Abstract
Background
The MYC family promotes neuroblastoma tumorigenesis at least in part through the induction of aerobic glycolysis by promoting the transcription of key glycolytic enzymes, such as LDHA. FX11 is a selective inhibitor of LDHA that has demonstrated preclinical efficacy in adult cancers. Herein, we hypothesized that FX11 would inhibit aerobic glycolysis and block growth of neuroblastoma cells.
Methods
We surveyed three MYCN-single copy and five MYCN-amplified neuroblastoma cell lines to correlate C-MYC/N-MYC protein levels with LDHA expression. Cell viability was measured with FX11 using a tetrazolium-based assay. Cell cycle analysis using propidium iodide with flow cytometry was performed to evaluate for growth arrest. Immunoblotting demonstrated PARP and Caspase 3 cleavage as evidence of apoptosis.
Results
LDHA is frequently expressed in both MYCN-amplified and MYCN-single copy cell lines. N-MYC and C-MYC protein levels did not correlate with LDHA protein expression. FX11 inhibits aerobic glycolysis and growth in three MYCN-amplified and one MYCN-single copy neuroblastoma cell lines. FX11 induces modest G1 cell cycle arrest with selective induction of apoptosis.
Conclusions
Small molecule LDHA inhibition is capable of blocking aerobic glycolysis and growth of neuroblastoma cell lines in vitro and merits further in vivo evaluation of its preclinical efficacy in neuroblastomas.
Keywords: FX11, aerobic glycolysis, neuroblastoma, LDHA
TOC Statement
We report that the LDHA inhibitor FX11 blocks aerobic glycolysis and induces cell cycle arrest and apoptosis in neuroblastomas. The significance of this finding is to demonstrate the potential efficacy of LDHA antagonists against neuroblastomas.
INTRODUCTION
Neuroblastomas are the most common extracranial pediatric solid tumors and account for a disproportionate (~15%) amount of all pediatric cancer-related deaths.1, 2 Afflicted children are risk stratified into low, intermediate, and high-risk disease, depending upon their age at diagnosis, stage at presentation, and biologic characteristics.1 MYCN-amplification status is a feature of high risk tumors that is present in ~30% of all neuroblastomas.2 N-MYC is a critical driver of neuroblastoma progression by promoting angiogenesis, metastasis, chemoresistance, genomic instability, and aerobic glycolysis, and has been shown to be sufficient for spontaneous neuroblastoma-like tumor formation in both murine and zebrafish animal models.3–5
Developing small molecules capable of directly targeting the MYC family of proteins (C-MYC, N-MYC, and L-MYC) has proven to be a vexing drug discovery effort.5, 6 An alternative strategy is to target downstream mediators of.7 MYC is a critical mediator of aerobic glycolysis, a phenomenon also known as the Warburg effect, where cancer cells preferentially utilize glycolysis over mitochondrial respiration even in the presence of adequate oxygen tension.4,5,8 MYC promotes aerobic glycolysis by promoting the transcription of key glycolytic genes, including GLUT1, HK2, PDK1, and LDHA.8 Despite the critical role of the MYC family in neuroblastoma, few studies have evaluated the role of aerobic glycolysis in neuroblastoma tumorigenesis.
Gossypol is an antimalarial compound derived from cottonseed that non-selectively inhibits LDH and other NAD+-binding enzymes including GAPDH.8, 9 FX11 [3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid] is a gossypol derivative that demonstrates selectivity for LDHA (Ki = 8μM).9, 10 FX11 inhibits LDHA and demonstrates preclinical efficacy in lymphoma, pancreatic, and prostate cancer.10, 11 However, whether or not FX11 could serve as an inhibitor of neuroblastoma progression has not been explored. Given the critical role of MYC in neuroblastoma progression, we hypothesized that inhibition of aerobic glycolysis using the LDHA antagonist FX11 would block neuroblastoma growth.
MATERIALS AND METHODS
Antibodies and Reagents
Primary antibodies for C-MYC and Caspase 3 were obtained from Cell Signaling Technology. LDHA and PARP primary antibodies were from Abcam. N-MYC antibodies were from Santa Cruz Biotech, and β-actin was obtained from Sigma. FX11 [3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid] was purchased from Millipore Calbiochem, dissolved in dimethyl sulfoxide (DMSO), and further diluted in culture media to desired concentrations. All other reagents were obtained from Sigma.
