Patients with VVS who did not have POTS had significant HR increases when upright; HR increases alone do not confer a diagnosis of POTS.
Abstract
BACKGROUND AND OBJECTIVES:
Recurrent postural vasovagal syncope (VVS) is caused by transient cerebral hypoperfusion from episodic hypotension and bradycardia; diagnosis is made by medical history. VVS contrasts with postural tachycardia syndrome (POTS), defined by chronic daily symptoms of orthostatic intolerance with excessive upright tachycardia without hypotension. POTS has recently been conflated with VVS when excessive tachycardia is succeeded by hypotension during tilt testing. We hypothesize that excessive tachycardia preceding hypotension and bradycardia is part of the vasovagal response during tilt testing of patients with VVS.
METHODS:
We prospectively performed head-up tilt (HUT) testing on patients with recurrent VVS (n = 47, 17.9 ± 1.1 y), who fainted at least 3 times within the last year, and control subjects (n = 15, 17.1 ± 1.0 y), from age and BMI-matched volunteers and measured blood pressure, heart rate (HR), cardiac output, total peripheral resistance, and end tidal carbon dioxide.
RESULTS:
Baseline parameters were the same in both groups. HR (supine versus 5 and 10 minutes HUT) significantly increased in control (65 ± 2.6 vs 83 ± 3.6 vs 85 ± 3.7, P < .001) and patients with VVS (69 ± 1.6 vs 103 ± 2.3 vs 109 ± 2.4, P < .001). HUT in controls maximally increased HR by 20.3 ± 2.9 beats per minute; the increase in patients with VVS of 39.8 ± 2.1 beats per minute was significantly greater (P < .001). An increase in HR of ≥40 beats per minute by 5 and 10 minutes or before faint with HUT, occurred in 26% and 44% of patients with VVS, respectively, but not in controls.
CONCLUSIONS:
Orthostasis in VVS is accompanied by large increases in HR that should not be construed as POTS.
What Is Known on This Subject:
Recurrent postural vasovagal syncope (VVS) is defined by episodic loss of consciousness resulting from hypotension. Upright heart rate is not specified for VVS. Postural tachycardia syndrome (POTS) is defined by chronic symptoms and excess tachycardia while upright, without hypotension. The definitions are mutually exclusive.
What This Study Adds:
Orthostatic heart rate (HR) increases in many patients with recurrent VVS. Although our patients with VVS did not have POTS, they had significant HR increases when upright at 5 and10 minutes. HR increases alone do not confer a diagnosis of POTS.
Imposition of an orthostatic stress, such as standing up, causes a rapid downward displacement of ∼500 to 700 mL from central stores into the splanchnic and lower extremity vascular beds.1 The resulting excessive blood pressure (BP) decreases, and if uncompensated can result in orthostatic hypotension (OH) and orthostatic intolerance (OI). Rapid circulatory compensation for orthostasis occurs via the sympathetic and parasympathetic arms of the autonomic nervous system for appropriate heart rate (HR) and BP control.2–6 The normal baroreflex response to decreased BP involves peripheral vasoconstriction and a reflex tachycardia.7,8
OI is the inability to tolerate upright posture that is relieved by recumbency, and is accompanied by signs and symptoms that include loss of consciousness (LOC), cognitive deficits, loss of vision or hearing, lightheadedness, headache, fatigue, nausea and abdominal pain, sweating, and tremulousness.9 Two common causes of OI in younger patients are vasovagal syncope (VVS) and postural tachycardia syndrome (POTS).10,11
Patients with POTS experience chronic OI plus excessive tachycardia when upright in the absence of hypotension. Symptoms occur daily, almost always interfere with work and/or school activities, and the illness must be present for several months to be diagnosed.12–14 Excessive tachycardia in POTS is defined by an increase of HR to >120 beats per minute during a 10-minute tilt or an increase of >30 beats per minute in adults or an increase of >40 beats per minute in those younger than 19 years of age.15
Syncope is defined as a “total loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery,”16 which is almost always due to hypotension. The most common form is VVS, in which ∼40% of people experience at least 1 episode throughout life, most presenting initially during adolescence.17,18 VVS can be elicited by upright posture and by emotional stress (eg, “blood phobia”).19 VVS, if recurrent, is characteristically episodic in otherwise healthy patients. Unlike POTS, which is a form of chronic OI, VVS is rarely present on a daily basis.
