In a multicenter study across the United States, maternal illness, mode of delivery, and perinatal exposure to pesticides were associated with risk of pediatric multiple sclerosis.
Abstract
OBJECTIVE:
To determine if prenatal, pregnancy, or postpartum-related environmental factors are associated with multiple sclerosis (MS) risk in children.
METHODS:
This is a case-control study of children with MS or clinically isolated syndrome and healthy controls enrolled at 16 clinics participating in the US Network of Pediatric MS Centers. Parents completed a comprehensive environmental questionnaire, including the capture of pregnancy and perinatal factors. Case status was confirmed by a panel of 3 pediatric MS specialists. Multivariable logistic regression analyses were used to determine association of these environmental factors with case status, adjusting for age, sex, race, ethnicity, US birth region, and socioeconomic status.
RESULTS:
Questionnaire responses were available for 265 eligible cases (median age 15.7 years, 62% girls) and 412 healthy controls (median age 14.6, 54% girls). In the primary multivariable analysis, maternal illness during pregnancy was associated with 2.3-fold increase in odds to have MS (95% confidence interval [CI] 1.20–4.21, P = .01) and cesarean delivery with 60% reduction (95% CI 0.20–0.82, P = .01). In a model adjusted for these variables, maternal age and BMI, tobacco smoke exposure, and breastfeeding were not associated with odds to have MS. In the secondary analyses, after adjustment for age, sex, race, ethnicity, and socioeconomic status, having a father who worked in a gardening-related occupation (odds ratio [OR] 2.18, 95% CI 1.14–4.16, P = .02) or any use in household of pesticide-related products (OR 1.73, 95% CI 1.06–2.81, P = .03) were both associated with increased odds to have pediatric MS.
CONCLUSION
Cesarean delivery and maternal health during pregnancy may influence risk for pediatric-onset MS. We report a new possible association of pesticide-related environmental exposures with pediatric MS that warrants further investigation and replication.
What’s Known on This Subject:
Previous studies of autoimmune diseases and pregnancy-related risk factors have implicated breastfeeding as protective. Many of these studies have been done in adults with potential for recall bias and without adjustment for other early risk factors.
What This Study Adds:
Our study does not confirm a role for breastfeeding in MS risk, but suggests that maternal illness, mode of delivery, and perinatal exposure to pesticides may contribute to risk.
The relative contributions and timing of environmental exposures that promote multiple sclerosis (MS) onset are not clearly defined. In particular, it is not known how early-life factors near time of gestation may participate. Exposures during pregnancy and breastfeeding have been associated with juvenile diabetes and arthritis,1–9 suggesting that early-life environment may be critical to the development of autoimmune disorders. Understanding the role of these factors is important for advancing knowledge of the molecular processes contributing to MS and ultimately the design of prevention strategies.10
In the few studies available for perinatal risk factors in adult-onset MS, lack of and reduced duration of breastfeeding have been associated with MS risk,11,12 and 2 previous publications on the mode of delivery and MS risk had differing results, one showing increased risk with cesarean delivery and the other demonstrating no effect.13,14 These studies, however, have not consistently adjusted for other upstream maternal factors, such as maternal illness in pregnancy or maternal age and BMI, which may influence delivery complications and breastfeeding choices. The potential impact of maternal illness during pregnancy and resulting physiologic stress or antigen exposure to the fetus has not been directly addressed in studies of MS risk. Also unaddressed have been potential toxic exposures during gestation. Previous data in adults have suggested that exposure to organic solvents may increase risk for MS.15–17 It is not known whether certain occupational, behavioral, or household exposures during gestation influence MS risk. Smoking cigarettes is a well-known MS risk factor,18,19 but whether maternal or paternal smoking contributes to risk is not as clear.
Studying the effects of pregnancy-related exposures in pediatric-onset MS offers several advantages. Mothers of patients directly contribute to data acquisition rather than relying on memories of adult patients with MS. There is closer proximity of the exposure to symptom onset and fewer effects from diminished quality of recall over time. Finally, it is possible that MS onset in childhood, rather than adulthood, is in part related to higher environmental exposures that may thus be easier to detect.
In one of the largest well-characterized cohorts of new-onset pediatric MS, we sought to determine if prenatal, pregnancy, or postpartum-related environmental factors are associated with MS risk in children.