Cell Culture
Neuroblastoma cell line, LAN-1, was a gift from Dr. Robert C. Seeger (University of Southern California, Los Angeles, CA). All other neuroblastoma cell lines (BE(2)-C, BE(2)-M17, IMR-32, SK-N-SH, SH-SY5Y, SK-N-AS, and SK-N-DZ) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in RPMI 1640 with glutamine and 10% FBS at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Cell Viability Assay
Neuroblastoma cells were seeded onto 96-well plates at equivalent density (ranging from 3–10×103 depending upon cell line) in RPMI culture medium with 10% FBS. Cells were permitted to attach overnight and were treated with either FX11 (10 or 20 μM) or vehicle control daily. Cell viability measurements using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies) were obtained daily.
Immunoblotting
Whole cell lysates were collected from neuroblastoma cells using cell lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, aprotinin, leupeptin, and 1 mM sodium orthovanadate) supplemented with proteinase inhibitors (Roche). PMSF (1 mM) was added immediately prior to use. Protein (30–50 μg) was run on a SDS-PAGE gel, transferred onto a PVDF membrane, and probed with antibodies. Blots were developed using an enhanced chemiluminescence system (Amersham Biosciences).
Measurement of Lactate Production
Extracellular L(+)-lactate production was measured in accordance with manufacturer’s protocol from Biovision. Specifically, an equal number of neuroblastoma cells (1–2 × 106 cells) were plated and permitted to attach overnight in standard media. Attached cells were then treated with FX11 (10 or 20 μM) or vehicle control (v/v%) dissolved in RPMI 1640 with 1% FBS in accordance with manufacturer’s recommendations. Following 24 h of incubation, the supernatant was collected and deproteinized using a 10kDa spin column (Vivaspin). Colorimetric absorbance was measured at 570 nM following 30 min incubation and background absorbance was subtracted from sample readings.
Cell Cycle Analysis
Cell cycle distribution was analyzed using flow cytometry with propidium iodide (Sigma Aldrich). Neuroblastoma cells were plated at equal numbers (1–2 × 106 cells) and treated daily with either FX11 (10 μM) or vehicle control. At 72 h of treatment, cells were trypsinized, washed once with PBS, and fixed in 70% ethanol. Fixed cells were washed with PBS, incubated with 100 μg/ml RNAase for 30 min at 37°C, stained with propidium iodide (50 μg/ml), and analyzed on a 3-laser BD LSRII (BD Biosciences, San Jose, CA). Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource.
Statistical Analysis
For in vitro experiments, conditions were compared by using Student’s t-test or one-way ANOVA with Tukey correction for two or ≥3 group experiments, respectively. For all experiments, p < 0.05 was considered significant.
RESULTS
Basal MYC Protein Levels Failed to Correlate with LDHA expression
The MYC family of oncogenic transcription factors is a critical driver of aerobic glycolysis that promotes the expression of key glycolytic enzymes, including LDHA.8,12 N-MYC has previously been shown to be a critical driver of LDHA expression with MYCN-amplification associated with increased LDHA mRNA levels in tumors and RNA interference of MYCN abrogating LDHA expression.13 We sought to evaluate this relationship at the protein expression level in 8 established neuroblastoma cell lines, including 3 MYCN-single copy cell lines (SK-N-SH, SK-N- AS, and SH-SY5Y) and 5 MYCN-amplified cell lines (SK-N-DZ, BE(2)-M17, BE(2)-C, IMR-32, and LAN-1). As anticipated, all of the MYCN-single copy cell lines expressed C-MYC, while all of the MYCN-amplified cell lines expressed N-MYC. Intriguingly, the basal expression of LDHA was relatively homogeneous among six of the eight cell lines with very low levels of LDHA expression noted in two of the MYCN-amplified neuroblastoma cell lines (BE(2)-C and BE(2)-M17) only with prolonged chemiluminescent exposure (Fig. 1A; Supplemental Fig). These findings suggest that LDHA is frequently highly expressed in neuroblastoma cell lines, and basal protein expression of C-MYC and N-MYC fail to correlate with LDHA expression.
Figure 1. FX11 inhibited aerobic glycolysis in neuroblastomas.