In VVS due to orthostasis (ie, postural VVS), changes in HR and BP can occur in the following manner. Upon standing up, there is a brief period during which autonomic adjustments are initialized.20 Thereafter, BP stabilizes and HR increases due to increased sympathetic activity, compensatory vasoconstriction with venous emptying, and vagal withdrawal.21–23 Usually, BP slowly decreases and HR reflexively increases. Symptoms of OI can begin within minutes of standing upright, and if not relieved by recumbency, a later precipitous fall in BP caused by vasodilation followed by a fall in HR results in LOC.11,19
During an upright tilt test of VVS, if reflex tachycardia were excessive, as defined above, an erroneous diagnosis of POTS might be made.24,25 Such a diagnosis is incorrect on clinical grounds because POTS is chronic OI, whereas episodic VVS is not. Although a diagnosis of syncope by laboratory testing requires a finding of hypotension, this specifically excludes a diagnosis of POTS.
We therefore hypothesized that excessive tachycardia preceding hypotension and bradycardia is often part of the vasovagal response, and can be observed during tilt table testing of patients diagnosed with VVS, who are free of chronic day to day symptoms of OI, but do have postural hypotension and therefore do not have POTS. And because we have observed orthostatic HR increases in many patients with recurrent VVS without POTS, we sought to show that HR increases alone do not confer a diagnosis of POTS. To test this hypothesis, the following experiments were performed.
Methods
Subjects
To test this hypothesis, we performed a prospective study over a 3-year period at the Center for Hypotension, which is an outpatient facility of the Department of Pediatrics at New York Medical College, Valhalla, New York. We recruited VVS subjects with a history of recurrent fainting who had fainted at least 3 times within the last year (47 patients [29 female] ranging in age from 12 to 20 years). We also recruited 15 healthy nonfainting control subjects (9 female), ranging in age from 11 to 22 years.
Patients who fainted were referred to our center for investigation after experiencing at least 3 episodes of fainting within the last 12 months. Data were analyzed for those patients who experienced VVS during our upright tilt procedure, which duplicated their vasovagal episodes that occurred in the real world, and who experienced a pre- and the postsyncopal postdrome as described below. None of the patients with VVS had daily orthostatic symptoms, and all were fully functional between syncopal episodes. We also recruited control subjects from age- and BMI-matched volunteers to also undergo a 70° head-up tilt (HUT). There were no differences in the ages, weight, and BMI between the groups.
Patients with VVS gave a medical history and underwent a physical examination, electrocardiography, echocardiography, and prolonged electrocardiography monitoring if needed to exclude cardiac causes for syncope. Control subjects, recruited by online advertising to students at our institution, reported no clinical illness, no OI, and had never fainted.
The diagnosis of VVS was based on clinical history and diagnostic features encompassed predisposing situations, prodromal symptoms, physical signs, and postdrome recovery and symptoms in the absence of heart disease.10 In all patients, past fainting had been induced by prolonged standing, and prodromal symptoms included pallor, lightheadedness, nausea with abdominal discomfort, diaphoresis, a feeling of warmth, visual scotomata, or frank loss of vision. After the faint, unconsciousness usually lasted less than 30 seconds once supine and most patients experienced postsyncopal fatigue.
Exclusion criteria included any infectious or systemic illness, competitive athletic training, recent long-term bed rest, use of nicotine containing products or pregnancy within the last year. Previous medication for syncope, if any, was discontinued for at least 2 weeks before participation in this study. No subjects were taking neurally active or vasoactive drugs at the time of evaluation.
All subjects refrained from caffeine and xanthine-containing products for at least 72 hours before testing. All subjects were instructed to fast for at least 4 hours before testing. This study was approved by the Institutional Review Board of New York Medical College. All subjects 18 or older signed an informed consent; those younger than 18 assented to participate and their parent or legal guardian signed an informed consent.
Protocol
Subjects arrived at our center at 9:30 am and were prepared for study while supine on an electronic motorized tilt table (Colin Medical Instruments Corp, San Antonio, TX) with a footboard. Beat-to-beat BP was measured by Finometer finger photoplethysmograph (FMS, Amsterdam, The Netherlands) on the right forefinger or middle finger. The Finometer uses the ModelFlow algorithm to estimate beat-to-beat cardiac output (CO) by pulse-wave analysis. Before experiments began, ModelFlow CO was calibrated against an Innocor inert gas rebreathing CO measurement (Innovision, Denmark) performed while the subject was supine. Mean arterial pressure (MAP) was calculated from systolic BP (SBP) and diastolic BP as (SBP + 2*diastolic BP)/3. We continuously computed total peripheral resistance (TPR) by dividing MAP by the ModelFlow CO averaged over each cardiac cycle. All measurements were made as previously reported.26 Signals were acquired at 200 samples/s, multiplexed, and A/D converted by using custom software.