Methods
Subjects and Design
This was a case-control study of children with MS or clinically isolated syndrome and healthy controls enrolled at 16 clinics, most of which participate in the US Network of Pediatric MS Centers. Institutional review board approval was obtained at all participating sites and written informed consent was obtained from parents and assent from adolescent subjects. Case status was determined by the treating neurologist and confirmed by a panel of 3 pediatric MS specialists by using published diagnostic criteria for pediatric demyelinating diseases.20,21 Cases were enrolled within 4 years of disease onset and had to have at least 2 silent T2-bright foci on magnetic resonance imaging. Healthy pediatric subjects were recruited at primary care, urgent care, and other clinics at the same institutions from which cases were enrolled. Inclusion criteria for healthy subjects were (1) absence of any autoimmune disease, except asthma and eczema; and (2) not having a parent with MS. Parents of subjects reported demographic data, including race and ethnicity according to National Institutes of Health guidelines, age, and socioeconomic factors, including level of education of both parents.
Environmental Questionnaire
Parents completed a comprehensive environmental questionnaire (http://www.usnpmsc.org/Documents/EnvironmentalAssessment.pdf), including the capture of pregnancy and perinatal factors. These questionnaires were completed on either a paper form or by electronic entry. Study staff reviewed for completeness and queries were made for missing data. This environmental questionnaire was developed by Drs Waubant and Barcellos based on previous questionnaires used in MS and other autoimmune disorders, such as diabetes.6 Questionnaires were tested in 30 families at the University of California, San Francisco, before finalization to identify wording problems that could jeopardize understanding of specific questions and areas with poor completion, which were then removed. Afterward, the coauthors of this work reviewed the questionnaire to shorten it and removed questions of interest for which recall would be extremely unreliable. For this study, only questions pertaining to time periods from 3 months before conception through first of life with adequate response rates (>85%) were included. As appropriate, summary variables were created a priori (before analysis) to reflect sum of similar exposures. For example, different plant pesticides used were combined into 1 variable. A variable for maternal illness in pregnancy was created that included having the flu, pneumonia, sore throat, tonsillitis, bronchitis, chronic earache, severe asthma, sinus infection, diarrhea/gastroenteritis, rash, skin infection, kidney infection, jaundice, high blood pressure (other than preeclampsia), anemia, fever, incompetent cervix, abruptio placenta, premature rupture of membranes, or prolonged labor. Given frequency of the diseases, preeclampsia and maternal diabetes were analyzed separately.
Statistical Methods
All analyses were performed by using SAS Version 9.4 (SAS Institute, Cary, NC). We described the cases and controls by using frequencies and percentages for the categorical variables and medians, interquartile ranges (25th percentile and 75th percentile), for continuous variables. We compared characteristics between cases and controls by using χ2 tests or Kruskal-Wallis tests. Due to varying rates of missing data, we reported the counts for patients with data available. Descriptions included the child’s age at onset (for cases), child’s age at consent, sex, race, ethnicity, primary payer type group, US birth region, and the highest education group the biological mother and father had achieved at the time of the child’s birth. Pregnancy-related risk factors were also compared between cases and controls by using χ2 tests or Kruskal-Wallis tests. Risk factors included mother’s prepregnancy BMI, mother’s age at time of pregnancy, birth order, breastfeeding within first 2 years of life, delivery method, birth complications, pregnancy-related illness, maternal diabetes, maternal preeclampsia, day care exposure, various smoking-related risks, and vitamin D supplementation.
Multivariable logistic regression analyses were used to determine association of perinatal environmental factors with case status adjusting for age, sex, race, ethnicity, mother’s education, and US region. For the primary analysis of hypothesis-driven pregnancy-related risk factors, mutual adjustments for all predictors of interest were made in the final model. We checked summaries of model fit and diagnostics, including residuals, observation influence, and consistency of the results as variables were added or removed. Variables strongly collinear (eg, birth order) with primary factors of interest (eg, maternal age) were not included in the final multivariable model out of concern for unstable model effects. We also performed exploratory analyses for other environmental exposures during the period of 3 months before conception through the first year of life, again adjusting for age, sex, race, ethnicity, and mother’s education.