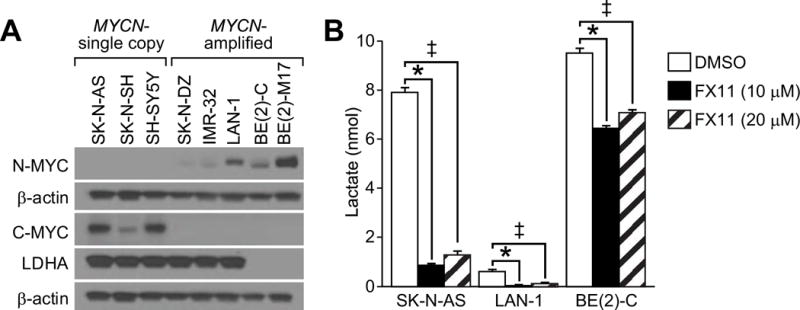
A) Immunoblotting for C- MYC, N-MYC, and LDHA demonstrated a lack of correlation between basal MYC and LDHA protein levels in neuroblastoma cell lines β-actin was used for protein loading control. B) FX11 inhibited aerobic lactate production in neuroblastoma cell lines (*=p<0.05 for 10 μM FX11 treatment vs. control; ‡=p<0.05 for 20 μM FX11 treatment vs. control).
FX11 Inhibited Aerobic Glycolysis in Neuroblastoma
We measured the production of lactic acid, the terminal product of glycolysis to demonstrate that FX11 blocks aerobic glycolysis in neuroblastoma cells. We measured extracellular lactate production in a MYCN-single copy, high LDHA expressing cell line (SK-N-AS), a MYCN-amplified, high LDHA expressing cell line (LAN-1), and a MYCN-amplified, low LDHA expressing cell line (BE(2)-C). The secreted lactate was measured from the supernatant following 24 h of treatment with FX11 (10 μM) or vehicle control in ambient oxygen. FX11 successfully decreased the lactate production in all three of the cell lines. Intriguingly, low LDHA-expressing BE(2)-C cells demonstrated relatively lower lactate inhibition (32%) than the high LDHA-expressing SK-N-AS and LAN-1 cells (89% and 95%) (Fig. 1B). Of importance, treatment with higher doses of FX11 (20 μM) did not augment levels of lactate inhibition. Together, these findings demonstrate that FX11 successfully blocked aerobic glycolysis at low micromolar concentrations. FX11 blocks lactate production in neuroblastomas at concentrations (10 μM) comparable to the reported Ki of FX11 for LDHA (8 μM), suggesting that FX11 selectively blocks LDHA in our experimental conditions10.
FX11 Inhibited Neuroblastoma Cell Growth
Having demonstrated that FX11 successfully inhibits aerobic glycolysis, we next sought to determine whether FX11 blocks neuroblastoma growth in vitro. We observed decreased cell viability in five cell lines (SK-N-AS, SK-N-SH, IMR-32, LAN-1, and BE(2)-C) using a tetazolium-based proliferation assay (Fig. 2). The two MYCN-amplified, high LDHA-expressing (LAN-1, IMR-32) and the MYCN-single copy SK-N-AS cell line demonstrated decreased cell viability starting at 48 h following treatment (p<0.05), while inhibition of SK-N-SH and BE(2)-C growth was first noted 72 h following treatment (p<0.05). Notably, all of the MYCN-amplified cell lines experienced a steep drop-off in cell viability between days 3 and 4, which may be suggestive of the onset of cell death (Fig. 2). Treatment with higher doses of FX11 (20 μM) augmented the degree of growth inhibition in both MYCN-amplified and MYCN-single copy cell lines. Given that higher doses of FX11 (20 μM) failed to further enhance the degree of lactate inhibition, these data suggest that additional cell death may be due to off-target or potentially toxic effects of FX11.
Figure 2. Neuroblastoma growth is blocked with FX11 treatment.
