Scholar]
- 12.Chung WS, Lin CL, Lin CL, et al. Bronchiectasis and the risk of cancer: a nationwide retrospective cohort study. Int J Clin Pract. 2015;69:682–688. doi: 10.1111/ijcp.12599. [DOI] [PubMed] [Google Scholar]
- 13.Hurst JR, Elborn JS, De Soyza A. COPD-bronchiectasis overlap syndrome. Eur Respir J. 2015;45:310–313. doi: 10.1183/09031936.00170014. [DOI] [PubMed] [Google Scholar]
- 14.Perry E, Eggleton P, De Soyza A, et al. Increased disease activity, severity and autoantibody positivity in rheumatoid arthritis patients with co-existent bronchiectasis. Int J Rheum Dis. 2015 doi: 10.1111/1756-185X.12702. [DOI] [PubMed] [Google Scholar]
- 15.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009;34:843–849. doi: 10.1183/09031936.00003709. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43:1357–1367. doi: 10.1183/09031936.00026313. [DOI] [PubMed] [Google Scholar]
- 18.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350 doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 23.Powell HA, Iyen-Omofoman B, Baldwin DR, et al. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8:6–11. doi: 10.1097/JTO.0b013e318274a7dc. [DOI] [PubMed] [Google Scholar]
- 24.Fabbri LM, Luppi F, Beghe B, et al. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 25.Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12:303–312. doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 26.Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med. 2014;2:455–463. doi: 10.1016/S2213-2600(14)70050-5. [DOI] [PubMed] [Google Scholar]
- 27.Hnin K, Nguyen C, Carson KV, et al. Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst Rev. 2015;8:CD001392. doi: 10.1002/14651858.CD001392.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 29.Vasquez A, Logomarsino JV. Anemia in Chronic Obstructive Pulmonary Disease and the Potential Role of Iron Deficiency. COPD. 2015:1–10. doi: 10.3109/15412555.2015.1043519. [DOI] [PubMed] [Google Scholar]
- 30.Rademacher J, Ringshausen FC, Suhling H, et al. Lung transplantation for non-cystic fibrosis bronchiectasis. Respir Med. 2016;115:60–65. doi: 10.1016/j.rmed.2016.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.