Results
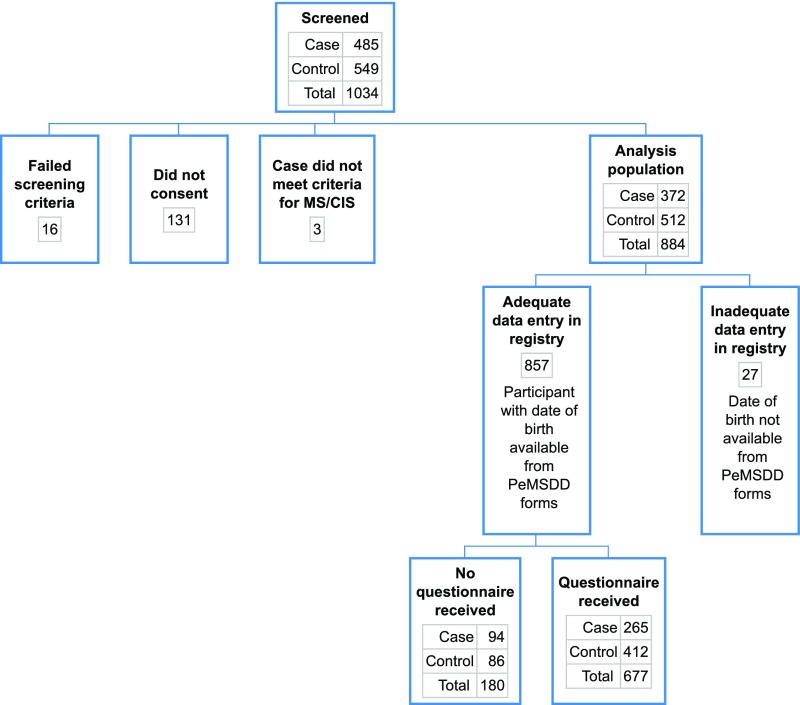
Questionnaire responses were available for 265 eligible cases (median age 15.7 years, 62% girls) and 412 healthy controls (median age 14.6 tears, 54% girls) enrolled between November 2011 and August 2015 (Fig 1). When comparing the demographics of the survey responders with the nonresponders, we found that more controls (83%) responded than cases (74%). There was no significant difference by sex, but responses varied by race, and Hispanic individuals (76%) were less likely to respond than non-Hispanic individuals (86%). Table 1 describes the subjects’ characteristics. There were no significant differences in race between cases and controls (P = .38, Table 1), but cases were more likely to be of Hispanic ethnicity than controls (29% vs 17%, P < .01). Both the mother’s (P < .01) and the father’s (P < .01) highest level of education differed between cases and controls, as well as US birth region (P = .03) (Table 1).
FIGURE 1.
Case and control enrollment and inclusion. CIS, clinically isolated syndrome; PeMSDD, Pediatric MS Database.
TABLE 1.
Subject Characteristics
| Control (n = 412) | Case (n = 265) | P | |
|---|---|---|---|
| Child’s age at disease onset, median (IQR) | — | 15.0 (12.6–16.4) | |
| Child’s age at consent, median (IQR) | 14.6 (11.8–17.1) | 15.7 (13.5–17.3) | .01a |
| The biological mother’s age at her birthday in the year the child was born, median (IQR) | 29 (24–33) | 28 (23–32) | .02a |
| Sex (% female) | 224 (54) | 165 (62) | .04b |
| Subject race category, n (%) | .38b | ||
| White | 280 (71) | 173 (70) | |
| Black | 71 (18) | 41 (17) | |
| Asian | 19 (5) | 9 (4) | |
| Other | 26 (7) | 25 (10) | |
| Subject ethnicity, n (%) | <.01b | ||
| Hispanic or Latino | 68 (17) | 78 (29) | |
| Not Hispanic or Latino | 332 (81) | 178 (67) | |
| Unknown or not reported | 12 (3) | 9 (3) | |
| Primary payer type group, n (%) | .07b | ||
| Commercial insurance | 257 (62) | 143 (54) | |
| Other, including self-pay and government insurance | 139 (34) | 106 (40) | |
| Unknown | 16 (4) | 16 (6) | |
| Highest education category the biological mother had achieved at the time of the child’s birth, n (%) | <.01b | ||
| High school or lower | 71 (19) | 82 (34) | |
| College | 237 (63) | 125 (51) | |
| Graduate school | 44 (12) | 22 (9) | |
| Other | 25 (7) | 15 (6) | |
| Highest education category of the biological father, n (%) | <.01b | ||
| High school or lower | 86 (25) | 88 (41) | |
| College | 178 (51) | 107 (49) | |
| Graduate school | 62 (18) | 15 (7) | |
| Other | 20 (6) | 7 (3) | |
| US birth region, n (%) | .03b | ||
| Northeast | 102 (27) | 94 (39) | |
| South | 96 (25) | 53 (22) | |
| Midwest | 40 (11) | 24 (10) | |
| West | 139 (37) | 73 (30) |
IQR, interquartile range.
Kruskal-Wallis test.
χ2 test of no association.
Maternal Factors in Pregnancy: Primary Analysis
Differences in frequencies of pregnancy-related maternal factors, including mode of delivery, illnesses during pregnancy, tobacco smoke exposures, vitamin D use, and breastfeeding, are presented in Table 2. Mother’s education was significantly associated with risk of MS, with an odds ratio of 0.33 for college education versus high school or lower (95% confidence interval [CI] 0.15–0.72, P = .01) and was included in all models. As previously reported, we observed an association of birth order with case status (Table 2). Birth order, however, was collinear with maternal age and not significantly associated with other maternal factors and thus was not included in the final multivariable model in Table 3. In multivariable analysis, with mutual adjustment of pregnancy risk factors of interest as well as adjustment for sex, race, ethnicity, US region, and socioeconomic status (Table 3), maternal illness other than diabetes and preeclampsia during pregnancy was associated with 2.3-fold increase in odds to have MS (95% CI 1.20–4.21, P = .01). Furthermore, cesarean delivery was associated with a 60% reduction (95% CI 0.20–0.82, P = .01). In a model adjusted for these variables, maternal age and BMI, tobacco smoke exposure, day care, and breastfeeding were not associated with MS risk (Table 3).