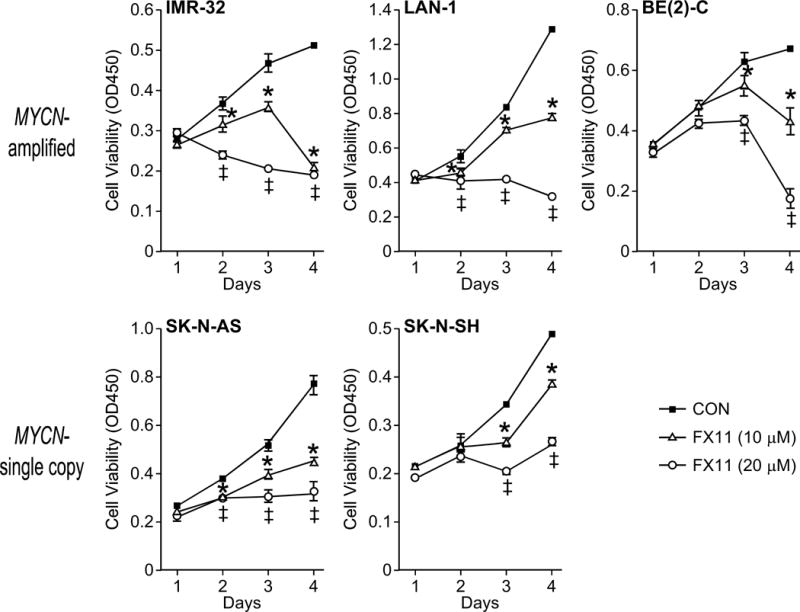
Serial measurements of cell viability were performed using a tetrazolium-based assay (CCK-8) to evaluate the growth effects of daily FX11 treatment in MYCN-amplified (IMR-32, LAN-1, and BE(2)-C) and MYCN- single copy (SK-N-AS and SK-N-SH) cell lines (*=p<0.05 for 10 μM FX11 treatment vs. control; ‡=p<0.05 for 20 μM FX11 treatment vs. control).
To further test whether FX11 was directly altering neuroblastoma cell proliferation, we evaluated cell cycle distribution of treated cells compared to vehicle control. We observed a significant but modest increase in the G1 cell cycle phase in all of the tested cell lines, ranging from a 2–11% increase in the G1 cell population compared to vehicle-treated controls with MYCN-amplified BE(2)-C and LAN-1 cells demonstrating the largest G1 cell cycle arrest (Fig. 3). Four of the five tested neuroblastoma cell lines (BE(2)-C, LAN-1, SK-N-SH, and SK-N-AS) demonstrated a significant decrease in the S-phase population, ranging from 2–7% decrease in S phase population (Fig. 3). Taken together, these findings suggest that low micromolar concentration (10 μM) of FX11 blocks progression through the cell cycle with resultant decrease in overall cellular proliferation.
Figure 3. FX11 induced modest G1 cell cycle arrest.
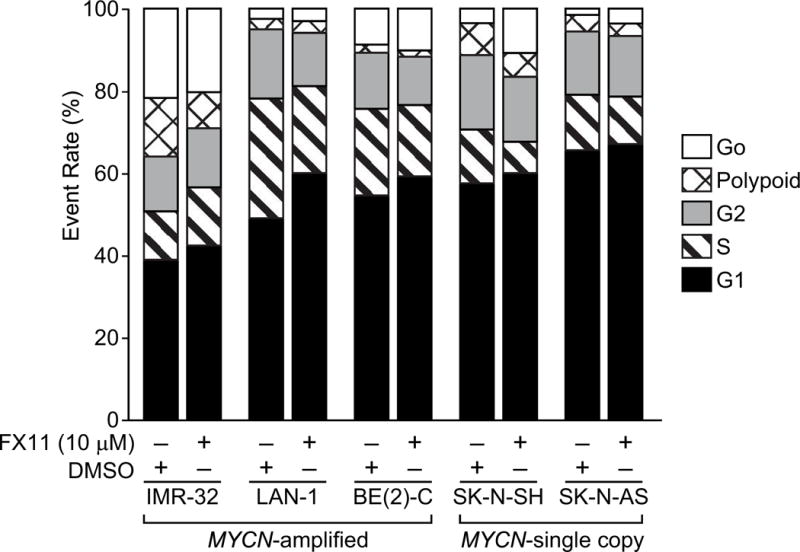
Cell cycle analysis was completed using propidium iodide and flow cytometry 72 h following treatment of five experimental cell lines with daily FX11 (10 μM). Cell cycle analysis was completed in triplicate for each cell line with 10,000 events per replicate.
FX11 Selectively Initiated Apoptosis in Neuroblastomas
Given the dramatic decrease in cell viability observed in the MYCN-amplified cell lines observed between days 3 and 4 of FX11 treatment (Fig. 2), we hypothesized that FX11 may also induce apoptosis in neuroblastoma cells. PARP (89 kDa) and Caspase 3 (17/19 kDa) cleavage products were our markers of apoptosis. These apoptotic markers were visualized by Western blotting at 24, 48, 72, and 96 h intervals following treatment with either FX11 (10 μM) or vehicle control. Caspase 3 and PARP cleavage was noted at 72 h in all of the MYCN-amplified and in the MYCN-single copy SK-N-SH cell line (Fig. 4). No PARP or Caspase 3 cleavage products were noted in the SK-N-AS cell line, suggesting these cells fail to undergo significant apoptosis in the presence of FX11 (Fig. 4). Thus, FX11 induces apoptosis in neuroblastoma cells at low micromolar doses.
