Abstract
Arsenic methyltransferase (As3mt) catalyzes the conversion of inorganic arsenic (iAs) to its methylated metabolites, including toxic methylarsonite (MAsIII) and dimethylarsinite (DMAsIII). Knockout (KO) of As3mt was shown to reduce the capacity to methylate iAs in mice. However, no data are available on the oxidation states of As species in tissues of these mice. Here, we compare the oxidation states of As species in tissues of male C57BL/6 As3mt-KO and wild-type (WT) mice exposed to arsenite (iAsIII) in drinking water. WT mice were exposed to 50 mg/L As and As3mt-KO mice that cannot tolerate 50 mg/L As were exposed to 0, 15, 20, 25 or 30 mg/L As. iAsIII accounted for 53% to 74% of total As in liver, pancreas, adipose, lung, heart, and kidney of As3mt-KO mice; tri- and pentavalent methylated arsenicals did not exceed 10% of total As. Tissues of WT mice retained iAs and methylated arsenicals: iAsIII, MAsIII and DMAsIII represented 55%–68% of the total As in the liver, pancreas, and brain. High levels of methylated species, particularly MAsIII, were found in the intestine of WT, but not As3mt-KO mice, suggesting that intestinal bacteria are not a major source of methylated As. Blood of WT mice contained significantly higher levels of As than blood of As3mt-KO mice. This study is the first to determine oxidation states of As species in tissues of As3mt-KO mice. Results will help to design studies using WT and As3mt-KO mice to examine the role of iAs methylation in adverse effects of iAs exposure.
Keywords: Arsenic speciation analysis, Hydride, generation-cryotrapping-atomic, absorption spectrometry, Arsenic (+3 oxidation state), methyltransferase, As3mt knockout mice
Introduction
Inorganic arsenic (iAs), a potent human carcinogen, is ubiquitous in the environment and accumulates in aquifers naturally and through anthropogenic activities. The ingestion of iAs through contaminated drinking water, most commonly as arsenite (iAsIII) and arsenate (iAsV), has been associated with numerous adverse effects, including peripheral vascular disease, hypertension, and cancer of the lungs, liver, and bladder (Tseng et al., 2007; Wang et al., 2007a; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans et al., 2004). A recent National Toxicology Program workshop examining the effects of environmental chemicals on the development of diabetes and obesity concluded that there was sufficient evidence to link iAs exposures to an increased risk of diabetes in populations exposed to high levels of iAs in drinking water (Maull et al., 2012).
The enzyme, arsenic (+3 oxidation state) methyltransferase (AS3MT) catalyzes the S-adenosylmethionine (SAM)-dependent methylation of iAs to tri- and pentavalent methylated metabolites (Thomas, 2004). AS3MT mRNA has been found in several human and rodent tissues, including, liver, kidney, urinary bladder, heart, lung, testes, and adrenal gland (Lin et al., 2002). Once ingested, iAs is sequentially methylated by AS3MT producing methylarsonite (MAsIII), methylarsonate (MAsV), dimethylarsinite (DMAsIII), dimethylarsinate (DMAsV), and trimethylarsine oxide (TMAsVO). Growing evidence suggests that the methylated trivalent As (AsIII) species, MAsIII and DMAsIII, produced in the course of iAs metabolism, are more toxic than iAs or their pentavalent counterparts (Thomas et al., 2001; Lin et al., 2001; Drobna et al., 2003; Wang et al., 2007b; Douillet et al., 2013).
Laboratory-based studies have shown that iAs exposure alters glucose homeostasis and several mechanisms regulating glucose metabolism. Specifically, in our studies, exposure to subtoxic concentrations of iAsIII, MAsIII or, DMAsIII inhibited glucose-stimulated insulin secretion by isolated murine pancreatic islets without affecting basal insulin secretion or insulin content and expression, suggesting that AsIII species inhibit insulin transport vesicle packaging or translocation to the plasma membrane (Douillet et al., 2013). In β-cell lines exposed to iAs, an impairment of glucose-stimulated insulin secretion has been associated with reduced insulin expression (Díaz-Villaseñor et al., 2006) alterations in Ca2+ oscillations (Diaz-Villasenor et al., 2008), or with an Nrf2-mediated antioxidant response suppressing endogenous reactive oxygen species (Yen et al., 2007; Fu et al., 2010) that may be required for insulin secretion (Pi and Collins, 2010). In other cell culture models, iAsIII has been shown to inhibit differentiation of adipocytes (Trouba et al., 2000; Wauson et al., 2002) and myotubes (Steffens et al., 2011), the cell types that are involved in glucose utilization in vivo. Moreover, we have shown that AsIII species inhibit insulin signaling and insulin-stimulated glucose uptake in cultured differentiated adipocytes (Paul et al., 2007a; Walton et al., 2004). We have also shown that in C57BL/6 mice exposure to 50 mg/L As as iAsIII in drinking water resulted in impaired glucose tolerance (Paul et al., 2007b, 2011). Notably, mice chronically exposed to iAsIII in combination with high-fat diet produced a unique diabetic phenotype characterized by impaired glucose tolerance in the absence of significant obesity and insulin resistance (Paul et al., 2011), suggesting that the mechanisms underlying As-induced diabetes differ from those responsible for development of the obesity-associated type 2 diabetes.