TABLE 2.
Unadjusted Pregnancy-related Risk Factors
| Controls | Cases | P | |
|---|---|---|---|
| Maternal BMI before pregnancy, median (IQR) | 23.0 (20.6–26.6) | 23.5 (20.6–27.5) | .21a |
| Maternal age at birth of child, median (IQR) | 29 (24–33) | 28 (23–32) | .02a |
| Birth order, median (IQR) | 2 (1–2) | 2 (1–3) | .01a |
| Breastfeeding in first 2 years of life | 306 (75) | 176 (68) | .05b |
| Delivery by cesarean | 90 (23) | 43 (17) | .05b |
| Any birth complications in the first 2 weeks of life | 140 (37) | 92 (39) | .64b |
| Maternal illness without diabetes or preeclampsia | 166 (54) | 126 (60) | .16b |
| Maternal diabetes (including gestational diabetes) | 10 (3) | 10 (4) | .29b |
| Preeclampsia | 11 (3) | 6 (2) | .75b |
| Day care first year of life | 236 (60) | 133 (53) | .06b |
| Tobacco smoke exposure | 127 (35) | 96 (44) | .03b |
| Vitamin D supplementation during pregnancy | 34 (11) | 14 (7) | .08b |
Values are n (%) unless otherwise noted.
Kruskal-Wallis Test.
χ2 test of no association.
TABLE 3.
Multivariable Analysis of Pregnancy-Related Risk Factors With Mutual Adjustment
| OR | 95% CI | P | |
|---|---|---|---|
| Maternal BMI before pregnancy | 1.04 | 0.98–1.09 | .18 |
| Maternal age at birth of child | 1.00 | 0.95–1.06 | .93 |
| Breastfeeding in first 2 years of life | 0.85 | 0.45–1.61 | .62 |
| Delivery by cesarean | 0.40 | 0.20–0.82 | .01 |
| Any birth complications in the first 2 weeks of life | 1.37 | 0.76–2.47 | .30 |
| Maternal illness without diabetes or preeclampsia | 2.25 | 1.20–4.21 | .01 |
| Maternal diabetes, including gestational diabetes | 3.32 | 0.82–13.48 | .09 |
| Preeclampsia | 1.23 | 0.31–4.87 | .76 |
| Day care first year of life | 0.75 | 0.41–1.34 | .33 |
| Tobacco smoke exposure | 0.63 | 0.34–1.17 | .14 |
| Vitamin D supplementation during pregnancy | 0.40 | 0.13–1.23 | .11 |
Also adjusted for age, sex, race, ethnicity, mother’s education, and US region.
Although appropriate adjustments were made in the multivariable model, to further verify that ethnic and socioeconomic differences in the cases and controls did not drive the main effects observed for maternal illness and mode of delivery, we performed a stratified analysis for mothers with college education and above versus those with high school or less education and for Hispanic and non-Hispanic children. The stratified analyses provided results that were consistent with the results for the full cohort (Supplemental Tables 6–9).
Exploratory Analyses
We performed additional exploratory analyses of environmental exposures during the period of 3 months before conception through the first year of life. For all potential risk factors, we adjusted for age, sex, race, ethnicity, and socioeconomic status. Factors associated with the odds to have pediatric-onset MS that reached nominal statistical significance of P < .05 are listed in Table 4. As these were exploratory analyses, the P values presented were not corrected for multiple comparisons. The additional predictors that were evaluated, but which had P ≥ .05, are presented in Supplemental Table 5.
TABLE 4.
Exploratory Analysis of Perinatal Exposures
| Cases Exposeda | Controls Exposed | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Infant prolonged hospital stay | 30 | 32 | 1.79 | 1.00–3.19 | .05 |
| Other children in the household before age 6 | 208 | 297 | 1.77 | 1.13–2.79 | .01 |
| Liked all foods versus picky eating first 2 y of life | 119 | 162 | 1.53 | 1.08–2.18 | .02 |
| Father’s BMI before pregnancy (per unit BMI) | — | — | 1.05 | 1.00–1.10 | .04 |
| Mother has freckles | 64 | 152 | 0.50 | 0.34–0.74 | <.01 |
| Father has worked regularly in gardening/farm work | 24 | 22 | 2.18 | 1.14–4.16 | .02 |
| Household use of plant/tree-related pesticides in perinatal period through first year of life | 12 | 10 | 1.73 | 1.06–2.81 | .03 |
| Dog slept in house during pregnancy | 69 | 94 | 1.92 | 1.03–3.60 | .04 |
| Prenatal use of B vitamins | 27 | 77 | 0.57 | 0.34–0.95 | .03 |
| Use of nausea medication in pregnancy | 8 | 35 | 0.33 | 0.15–0.74 | <.01 |
Adjusted for age, sex, race, ethnicity, and mother’s highest level of education.