Figure 4. LDHA blockade with FX11 selectively induced apoptosis.
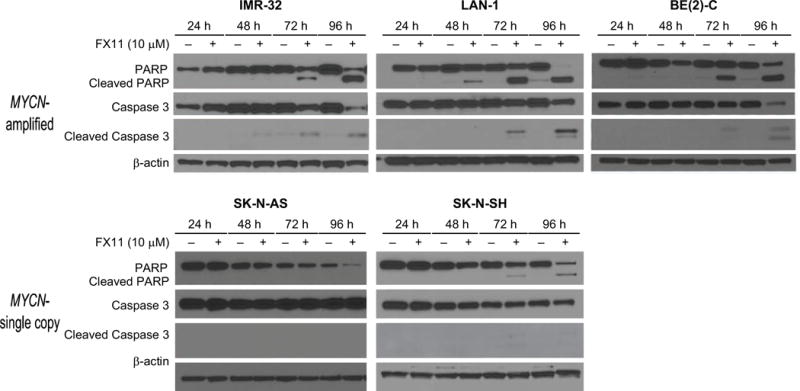
Western blotting was performed to detect PARP and Caspase 3 cleavage products (1–4 d) following daily treatment with FX11 (10 μM). β-actin was used for protein loading control.
DISCUSSION
The MYC family of oncogenic transcription factors are critical drivers of neuroblastoma transformation and promote aerobic glycolysis by enhancing the expression of LDHA.4 Elevated serum lactate dehydrogenase (LDH) is a poor prognostic factor in neuroblastoma with a reported pooled hazard ratio of 3.36 for overall survival (1.72–6.57).14 LDH is a key regulatory enzyme in glycolysis that facilitates the interconversion of pyruvate and lactate. It is composed of four subunits encoded by two genes LDHA and LDHB that assemble into 5 different isoenzymes (LDHB4, LDHA1B3, LDHA2B2, LDHA3B1, LDHA4).8 LDHA subunits catalyze the reduction of pyruvate to lactate, while LDHB subunits facilitate the reverse reaction whereby lactate is oxidized to pyruvate with NAD+ serving as the electron recipient.8,12 Thus, LDHA-containing tetramers favor glycolysis by promoting the production of lactate. A previous report by Qing et al. utilizing RNA interference demonstrated that N-MYC regulates LDHA expression.13 Furthermore, LDHA mRNA levels were found to be increased in high risk MYCN-amplified neuroblastomas as compared to high risk MYCN-single copy tumors, suggesting that LDHA is a potential therapeutic target for MYCN-amplified neuroblastomas.13
In our current study, we have demonstrated that the LDHA antagonist FX11 inhibits aerobic glycolysis and induces cell cycle arrest and apoptosis in both MYCN-single copy and MYCN-amplified cell lines. FX11 has previously demonstrated preclinical efficacy in lymphoma, pancreatic, osteosarcoma and prostate cancer.11,13 In the present study, we demonstrated that FX11 inhibits aerobic glycolysis at low micromolar concentrations in neuroblastoma cell lines (10 μM) that are comparable to its Ki for LDHA (8 μM), suggesting that our experimental conditions selectively inhibited LDHA. The potency of FX11 in blocking the in vitro growth of neuroblastoma cell lines is comparable to prior reports in pancreatic cancer, lymphoma, prostate cancer, and pediatric osteosarcoma.10,11,15 Increasing the dose of FX11 (20 μM) enhanced blockade of neuroblastoma cell line growth but failed to enhance the blockade of lactate production beyond that observed at lower doses (10 μM). These findings suggest that FX11 (20 μM) initiates either off-target or cytotoxic effects with treatments at higher concentrations. No specific off-target effects have been reported in other trials with FX11, but the compound has been reported to be well-tolerated in murine xenograft models.10,11,15
A clear limitation of our current study is the use of in vitro culture conditions and neuroblastoma cell lines to evaluate the role of FX11 in both the evaluation of its ability to inhibit aerobic glycolysis and alter neuroblastoma growth and cell death. While in vitro conditions facilitate precise metabolic measurements, such as aerobic lactate production, as potential measures of metabolic flux, it is unknown whether such conditions accurately reflect intratumoral conditions. In our current model, we note consistently high levels of LDHA protein expression across six of the eight tested cell lines, but low levels of LDHA protein expression were noted in two of the MYCN-amplified cell lines [BE(2)-C and BE(2)-M17)]. Further evaluation demonstrated that all of the MYCN-amplified and one MYCN-single copy cells lines underwent growth arrest and induction of apoptosis in the presence of FX11. Overall, these findings support the assertion of Qing et al. that MYCN-amplified neuroblastoma express high levels of LDHA and susceptibility to LDHA RNA interference.13 However, this previous study did not evaluate the role of LDHA inhibition in MYCN-single copy neuroblastomas. Herein, we assert that both MYCN-amplified and MYCN-single copy neuroblastomas may be sensitive to LDHA inhibition as both are capable of expressing high levels of LDHA protein levels, and we have demonstrated in vitro sensitivity of SK-N-SH cell lines to FX11 at low micromolar doses.