Genetically altered, C57BL/6 As3mt-knockout (KO) mice have been recently developed and partially characterized (Drobna et al., 2009). When exposed to iAs these mice retained significantly more As than WT mice (Chen et al., 2011; Drobna et al., 2009; Hughes et al., 2010) and exhibited increased sensitivity to iAs toxicity (Yokohira et al., 2010, 2011). Chemical analyses have shown that iAs was the predominant species in tissues of As3mt-KO mice exposed to iAs; however, methylated As metabolites were detected in liver and plasma, suggesting the methylation of iAs by other methyltransferases or by intestinal microbiota (Drobna et al., 2009; Naranmandura et al., 2012). The oxidation states of iAs or the methylated As species found in tissues of As3mt-KO mice have never been determined. In spite of this information gap, the As3mt-KO mice have been used as a laboratory model to explore the role of iAs methylation and the contribution of trivalent methylated arsenicals in the development of iAs-induced diseases.
Hydride generation-cryotrapping-atomic absorption spectrometry (HG-CT-AAS) is uniquely suited for the oxidation state specific speciation analysis of As in complex biological matrices. The analysis using HG-CT-AAS does not require sample pretreatment or extraction, thus preserving the methylation state of unstable MAsIII and DMAsIII (Matoušek et al., 2008; Hernández-Zavala et al., 2008; Currier et al., 2011a, 2011b). This method has been successfully used to determine concentrations of the methylated trivalent arsenicals, MAsIII and DMAsIII, in human urine (Del Razo et al., 2001, 2011; Valenzuela et al., 2004, 2009), mouse tissues (Currier et al., 2011a, 2011b), in vitro cell cultures (Del Razo et al., 2001; Hernández-Zavala et al., 2008) and in vitro mixtures for methylation of iAs by recombinant AS3MT (Hernández-Zavala et al., 2008; Ding et al., 2012). In this study, we used HG-CT-AAS to characterize the retention of tri-and pentavalent arsenicals in tissues of wild-type (WT) and As3mt-KO C57/BL6 mice after exposure to iAsIII.
1. Materials and methods
1.1. Arsenicals
The following pentavalent arsenicals were used for calibration during the HG-CT-AAS analysis: sodium arsenite (NaAsIIIO2) and sodium arsenate (Na2HAsVO4) (both ≥ 99% pure) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methylarsonic acid, disodium salt (CH3AsVO(ONa)2), and dimethylarsinic acid ((CH3)2AsVO(OH)), both better than 98% pure, were purchased from Chem Service (West Chester, PA, USA). The As content in each of the standards was determined by graphite furnace-AAS (Matoušek et al., 2008).
1.2. Mice and treatments
All procedures involving mice were approved by the University of North Carolina Institutional Animal Care and Use Committee. The As3mt-KO mouse colony has been established at UNC using breeding pairs provided by Dr. David Thomas, U.S. EPA. In this study, 13- to 18-week old male C57BL/6 As3mt-KO mice were used along with 13–17 week old male WT C57BL/6J mice from Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed in polycarbonate cages (5 per cage) with corn cob bedding at the University of North Carolina Animal Facility (12 hr light/dark cycle, 22 ± 1°C and humidity 50% ± 10%). Mice were fed Lab Diet 5058 (Nutrition International, Brentwood, MO, USA) and drank pure deionized water (DIW) or DIW containing iAsIII ad libitum for the 4-week study period. As3mt-KO mice were exposed to 0, 15, 20, 25, or 30 mg/L As as iAsIII (n = 5 per treatment group). WT mice were exposed to 50 mg/L As (n = 10), the dose that previously produced a diabetic phenotype in this mouse strain (Paul et al., 2007b). The concentration of iAs in water in all treatment groups was verified by HG-CT-AAS analysis. Mice were euthanized by cervical dislocation. Freshly dissected tissues were processed as previously described, and tissue homogenates were prepared in ice cold DIW (10%, W/V) using an electrical overhead stirrer equipped with a Teflon pestle tissue homogenizer (Wheaton Industries, Inc., Millville, NJ, USA) (Currier et al., 2011b).
1.3. Speciation analysis of As by HG-CT-AAS
Arsenic species were analyzed by an optimized method for the oxidation state specific analysis of As species in biological matrices (Matoušek et al., 2008; Hernández-Zavala et al., 2008; Currier et al., 2011a, 2011b). Briefly, HG-CT-AAS analysis was performed using a FIAS 400 flow injection accessory (Perkin-Elmer, Norwalk, CT, USA) coupled to a cryotrapping unit and an AAnalyst 800 spectrometer (Perkin-Elmer) equipped with a multiple microflame quartz tube atomizer (multiatomizer) (Matoušek et al., 2008; Hernández-Zavala et al., 2008). To measure the oxidation state of As species, two aliquots of each sample are analyzed; arsine gases from trivalent arsenicals (iAsIII, MAsIII and DMAsIII) and from TMAsVO are generated directly at pH 6, while arsines from both tri- and pentavalent arsenicals (iAsIII+V, MAsIII+V and DMAsIII+V) are measured after pre-reduction for 1 hr at room temperature with 2% L-cysteine (EMD Chemicals Inc., Gibbstown, NJ, USA) (Matoušek et al., 2008; Hernández-Zavala et al., 2008). The pre-reduction, however, results in loss of TMAsVO. The concentrations of the pentavalent iAsV, MAsV, and DMAsV are calculated by subtracting the AAS peak area signals obtained for L-cysteine-treated and directly analyzed, untreated sample aliquots. Calibration curves for quantification of tri- and pentavalent As species were generated using aqueous solutions of the pentavalent standards (iAsV, MAsV, and DMAsV) pre-treated with 2% L-cysteine. Notably, results of our published work shows that HG-CT-AAS analyses of crude tissue homogenates result in high (~80%–100%) recoveries of As (Currier et al., 2011b).