Exposure counts are out of total of n = 265 cases and n = 412 controls.
Variables of interest in this exploratory analysis included those related to exposure to gardening and pesticides. Having a father who worked in a gardening-related occupation (odds ratio [OR] 2.18, 95% CI 1.14–4.16, P = .02) or any use in subject’s household of plant-related pesticide product from 3 months before pregnancy through the first year of life (OR 1.73, 95% CI 1.06–2.81, P = .03) were both associated with increased odds to have pediatric MS after adjustment for age, sex, race, ethnicity, and socioeconomic status. Other variables associated with increased odds to have MS included having a dog that slept in the house, higher father’s BMI, number of children in the household, and a prolonged hospital stay for the infant after birth. Month of birth (P = .95) and birth weight (P = .46) were not associated with odds of MS (Supplemental Table 5). We examined exposures to adhesives or paint thinner petroleum products. Although there was no statistically significant association of these exposures in the household during the peripregnancy period (OR 1.22, 95% CI 0.79–1.89, P = .36, Supplemental Table 5), exposure after age 1 was strongly associated with a twofold higher risk of MS (OR 2.02, 95% CI 1.23–3.29, P < .01).
Discussion
Our study leverages a unique dataset of early-life exposures. We have identified maternal illness during pregnancy as a potential risk factor for pediatric-onset MS, whereas delivery by cesarean may be protective. We did not confirm breastfeeding as a protective factor once adjustment was made for the previously mentioned factors. Our exploratory analyses suggest exposure to pesticides in the perinatal period may increase MS risk and exposure to petroleum-based organic solvents may be most relevant after the first year of life.
Mechanisms by which maternal illness in pregnancy may be associated with MS risk include stress-mediated changes, such as elevated glucocorticoid levels, placental insufficiency, metabolic abnormalities, including hyperglycemia, or infectious or immune-mediated exposures.5,22–24 Data from animal models, including the murine model of MS, experimental autoimmune encephalitis, have suggested that pathogen exposures during gestation may have prolonged effects on immune responses in the offspring. Certain antigens may increase proinflammatory cytokines, including interleukin-6 and drive a T-helper cell 17 phenotype that may persist in the offspring even into adulthood.25,26
Although we found a protective effect of cesarean delivery on the odds of having pediatric-onset MS, this contrasts to the previously reported deleterious or neutral effect of cesarean delivery on adult-onset MS risk. Being delivered by cesarean was associated with increased risk of developing adult-onset MS in a sibling-matched case-control study from Iran,13 whereas no association was reported in a nationwide Danish cohort.14 The discrepancy between these studies might be due to racial or age of MS onset differences, but also control characteristics. Patients of the first study were all Iranian, with a mean age of MS onset of 27.4 years, whereas patients in the second study were all of Danish-born parents and most of them had adult-onset MS. Most patients in our study were white, but a sizeable proportion was Hispanic or nonwhite. It is conceivable that the mode of delivery in various populations has different or opposing effects. The effect of cesarean delivery also may be expected to have more direct impact on pediatric onset given proximity to the exposure. Chance, measurement bias, selection bias, and the effect of unmeasured confounders in these studies might explain some of the differences. In the Iranian study, the mode of delivery was recorded through the adult patient’s self-report, which may be more prone to recall bias. The Danish study used the national medical Birth Register, whereas in our study mode of delivery was reported by parents. Last, we adjusted for additional peripregnancy factors that are often upstream of and lead to cesarean delivery (maternal age and BMI, diabetes, preeclampsia) that were not accounted for in these other studies.
Despite the differences in the previously described studies, a potential protective effect of cesarean delivery on early onset of MS is of interest in light of recent findings regarding the microbiome and autoimmune diseases.27–29 Cesarean delivery is associated with differences in gut colonization in the first few years of life.30,31 These differences may be most relevant in disease development in childhood and become less relevant in later adult life. In a recent pediatric MS study, we reported substantial differences in type and abundance of taxa in cases versus controls with some taxa such as Christensenellaceae possibly relevant to birth method and impact on disease risk.32,33 Future studies will confirm if history of cesarean delivery is associated with microbiota in pediatric MS.