After preparation for study, subjects remained awake and supine for 30 minutes to acclimate. Baseline data comprised continuously measured HR, BP, CO, and TPR. For purposes of comparison, we used averaged data for the 10 minutes immediately preceding the tilt test to determine baseline supine values. After supine data collections were complete, subjects were tilted upright to 70°, and all HUT studies were performed without pharmacologic provocation. The duration of upright tilt was 10 minutes in healthy volunteer controls, whereas patients with VVS remained upright until fainting was imminent and was not limited to 10 minutes. We have previously demonstrated that 10 minutes in controls is sufficient time for the comparison of orthostatic changes in patients with VVS.27,28 Also, near fainting occurred on average between 10 and 11 minutes after starting upright tilt in patients with VVS. Continuously measured HR, BP, CO, and TPR were recorded for offline analysis.
We compared data for both control subjects and patients with VVS obtained during the baseline period, and at 5 and 10 minutes after HUT. All data were collected for 30 seconds before and after the time periods.
Patients with VVS were tilted back to supine when fainting was imminent; imminent postural VVS was defined by a tilt-induced decrease in MAP to <60 mm Hg or a decrease in SBP <70 mm Hg associated with symptoms of impending LOC, severe lightheadedness, nausea, heat, and diaphoresis. By design all of the patients who fainted developed a classic vasovagal faint with hypotension followed by bradycardia during the tilt.
Statistical Methods
Baseline data for BP, HR, CO, TPR, and end tidal carbon dioxide are shown in absolute units in Table 1. All tabular results are reported as mean ± SEM, and differences were evaluated by using t tests and repeated measures analysis of variance. In addition, HR data were binned by multiples of 7 to provide for clearer observations of underlying distributions and for ease of visualization. Significance was established at P < .05. To reduce interrater variability, all data were collected and analyzed by the same investigator throughout the entire study. Results were calculated by using GraphPad Prism version 4.0.
TABLE 1.
Cardiovascular Parameters of Control Subjects and Patients with VVS at Baseline, and 5 and 10 Minutes Following HUT
| Control, n = 15 | VVS, n = 47 | |||||
|---|---|---|---|---|---|---|
| Baseline | 5 min | 10 min | Baseline | 5 min | 10 min | |
| HR, beats per minute | 65 ± 2.6 | 83 ± 3.6a | 85 ± 3.7a | 69 ± 1.6 | 103 ± 2.3ab | 109 ± 2.4ab |
| SBP, mm Hg | 115 ± 2.2 | 120 ± 3.5 | 119 ± 3.9 | 113 ± 1.3 | 115 ± 2.4 | 97 ± 3.4ab |
| Diastolic BP, mm Hg | 58 ± 2.3 | 63 ± 1.5 | 63 ± 2.4 | 63 ± 1.0 | 68 ± 1.9a | 55 ± 2.1a |
| MAP, mm Hg | 77 ± 2.1 | 82 ± 2.5 | 82 ± 2.6 | 80 ± 1.1 | 85 ± 1.7a | 69 ± 2.3ab |
| CO, L/min | 5.5 ± 0.3 | 5.0 ± 0.3 | 5.0 ± 0.3 | 4.8 ± 0.2 | 5.3 ± 0.3 | 4.5 ± 0.2 |
| TPR, mm Hg /L/min | 17.9 ± 0.8 | 19.9 ± 0.9 | 22.2 ± 0.9a | 17.8 ± 0.9 | 16.8 ± 1.1 | 16.4 ± 0.7ǂ |
P < .05 compared with baseline value.
P < .05 compared with corresponding control value.
Results
There were no differences in the age of the 2 study groups, comparing control subjects (17.1 ± 1.0 y, n = 15) to patients with VVS (17.9 ± 1.1 y, n = 47) who had fainted at least 3 times within the last year, nor in the characteristics of the 2 study groups, as shown in Table 2. A comparison of HR while supine versus 5 and 10 minutes 70° HUT revealed a significant increase in both control (65 ± 2.6 vs 83 ± 3.6 vs 85 ± 3.7, P < .001) and patients with VVS (69 ± 1.6 vs 103 ± 2.3 vs 109 ± 2.4, P < .001). HUT in controls maximally increased HR by 20.3 ± 2.9 beats per minute; the increase in patients with VVS of 39.8 ± 2.1 beats per minute was significantly greater than control (P < .001). An increase in HR of >40 beats per minute by 5 and 10 minutes with HUT occurred in 26.7% and 44.4% of patients with VVS, respectively, but did not occur in controls.
TABLE 2.