Overall, we have demonstrated, in conjunction with previous works, that LDHA is a potential target for the development of novel neuroblastoma anticancer strategies. Germline mutations in LDHA have been reported demonstrating loss of LDHA protein expression associated only with mild exertional myopathy, highlighting the potential therapeutic window of specific LDHA inhibitors.16,17 Ongoing drug development efforts have been devoted to the identification of increasingly specific LDHA inhibitors, and our findings support efforts to evaluate the preclinical efficacy of novel LDHA antagonists in both MYCN-amplified and MYCN-single copy neuroblastomas.
Supplementary Material
Supplemental Figure. Western blotting demonstrating low levels of LDHA in BE(2)-C and BE(2)-M17 cells with prolonged chemiluminescent exposure (3 min). β-actin was used for protein loading control.
Acknowledgments
This work was supported by grants R01 DK61470 from the National Institutes of Health, American College of Surgeons Resident Research Scholarship and Rally Foundation for Cancer Research. Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). We also thank Karen Martin for her assistance with manuscript preparation.
Footnotes
Presented at the 11th Academic Surgical Congress in Jacksonville, FL on February 2, 2016.
We have no financial or other interests, which may be construed as a conflict of interest.
References
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. Embo J. 1997;16:2985–95. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tansey WP. Mammalian MYC Proteins and Cancer. New Journal of Science. 2014;2014:27. [Google Scholar]
- 5.Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson WC, Weiss WA. Myc proteins as therapeutic targets. Oncogene. 2010;29:1249–59. doi: 10.1038/onc.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Simon MC. Molecular Pathways: Targeting MYC-induced metabolic reprogramming and oncogenic stress in cancer. Clin Cancer Res. 2013;19:5835–41. doi: 10.1158/1078-0432.CCR-12-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deck LM, Royer RE, Chamblee BB, Hernandez VM, Malone RR, Torres JE, et al. Selective inhibitors of human lactate dehydrogenases and lactate dehydrogenase from the malarial parasite Plasmodium falciparum. J Med Chem. 1998;41:3879–87. doi: 10.1021/jm980334n. [DOI] [PubMed] [Google Scholar]
- 10.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ, Xue J, et al. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015;36:8093–100. doi: 10.1007/s13277-015-3540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65:904–10. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 13.Qing G, Skuli N, Mayes PA, Pawel B, Martinez D, Maris JM, et al. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res. 2010;70:10351–61. doi: 10.1158/0008-5472.CAN-10-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, et al. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res. 2004;10:4–12. doi: 10.1158/1078-0432.ccr-1051-2. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Tu DN, Li H, Jiang JX, Cao X, You JB, et al. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. Biomed Pharmacother. 2016;81:388–93. doi: 10.1016/j.biopha.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Kanno T, Sudo K, Maekawa M, Nishimura Y, Ukita M, Fukutake K. Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy. Clin Chim Acta. 1988;173:89–98. doi: 10.1016/0009-8981(88)90359-2. [DOI] [PubMed] [Google Scholar]
- 17.Maekawa M, Sudo K, Kanno T, Li SS. Molecular characterization of genetic mutation in human lactate dehydrogenase-A (M) deficiency. Biochem Biophys Res Commun. 1990;168:677–82. doi: 10.1016/0006-291x(90)92374-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Western blotting demonstrating low levels of LDHA in BE(2)-C and BE(2)-M17 cells with prolonged chemiluminescent exposure (3 min). β-actin was used for protein loading control.