Perinatal exposures may affect MS risk through epigenetic changes that in turn can alter the immune response.34 Embryogenesis is a particularly vulnerable time for DNA modification.35,36 Very early in development, for example, DNA methylation patterns are erased and then reestablished during periods of rapid cell division, sexual development, and organogenesis. Maternal illness may affect this process and also environmental toxins. Endocrine disruptors, persistent organic pollutants, arsenic, and several herbicides and insecticides have been associated with epigenetic modifications.37
Although our results of an association of pesticides with MS risk are exploratory and require replication, associations have been reported between pesticides and Parkinson and Alzheimer diseases.38–40 Pesticides also have been implicated in systemic autoimmune disorders, including systemic lupus erythematosus and are recognized to likely have the strongest impact on the immune system during embryogenesis or early childhood, including on the development of the lymphoid organs.41–43 The time of exposure to pesticides associated with these other disorders is unclear but could occur very early in life.
Inhaled organic solvents largely related to the painting industry have been previously associated with MS risk and this association has additionally been shown to be modified by smoking exposure and HLA-DRB1*15:01 carrier allele status (strongest genetic risk factor for MS).15–17 We did not find a significant association of this risk factor in the perinatal period, but did find twofold higher odds to have MS in those exposed after the first year of life. This fits with the hypothesis that these chemicals increase risk through inhalation and would be less likely to have effect through in utero exposure.
Our results suggest that lower socioeconomic status may be a risk factor for MS. This is consistent with data from a population-representative case-control study from the Northern California Region of the Kaiser Permanente Medical Care Plan44 and a recent cohort study demonstrating association of lower perinatal socioeconomic status and risk for rheumatoid arthritis.45 These results, however, are in contrast to previous studies that have demonstrated risk associated with higher socioeconomic status.46 A systematic review of the association of socioeconomic status with MS risk has found significant heterogeneity in methods and results for 21 studies from 13 countries.46 Part of the inconsistent results could in fact be related to the very different ranges of socioeconomic statuses in various regions of the world. We adjusted for socioeconomic status in all of our models, and performed stratified analyses to address concerns of confounding from this variable on the perinatal risk factor associations studied.
In addition to the positive associations discussed previously, some pertinent negative results also were found. In a multivariable model adjusting for other pregnancy-related factors, breastfeeding and maternal BMI were not associated with odds to have MS. Although illness-related stress may affect risk, stressful life events experienced by the mother, such as divorce, trauma, or job loss, were not associated with having MS.
Strengths of our study include that our relatively large dataset is one of few in the field of autoimmune diseases to look at rigorous multivariable models with mutual adjustment for several early-life exposures with potential immune effects. In our primary multivariable model of hypothesis-driven pregnancy-related factors, we were able to address effects of these risk factors simultaneously and avoid potential confounding among these often highly related factors. Additional strengths include enrollment of carefully ascertained cases shortly after MS onset (median less than 1 year), racial and ethnic diversity, and a rigorously phenotyped cohort.
Limitations of our study included the case-control design. Controls were recruited from the same institutions as cases and, thus, likely from the same underlying cohort of children in those catchment areas; however, the cases may derive from a broader geographic area than the healthy controls, as primary care is more widely available than MS specialty care. Although in this pediatric study, there were likely fewer effects from diminished quality of recall over time compared with adult studies, there still may be differential recall between parents with an ill child compared with parents of a healthy child. There were some differences in the participants who completed the questionnaires versus the nonresponders in terms of race and ethnicity. We have observed a discrepancy in socioeconomic status in MS cases versus controls. Despite adjustment in our models for mother’s education (adjustment for health insurance type did not further contribute to the models), residual confounding might have biased our results. It is known that cesarean delivery rates are higher in women with higher socioeconomic status and pesticide exposures also may differ.47 However, factors such as work-related exposures and differential health care may instead be mediators of the association of socioeconomic status and MS risk. In the exploratory analyses, results were not statistically significant after adjustment for multiple comparisons, but several variables pertaining to garden work demonstrated association with odds to have MS and in this rare dataset of early-life risk, several factors of interest have been identified that warrant further investigation and replication.
Studying patients with earlier than average age of disease onset provides an outstanding window of opportunity to unravel early-life exposures relevant to disease risk. Our results motivate continuing our recruitment efforts and developing international collaborations as the interactions among early-life exposures associated with autoimmune disease risk may be complex and necessitate large datasets for identification.2 For example, for diabetes risk, there may be opposing or synergistic effects of mode of delivery, early enteroviral infections, breastfeeding, early dairy product use, and day care exposure.2,48 Identifying the exact environmental exposures and interactions between those that are associated with pediatric MS will lead researchers back to the bench to unravel biological processes at play and possibly allow the development of prevention strategies, particularly for those families with higher risk.