1.4. Statistical analysis
All statistical analyses were performed using GraphPad Instat software package (GraphPad Software Inc., San Diego, CA, USA). Linear regression and correlation analyses were employed to characterize the calibration curves using aqueous pentavalent As standards. ANOVA followed by Bonferroni’s multiple comparison post-test was used to determine significant differences between As3mt-KO and WT mice for each of the measured endpoints. Statistical significance was considered at the level of p < 0.05.
2. Results and discussion
2.1. Water consumption and body weights
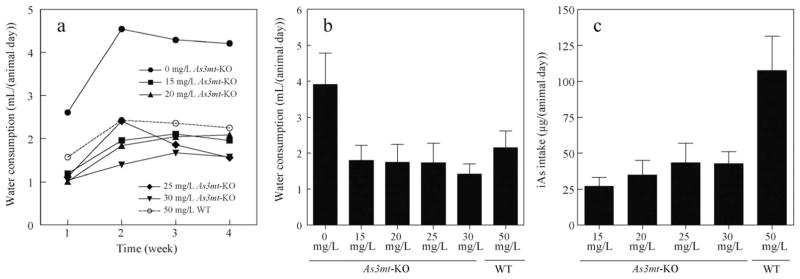
The consumption of water for each exposure group and individual body weights were measured weekly throughout the study. Fig. 1 depicts the estimated daily water consumption and the corresponding iAs intake for each exposure group over the 4-week study period. Water intake increased after the first week and then plateaued for the remaining study period except in As3mt-KO mice exposed to 25 mg/L As, which exhibited decreased water consumption in weeks 3 and 4 (Fig. 1a). The As3mt-KO mice exposed to pure DIW consumed significantly more water than all iAs-treated groups (Fig. 1a and b). As3mt-KO mice exposed to 15, 20, 25, or 30 mg/L As ingested approximately 27.1, 34.9, 43.4, and 42.7 μg of iAs/day, respectively, while WT mice exposed to 50 mg/L As ingested approximately 107.6 μg of iAs/day (Fig. 1c). There was no significant difference in iAs/day ingestion among the As3mt-KO groups, but the WT group ingested significantly more iAs than all other groups. We have previously shown that WT mice exposed to 50 mg/L As could taste the high concentration As and drank less water than WT mice at lower exposure levels or unexposed mice (Paul et al., 2007b, 2011). The current data suggest that KO mice are even more sensitive to As and further limit the water consumption.
Fig. 1.
Water consumption and iAs intake by As3mt-KO and WT mice. (a) Estimated daily water consumption by week for As3mt-KO mice exposed to 0, 15, 20, 25, or 30 mg/L As and wild-type (WT) mice exposed to 50 mg/L As. (b) Average daily water consumption per mouse for each treatment group. (c) Estimated daily iAs intake per mouse for each treatment group. Mean and SD values are shown; n = 5 for each of the As3mt-KO group and n = 10 for WT group. iAs: inorganic arsenic; As3mt: arsenic methyltransferase; KO: knockout; WT: wild-type.
The body weight of each mouse was measured prior to iAs exposure and weekly throughout the study. On average, all mice gained weight except for As3mt-KO mice exposed to 30 mg/L As (Fig. 2). Unexposed As3mt-KO mice gained an average of 3.2 g over the 4-week study period, while As3mt-KO mice exposed to 15, 20, or 25 mg/L As gained an average of 1.2, 1.6, and 1.1 g, respectively. As3mt-KO mice exposed to 30 mg/L As lost an average of 1.2 g over the study period. However, no other signs of toxicity were observed during the study or in dissected tissues. This is in contrast with previous studies reporting lethality and histopathological abnormalities in As3mt-KO mice exposed to 25 mg/L for 4 weeks (Yokohira et al., 2011; Chen et al., 2011).
Fig. 2.