Conclusions
We found evidence for maternal and perinatal factors having influence on developing pediatric MS. The biological mechanisms for our observed associations remain unknown and will be a focus of future study. Of additional interest are the potential mediating roles of the microbiome in these associations and in epigenetic changes that occur in this vulnerable developmental period.
Supplementary Material
Acknowledgments
We thank all the families and staff members of the pediatric MS centers participating in this study. Without them these investigations would not be possible.
Additional Members of the Network of Pediatric Multiple Sclerosis Centers:
Gregory Aaen, MD; Anita Belman, MD; Leslie Benson, MD; Candee Meghan, MD; Mark Gorman, MD; Manu Goyal, MD; Benjamin Greenberg, MD; Yolanda Harris, BS, MA; Ilana Kahn, MD; Timothy Lotze, MD; Mar Soe, MD; Manikum Moodley, MD; Jayne Ness, MD, PhD; Mary Rensel, MD; Shelly Roalstad, PhD; Moses Rodriguez, MD; John Rose, MD; Teri Schreiner, MD; Jan-Mendelt Tillema, MD; and Amy Waldman, MD.
Glossary
- CI
confidence interval
- MS
multiple sclerosis
- OR
odds ratio
Footnotes
Dr Graves designed the study, contributed to the statistical analysis plan, and wrote the manuscript; Drs Chitnis, Weinstock-Guttman, Rubin, and Zelikovich contributed to study design and edited the manuscript for content; Dr Nourbakhsh reviewed analyzed data and edited the manuscript for content; Mr Waltz and Mr Simmons performed statistical analysis and edited the manuscript for content; Dr Casper contributed to the statistical analysis plan and performed statistical analysis and edited the manuscript for content; Dr Waubant designed the study, obtained funding, and edited the manuscript for content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institutes of Health grant R01NS071463 (Principal Investigator Dr Waubant). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Stene LC, Gale EA. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56(9):1888–1897 [DOI] [PubMed] [Google Scholar]
- 2.Couper JJ. Environmental triggers of type 1 diabetes. J Paediatr Child Health. 2001;37(3):218–220 [DOI] [PubMed] [Google Scholar]
- 3.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–735 [DOI] [PubMed] [Google Scholar]
- 4.Larsson K, Elding-Larsson H, Cederwall E, et al. Genetic and perinatal factors as risk for childhood type 1 diabetes. Diabetes Metab Res Rev. 2004;20(6):429–437 [DOI] [PubMed] [Google Scholar]
- 5.Wahlberg J, Fredriksson J, Nikolic E, Vaarala O, Ludvigsson J; ABIS-Study Group . Environmental factors related to the induction of beta-cell autoantibodies in 1-yr-old healthy children. Pediatr Diabetes. 2005;6(4):199–205 [DOI] [PubMed] [Google Scholar]
- 6.Frederiksen B, Kroehl M, Lamb MM, et al. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr. 2013;167(9):808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stene LC, Barriga K, Norris JM, et al. Perinatal factors and development of islet autoimmunity in early childhood: the diabetes autoimmunity study in the young. Am J Epidemiol. 2004;160(1):3–10 [DOI] [PubMed] [Google Scholar]
- 8.Berkun Y, Padeh S. Environmental factors and the geoepidemiology of juvenile idiopathic arthritis. Autoimmun Rev. 2010;9(5):A319–A324 [DOI] [PubMed] [Google Scholar]
- 9.Carlens C, Jacobsson L, Brandt L, Cnattingius S, Stephansson O, Askling J. Perinatal characteristics, early life infections and later risk of rheumatoid arthritis and juvenile idiopathic arthritis. Ann Rheum Dis. 2009;68(7):1159–1164 [DOI] [PubMed] [Google Scholar]
- 10.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–633 [DOI] [PubMed] [Google Scholar]
- 11.Ragnedda G, Leoni S, Parpinel M, et al. Reduced duration of breastfeeding is associated with a higher risk of multiple sclerosis in both Italian and Norwegian adult males: the EnvIMS study. J Neurol. 2015;262(5):1271–1277 [DOI] [PubMed] [Google Scholar]
- 12.Conradi S, Malzahn U, Paul F, et al. Breastfeeding is associated with lower risk for multiple sclerosis. Mult Scler. 2013;19(5):553–558 [DOI] [PubMed] [Google Scholar]
- 13.Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18(4):468–471 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen NM, Bager P, Stenager E, et al. Cesarean section and offspring’s risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler. 2013;19(11):1473–1477 [DOI] [PubMed] [Google Scholar]
- 15.Barragán-Martínez C, Speck-Hernández CA, Montoya-Ortiz G, Mantilla RD, Anaya JM, Rojas-Villarraga A. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta-analysis. PLoS One. 2012;7(12):e51506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedstrom AK, Olsson T, Alfredsson L.. Smoking, organic solvents and MS susceptibility; interaction with HLA genotype. ECTRIMS Online Library. 2015;116640 [Google Scholar]
- 17.Riise T, Moen BE, Kyvik KR. Organic solvents and the risk of multiple sclerosis. Epidemiology. 2002;13(6):718–720 [DOI] [PubMed] [Google Scholar]
- 18.Hernán MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. 2001;154(1):69–74 [DOI] [PubMed] [Google Scholar]
- 19.Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;61(8):1122–1124 [DOI] [PubMed] [Google Scholar]
- 20.Krupp LB, Tardieu M, Amato MP, et al. ; International Pediatric Multiple Sclerosis Study Group . International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267 [DOI] [PubMed] [Google Scholar]
- 21.Belman AL, Krupp LB, Olsen CS, et al. ; US Network of Pediatric MS Centers . Characteristics of Children and Adolescents With Multiple Sclerosis. Pediatrics. 2016;138(1):e20160120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2). Available at: www.pediatrics.org/cgi/content/full/126/2/e401 [DOI] [PubMed] [Google Scholar]
- 23.Aoyama K, Seaward PG, Lapinsky SE. Fetal outcome in the critically ill pregnant woman. Crit Care. 2014;18(3):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zager A, Peron JP, Mennecier G, Rodrigues SC, Aloia TP, Palermo-Neto J. Maternal immune activation in late gestation increases neuroinflammation and aggravates experimental autoimmune encephalomyelitis in the offspring. Brain Behav Immun. 2015;43:159–171 [DOI] [PubMed] [Google Scholar]
- 26.Mandal M, Donnelly R, Elkabes S, et al. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun. 2013;33:33–45 [DOI] [PubMed] [Google Scholar]
- 27.Proal AD, Albert PJ, Marshall TG. The human microbiome and autoimmunity. Curr Opin Rheumatol. 2013;25(2):234–240 [DOI] [PubMed] [Google Scholar]
- 28.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14(10):492. [DOI] [PubMed] [Google Scholar]
- 29.Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):344. [DOI] [PubMed] [Google Scholar]
- 30.Hansen CH, Andersen LS, Krych L, et al. Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. J Immunol. 2014;193(3):1213–1222 [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremlett H, Fadrosh DW, Faruqi AA, et al. ; US Network of Pediatric MS Centers . Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23(8):1308–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023 [DOI] [PubMed] [Google Scholar]
- 34.Cortessis VK, Thomas DC, Levine AJ, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012;131(10):1565–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31(3):363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collotta M, Bertazzi PA, Bollati V. Epigenetics and pesticides. Toxicology. 2013;307:35–41 [DOI] [PubMed] [Google Scholar]
- 38.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54:141–164 [DOI] [PubMed] [Google Scholar]
- 39.Hayden KM, Norton MC, Darcey D, et al. ; Cache County Study Investigators . Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology. 2010;74(19):1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freire C, Koifman S. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology. 2012;33(5):947–971 [DOI] [PubMed] [Google Scholar]
- 41.Parks CG, De Roos AJ. Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus. 2014;23(6):527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mokarizadeh A, Faryabi MR, Rezvanfar MA, Abdollahi M. A comprehensive review of pesticides and the immune dysregulation: mechanisms, evidence and consequences. Toxicol Mech Methods. 2015;25(4):258–278 [DOI] [PubMed] [Google Scholar]
- 43.Corsini E, Sokooti M, Galli CL, Moretto A, Colosio C. Pesticide induced immunotoxicity in humans: a comprehensive review of the existing evidence. Toxicology. 2013;307:123–135 [DOI] [PubMed] [Google Scholar]
- 44.Briggs FB, Acuña BS, Shen L, et al. Adverse socioeconomic position during the life course is associated with multiple sclerosis. J Epidemiol Community Health. 2014;68(7):622–629 [DOI] [PubMed] [Google Scholar]
- 45.Parks CG, D’Aloisio AA, DeRoo LA, et al. Childhood socioeconomic factors and perinatal characteristics influence development of rheumatoid arthritis in adulthood. Ann Rheum Dis. 2013;72(3):350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goulden R, Ibrahim T, Wolfson C. Is high socioeconomic status a risk factor for multiple sclerosis? A systematic review. Eur J Neurol. 2015;22(6):899–911 [DOI] [PubMed] [Google Scholar]
- 47.Gould JB, Davey B, Stafford RS. Socioeconomic differences in rates of cesarean section. N Engl J Med. 1989;321(4):233–239 [DOI] [PubMed] [Google Scholar]
- 48.Hall K, Frederiksen B, Rewers M, Norris JM. Daycare attendance, breastfeeding, and the development of type 1 diabetes: the diabetes autoimmunity study in the young. Biomed Res Int. 2015;2015:203947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.