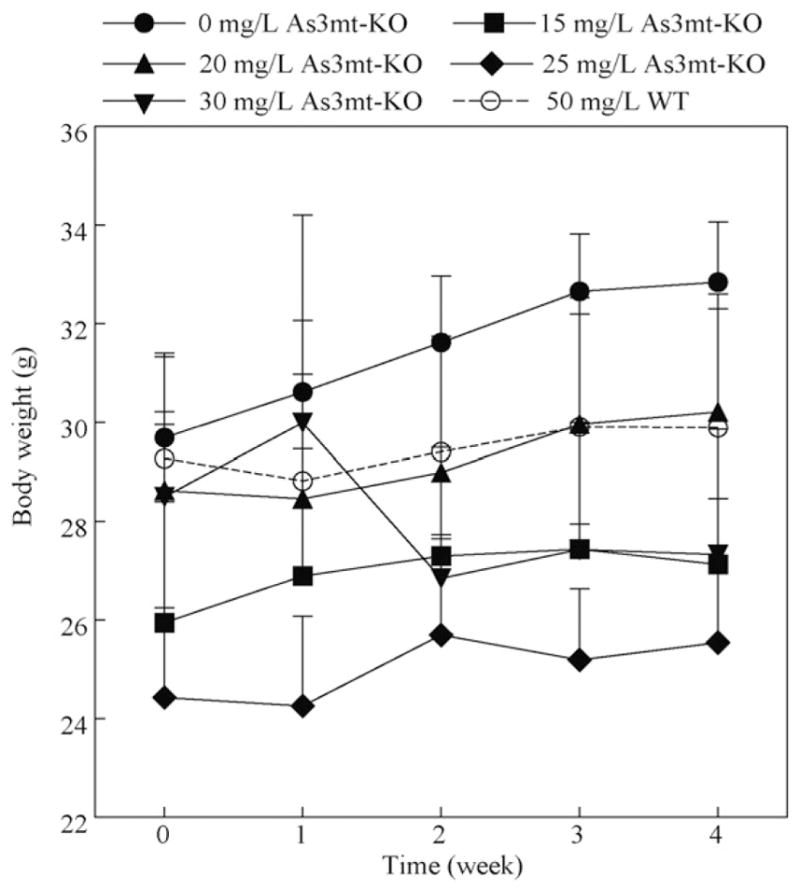
Change in body weights of As3mt-KO mice exposed to 0, 15, 20, 25, or 30 mg/L As and WT mice exposed to 50 mg/L As. Mean and SD, n = 5 for each of the As3mt-KO group and n = 10 for the WT group. There were no statistically significant differences in weekly average weights between the treatment groups.
2.2. Effect of genotype on As speciation in tissues
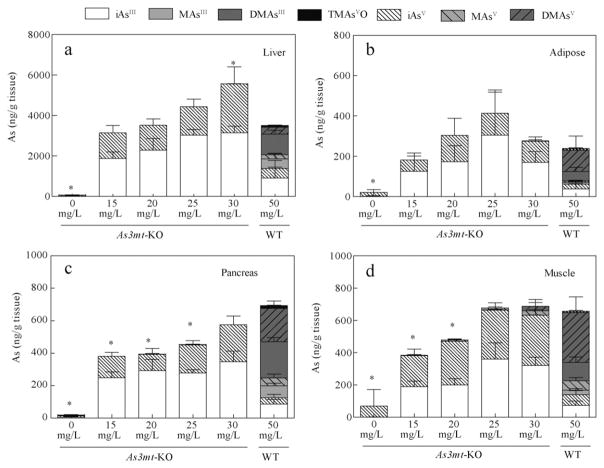
The speciation of As in tissues of As3mt-KO mice has previously been examined. However, the analysis was limited to plasma, red blood cells, liver, kidney, lung, and bladder, and did not determine the oxidation state of As in the detected As species (Drobna et al., 2009; Hughes et al., 2010; Chen et al., 2011). Only recent optimization of the HG-CT-AAS method has allowed for the analysis of tri-and pentavalent As species in tissue homogenates without sample extractions or digestions that could affect the oxidation state of As. This method was used in the present study to compare the speciation of As and to measure the concentration of AsIII and AsV species in tissues of As3mt-KO mice and WT mice. As expected, iAsIII and iAsV were the main species retained in tissues of As3mt-KO mice, while methylated As metabolites predominated in most tissues from WT mice. Fig. 3 describes the distribution of As species in tissues involved in regulation of glucose homeostasis (i.e., liver, adipose tissue, pancreas, and skeletal muscle). As3mt-KO mice exposed to 0 mg/L As retained small amounts of iAs, likely due to the presence of iAs in standard rodent chow (Paul et al., 2007b). In As3mt-KO mice exposed to iAs in drinking water, iAsIII was the predominate species in liver, adipose tissue, and pancreas, representing 57% to 74% of total speciated As (Fig. 3a–c). In skeletal muscle, iAsIII and iAsV ranged from 42% to 53% and 45% to 57% of total speciated As, respectively (Fig. 3d). The concentrations of As species in As3mt-KO mice exposed to 15, 20, 25, or 30 mg/L As increased in a dose-dependent manner in liver, skeletal muscle and pancreas. In adipose tissue, the amount of iAs increased only between 15 and 25 mg/L iAs, but decreased at 30 mg/L. It is possible that this decrease was associated with the overall toxicity that was manifested by decrease in body weight of the As3mt-KO mice exposed to 30 mg/L As. DMAsIII was the major As species in the liver and pancreas of WT mice, accounting for 29% and 32% of total speciated As respectively. DMAsV represented 45% and 47% of As in adipose and skeletal muscle tissues, respectively. Notably, the sum of trivalent species (iAsIII + MAsIII + DMAsIII) accounted for 55% and 68% of As in pancreas and liver of WT mice, respectively.
Fig. 3.
Oxidation state specific analysis of As in tissues critical for glucose homeostasis. The concentration (ng As/g wet tissue) of seven As species in liver (a), adipose tissue (b), pancreas (c), and skeletal muscle (d) of As3mt-KO mice exposed to 0, 15, 20, 25, and 30 mg/L and WT mice exposed to 50 mg/L As as iAsIII. Data is represented as means with SD for each arsenical (As3mt-KO, n = 5; WT, n = 10). *Statistically significant difference in total speciated As compared to 50 mg/L WT group (p > 0.05).
In a previous study of iAs metabolism in As3mt-KO mice, methylated species accounted for 28% to 32% of total As in liver and 22% to 28% in urine of As3mt-KO mice exposed to a single dose of iAsV, suggesting the role of alternative As methylation mechanisms (Drobna et al., 2009). However, a more recent study in As3mt-KO mice exposed to iAsIII through drinking water (Chen et al., 2011) supports our finding that iAsIII and iAsV are exclusive As species retained in liver, with no detectable levels of the methylated metabolites. The observed differences may explained by the dosing (single vs. chronic) or by the fact that mice were exposed to iAsIII in one study but to iAsV in the other. Notably, in the present study, the methylated arsenicals in tissues of As3mt-KO mice never exceeded 10% of the total speciated As and no methylated As species were detected in liver, pancreas, or adipose tissue.
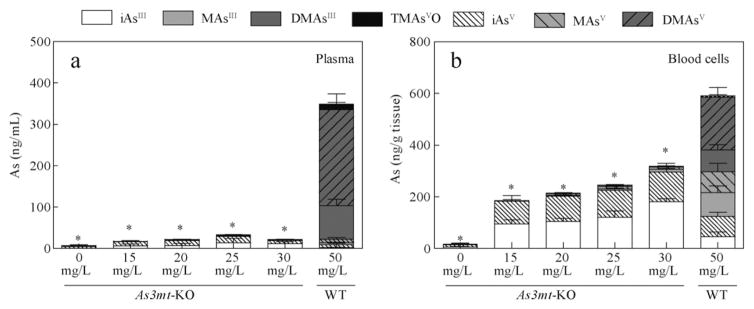
In addition to the analysis of AsIII and AsV species in the glucose regulating tissues, we also performed the oxidation-state analysis of As in the blood. Fig. 4 shows As species in blood plasma and blood cells of WT and As3mt-KO mice. Plasma and blood cells of WT mice exposed to 50 mg/L As contained significantly more As as compared to any As3mt-KO treatment group. In plasma and blood cells of As3mt-KO mice, iAsIII and iAsV were present at approximately equal concentrations. An average of 348 ng As/mL was present in plasma of WT mice exposed to 50 mg/L As compared with only 6, 17, 21, 33, and 22 ng As/mL in As3mt-KO mice exposed to 0, 15, 20, 25 and 30 mg/L As, a greater than 10-fold difference (Fig. 4a). In the blood cells of As3mt-KO mice, a dose-dependent increase in As from 15 to 317 ng As/g tissue was observed, while an average of 506 ng As/g tissue was retained in blood cells of WT mice (Fig. 4b). Our finding that WT mice retain more total As in blood cells than As3mt-KO mice conflicts with a previous study where WT and As3mt-KO mice were exposed to 1, 10, and 25 mg/L As as iAsIII in drinking water for 33 days (Chen et al., 2011). Here, the authors reported that greater levels of total As were retained by erythrocytes of As3mt-KO mice than WT mice. This difference could be associated with differences in sample preparation, analytical speciation technique, differences in As recovery, or with higher exposures in the present study for WT mice. Notably, the total As level in blood cells from As3mt-KO mice exposed to 25 mg/L As in our study (245 ng As/g tissue) is in good agreement with the level (~300 μg/L As) reported by Chen et al. (2011).
Fig. 4.
Oxidation state specific analysis of circulating As in As3mt-KO mice exposed to 0, 15, 20, 25, and 30 mg/L As and WT mice exposed to 50 mg/L As as iAsIII. The concentration of As species in plasma (a, ng As/mL) and blood cells (b, ng As/g wet tissue). Data is represented as means with SD for each arsenical. (As3mt-KO, n = 5; WT, n = 10). * Statistically significant difference in total speciated As compared to 50 mg/L WT group (p > 0.05).
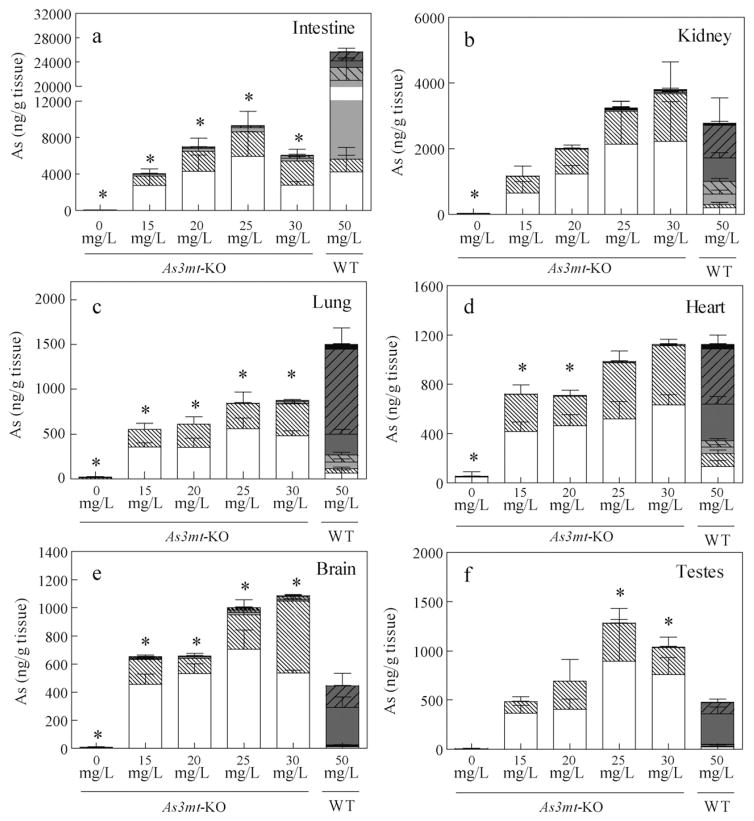
The concentrations of AsIII and AsV species in several other tissues from WT and As3mt-KO mice, including intestine, kidney, lung, heart, brain, and testes were also examined (Fig. 5). Notably, MAsIII accounted for 62% of As in the intestine (with intestinal content included) of WT mice exposed to 50 mg/L As (Fig. 5a). In contrast, iAsIII was the predominant species in the intestine from As3mt-KO mice while the methylated arsenicals represented less than 10% of total speciated As. It has been previously suggested that iAs methylation by intestinal bacteria is responsible for the formation of methylated As species in As3mt-KO mice (Drobna et al., 2009). Studies in other laboratories have shown that iAs can also be methylated by N-6 adenine-specific DNA methyltransferase 1 (Ren et al., 2011). Thus, methylation by intestinal microbiota or other methyltransferases may explain the presence of small amounts of the methylated As species in the intestine and in other tissues of As3mt-KO mice examined in the present study. Interestingly, total speciated As in the intestinal samples averaged between 4040 and 9273 ng As/g in As3mt-KO mice, but 24,879 ng As/g in WT mice. This data suggests that methylation increases intestinal content and/or excretion of As via feces. In a previous study where WT C57/BL6 and As3mt-KO mice were repeatedly dosed with 0.5 mg/kg iAsV, whole body As burden and daily excretion analysis revealed that WT mice excreted As at least 10 to 20 times faster than As3mt-KO mice (Hughes et al., 2010). Our results show that in addition to urine, excretion in feces contributes significantly to the clearance of methylated As species from the mouse body.
Fig. 5.
Oxidation state specific analysis of As in tissues from As3mt-KO mice exposed to 0, 15, 20, 25, and 30 mg/L As and WT mice exposed to 50 mg/L As as iAsIII. The concentration of seven As species (ng As/g wet tissue) in intestine with intestinal content (a), kidney (b), lung (c), heart (d), brain (e), and testes (f). Data is represented as means with SD for each arsenical. (As3mt-KO: n = 5; WT, n = 10).
In the kidney, lung, brain, and testes of As3mt-KO mice, iAsIII accounted for 49% to 81% of total speciated As (Fig. 5). In kidney, heart, lung of WT mice, DMAsV accounted for 36%, 40%, and 63% of speciated As, respectively, while DMAsIII predominated in brain (61%) and testes (67%). Notably, total speciated As level in lung from WT mice exposed to 50 mg/L As was significantly higher than in As3mt-KO mice in any treatment group. A recent report indicates significantly higher retention in lungs of As3mt-KO mice exposed to 1 and 10 mg/L iAsIII compared to WT mice at the same exposures, but not 25 mg/L (Chen et al., 2011). Our results suggest that at higher exposures, methylated arsenicals are more preferentially retained in lung tissue. Overall, these data indicate that trivalent As species are highly retained in most tissues of WT and As3mt-KO mice exposed to As and likely play a significant role in the development of iAs-induced diseases.
2.3. Comparison of the tissue levels of As
In the present study, we exposed WT C57BL/6J mice to 50 mg/L As in drinking water, the dose that produced diabetes in these mice in our previous studies (Paul et al., 2007b, 2011). Human exposures are order of magnitude lower. However, mice metabolize iAs more efficiently than humans, thus higher doses may be needed in studies using mice to produce the effects described in human studies. In fact, total As levels in the livers of mice exposed to 50 mg/L in our previous studies (Paul et al., 2007b, 2011) were in the range of the concentrations reported in livers of Bangladeshi residents exposed to 0.2–2 mg/L As (Mazumder, 2005), indicating that this concentration may be relevant for human exposures.
The As3mt-KO mouse model provides a unique platform to study the adverse effects of individual arsenicals because little internal methylation occurs. However, the As3mt genotype affects As retention and susceptibility to As-induced toxicity; studies in As3mt-KO mice have reported lethality at 50 mg/L As exposures and toxic effects at exposures as low as 25 mg/L, concentrations that are well tolerated by WT mice (Yokohira et al., 2010, 2011). Thus, to determine which As exposures in As3mt-KO mice yield equivalent internal As doses compared to WT mice, As retention in tissues critical for glucose homeostasis (i.e., pancreas, liver, skeletal muscle, and adipose tissue; Fig. 3) was examined. Here, differences between the sum of iAsIII+V, MAsIII+V, DMAsIII+V and TMAsVO for each As3mt-KO group and the WT group exposed to 50 mg/L As were evaluated by one-way ANOVA and Bonferroni’s multiple comparison test (Table 1). Total speciated As values for As3mt-KO exposure groups that were not significantly different from the 50 mg/L WT group were considered to produce approximately equivalent internal doses. In the pancreas, equivalent internal doses to WT mice were only achieved in As3mt-KO mice exposed to 30 mg/L As. Equivalent internal As doses in the liver were seen in As3mt-KO mice exposed to 15, 20, and 25 mg/L As and for all exposures in adipose tissue. In skeletal muscle, 25 and 30 mg/L As exposure in As3mt-KO mice resulted in equivalent internal As doses compared to WT mice exposed to 50 mg/L As.
Table 1.
Determination of equivalent internal As doses in tissues critical for glucose homeostasis.
| Treatment group | Total speciated Asa (ng As/g tissue)
|
|||
|---|---|---|---|---|
| Pancreas | Liver | Skeletal muscle | Adipose | |
| 0 mg/L As3mt-KO | 16 (7) | 67 (10) | 69 (102) | 21 (14) |
| 15 mg/L As3mt-KO | 381 (38) | 3137 (476)# | 386 (55) | 181 (107)# |
| 20 mg/L As3mt-KO | 394 (86) | 3525 (787)# | 479 (49) | 304 (112)# |
| 25 mg/L As3mt-KO | 452 (26) | 4497 (300)# | 677 (114)# | 413 (329)# |
| 30 mg/L As3mt-KO | 576 (83)# | 5564 (1024) | 688 (147)# | 277 (61)#y |
| 50 mg/L WT | 693 (141) | 3511 (1174) | 658 (185) | 239 (118) |
Total speciated As includes the sum of iAsIII+V, MAsIII+V, DMAsIII+V and TMAsVO determined by HG-CT-AAS. Mean (SD); As3mt-KO, n = 5 and WT, n = 10.
Statistically non-significant difference in total speciated As compared to 50 mg/L WT group (p > 0.05).
While exposure to 30 mg/L in As3mt-KO mice for 4 weeks produced equivalent total As tissue retention in the pancreas, skeletal muscle and adipose, decreased body weights were observed in this treatment group after 4 weeks of exposure (Fig. 2). In contrast to other studies, no signs of toxicity were observed in any of the other As3mt-KO treatment groups (Yokohira et al., 2010, 2011). However, study duration and potential toxicity should be carefully considered when planning experiments involving As3mt-KO mice. Therefore, exposure to both 25 and 30 mg/L would produce equivalent internal As doses in tissues critical for glucose homeostasis and will be used in future studies examining the effects of As on the development of diabetes.
3. Conclusions
In As3mt-KO mice exposed to 15, 20, 25, or 30 mg/L As, iAsIII is the most prevalent species in liver, pancreas, and adipose tissues. The majority of iAs and methylated As species retained in liver and pancreas of WT mice exposed to 50 mg/L As are in the trivalent form. DMAsV is the most prevalent species retained in skeletal muscle and adipose tissue of WT mice. For tissues critical to glucose homeostasis, doses of 25 and 30 mg/L As as iAsIII will produce in As3mt-KO mice total As levels approximately equivalent to those in WT mice exposed to the diabetogenic level of 50 mg/L As. These results should be considered when designing future studies using As3mt-KO mice to examine then role of iAs metabolism in adverse effects associated with iAs exposure.
Acknowledgments
We would like to dedicate this paper to our good friend and colleague Dr. William Cullen and thank him for his continuous support and advice. This work was supported by NIH grant No. 2 R01 ES010845 to M.S., the UNC Nutrition Obesity Research Center grant no. DK056350, and by NIH grant No. P30ES010126 to the UNC Center for Environmental Health and Susceptibility. The investigation by J.C. was supported by a pre-doctoral traineeship (National Research Service Award T32 ES007126) from the National Institute of Environmental Health Sciences, NIH. The authors thank Dr. David J. Thomas (U.S. EPA) who provided breeding pairs for the UNC As3mt-KO mouse colony. The authors also thank Mr. Jesse Saunders for his help with sample processing and Ms. Rachel Davis for her work in animal husbandry.
References
- Chen B, Arnold LL, Cohen SM, Thomas DJ, Le XC. Mouse arsenic (+3 oxidation state) methyltransferase genotype affects metabolism and tissue dosimetry of arsenicals after Arsenite Administration in Drinking Water. Toxicol Sci. 2011;124(2):320–326. doi: 10.1093/toxsci/kfr246. [DOI] [PubMed] [Google Scholar]
- Currier JM, Svoboda M, de Moraes DP, Matoušek T, Dědina J, Stýblo M. Direct analysis of methylated trivalent arsenicals in mouse liver by hydride generation-cryotrapping-atomic absorption spectrometry. Chem Res Toxicol. 2011a;24(4):478–480. doi: 10.1021/tx200060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JM, Svoboda M, Matoušek T, Dědina J, Stýblo M. Direct analysis and stability of methylated trivalent arsenic metabolites in cells and tissues. Metallomics. 2011b;3(12):1347. doi: 10.1039/c1mt00095k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, Styblo M, Cullen WR, Thomas DJ. Determination of trivalent methylated arsenicals in biological matrices. Toxicol Appl Pharmacol. 2001;174(3):282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Valenzuela OL, Castellanos E, Sánchez-Peña LC, Currier JM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health. 2011;10(1):73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Villaseñor A, Sánchez-Soto MC, Cebrián ME, Ostrosky-Wegman P, Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic β-cells. Toxicol Appl Pharmacol. 2006;214(1):30–34. doi: 10.1016/j.taap.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Diaz-Villasenor A, Burns A, Salazar A, Sordo M, Hiriart M, Cebrian M, et al. Arsenite reduces insulin secretion in rat pancreatic β-cells by decreasing the calcium-dependent calpain-10 proteolysis of SNAP-25. Toxicol Appl Pharmacol. 2008;231(3):291–299. doi: 10.1016/j.taap.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders RJ, Drobná Z, Walton FS, Xun P, Thomas DJ, et al. Methylation of arsenic by recombinant human wild-type arsenic (+3 oxidation state) methyltransferase and its methionine 287 threonine (M287T) polymorph: role of glutathione. Toxicol Appl Pharmacol. 2012;264(1):121–130. doi: 10.1016/j.taap.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol. 2013;267(1):11–15. doi: 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Jaspers I, Thomas DJ, Styblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. FASEB J. 2003;17(1):67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22(10):1713–1720. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, et al. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118(6):864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Zavala A, Matoušek T, Drobná Z, Paul DS, Walton F, Adair BM, et al. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer) J Anal At Spectrom. 2008;23(3):342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Edwards BC, Herbin-Davis KM, Saunders J, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249(3):217–223. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. Vol. 84. IARC; 2004s. [PMC free article] [PubMed] [Google Scholar]
- Lin S, Del Razo LM, Styblo M, Wang C, Cullen WR, Thomas DJ. Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes. Chem Res Toxicol. 2001;14(3):305–311. doi: 10.1021/tx0001878. [DOI] [PubMed] [Google Scholar]
- Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, et al. A NovelS-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277(13):10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- Matoušek T, Hernández-Zavala A, Svoboda M, Langrová L, Adair BM, Drobná Z, et al. Oxidation state specific generation of arsines from methylated arsenicals based on l-cysteine treatment in buffered media for speciation analysis by hydride generation-automated cryotrapping-gas chromatography-atomic absorption spectrometry with the multiatomizer. Spectrochim Acta B At Spectrosc. 2008;63(3):396–406. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120(12):1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharmacol. 2005;206(2):169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Naranmandura H, Rehman K, Le XC, Thomas DJ. Formation of methylated oxyarsenicals and thioarsenicals in wild-type and arsenic (+3 oxidation state) methyltransferase knockout mice exposed to arsenate. Anal Bioanal Chem. 2012;405(6):1885–1891. doi: 10.1007/s00216-012-6207-0. [DOI] [PubMed] [Google Scholar]
- Paul DS, Harmon AW, Devesa V, Thomas DJ, Stýblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007a;115(5):734–742. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dědina J, Matoušek T, et al. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol. 2007b;222(3):305–314. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect. 2011;119(8):1104–1109. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function. Diabetes Obes Metab. 2010;12:141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- Ren X, Aleshin M, Jo WJ, Dills R, Kalman DA, Vulpe CD, Smith MT, Zhang L. Involvement of N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) in arsenic biomethylation and its role in arsenic-induced toxicity. Environ Health Perspect. 2011;119(6):771–777. doi: 10.1289/ehp.1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens AA, Hong GM, Bain LJ. Sodium arsenite delays the differentiation of C2C12 mouse myoblast cells and alters methylation patterns on the transcription factor myogenin. Toxicol Appl Pharmacol. 2011;250(2):154–161. doi: 10.1016/j.taap.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. Elucidating the pathway for arsenic methylation*1. Toxicol Appl Pharmacol. 2004;198(3):319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176(2):127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Trouba KJ, Wauson EM, Vorce RL. Sodium arsenite inhibits terminal differentiation of murine C3H 10T1/2 PREADIPOCYTES. Toxicol Appl Pharmacol. 2000;168(1):25–35. doi: 10.1006/taap.2000.9012. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Chong CK, Tseng CP, Centeno JA. Blackfoot disease in Taiwan: its link with inorganic arsenic exposure from drinking water. AMBIO J Human Environ. 2007;36(1):82–84. doi: 10.1579/0044-7447(2007)36[82:bditil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, et al. Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ Health Perspect. 2004;113(3):250–254. doi: 10.1289/ehp.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela OL, Drobná Z, Hernández-Castellanos E, Sánchez-Peña LC, García-Vargas GG, Borja-Aburto VH, et al. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol Appl Pharmacol. 2009;239(2):200–207. doi: 10.1016/j.taap.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol. 2004;198(3):424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Wang CH, Hsiao CK, Chen CL, Hsu LI, Chiou HY, Chen SY, et al. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol. 2007a;222(3):315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Wang TC, Jan KY, Wang ASS, Gurr JR. Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutat Res Fundam Mol Mech Mutagen. 2007b;615(1–2):75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Wauson EM, Langan AS, Vorce RL. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol Sci. 2002;65(2):211–219. doi: 10.1093/toxsci/65.2.211. [DOI] [PubMed] [Google Scholar]
- Yen C, Lu F, Huang C, Chen W, Liu S, Linshiau S. The diabetogenic effects of the combination of humic acid and arsenic: in vitro and in vivo studies. Toxicol Lett. 2007;172(3):91–105. doi: 10.1016/j.toxlet.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Yokohira M, Arnold LL, Pennington KL, Suzuki S, Kakiuchi-Kiyota S, Herbin-Davis K, et al. Severe systemic toxicity and urinary bladder cytotoxicity and regenerative hyperplasia induced by arsenite in arsenic (+3 oxidation state) methyltransferase knockout mice. A preliminary report. Toxicol Appl Pharmacol. 2010;246(1–2):1–7. doi: 10.1016/j.taap.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Yokohira M, Arnold LL, Pennington KL, Suzuki S, Kakiuchi-Kiyota S, Herbin-Davis K, et al. Effect of sodium arsenite dose administered in the drinking water on the urinary bladder epithelium of female arsenic (+3 oxidation state) methyltransferase knockout mice. Toxicol Sci. 2011;121(2):257–266. doi: 10.1093/toxsci/kfr051. [DOI] [PubMed] [Google Scholar]