Demographic Characteristics of Control Subjects and Patients with VVS
| Control Subjects | VVS Subjects | |
|---|---|---|
| Number (F/M) | 15 (9/6) | 47 (29/18) |
| Age, y ± SEM | 17.1 ± 1.0 | 17.9 ± 1.1 |
| Age range, y | 11–22 | 12–20 |
| Height, cm | 164.1 ± 3.2 | 166.7 ± 1.2 |
| Weight, kg | 59.5 ± 3.1 | 64.3 ± 1.4 |
| BMI | 21.9 ± 1.1 | 23.0 ± 0.4 |
| BMI z score | 0.26 | 0.67 |
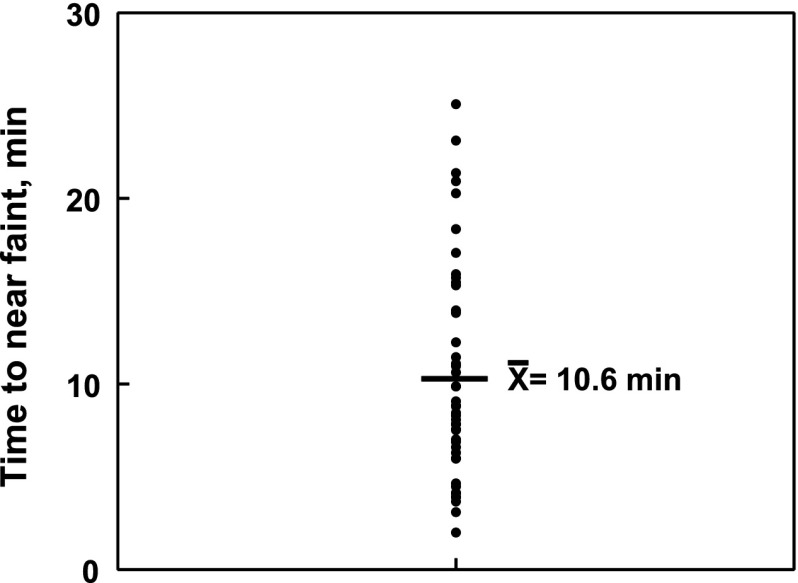
Healthy control subjects did not experience symptoms of OI nor did they faint during the 10-minute HUT. The average time to faint for the patients with VVS was 640 ± 70 seconds (10 minutes, 40 seconds); a scatterplot of these times for each patient with VVS is shown in Fig 1. A comparison of measured parameters is shown in Table 1, evaluated for control subjects and patients with VVS at baseline, and 5 and 10 minutes after HUT. These values were the same for both groups while supine (baseline) except that diastolic pressure first significantly increased at 5 minutes then significantly decreased at 10 minutes in patients with VVS compared with control (P < .05). There was also a significant increase in TPR in controls at 10 minutes after tilt compared with baseline (P < .001), and a significant decrease at 10 minutes for patients with VVS compared with baseline (P < .001). The imposition of an orthostatic challenge by HUT resulted in the anticipated significant increases in HR (P < .05) comparing supine to both 5- and 10-minute values for both controls and patients with VVS.
FIGURE 1.
A scatterplot of the time to near faint in patients with VVS. The average time to near faint  for all 47 patients was 10 minutes, 40 seconds.
for all 47 patients was 10 minutes, 40 seconds.
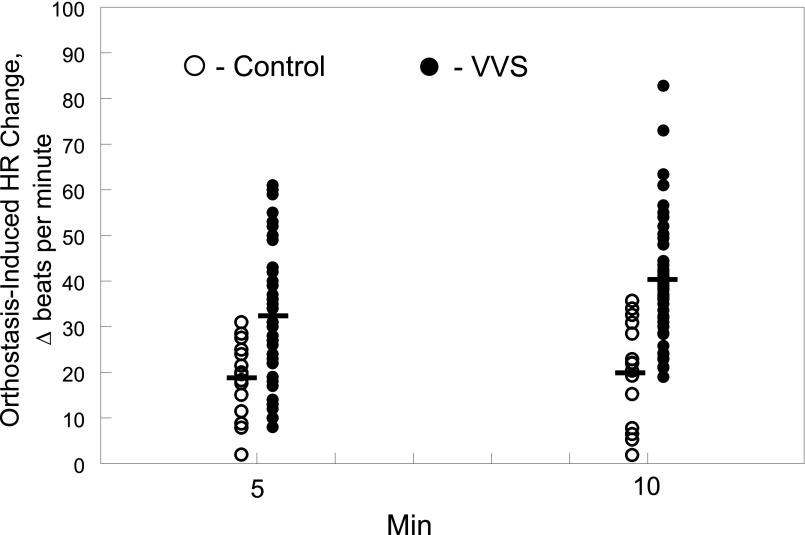
The range of HR change after HUT in controls and patients with VVS is shown in Fig 2 at both 5 and 10 minutes after HUT. Although average HR change increased significantly in both groups compared with their respective supine values (P < .001), the increase was significantly greater only in the patients with VVS comparing 5 to 10 minutes (Δ 33.3 ± 2.3 vs 39.8 ± 2.1, P < .05).
FIGURE 2.
The change in HR (in beats per minute) measured in each control subject (Ο) and patients with VVS (●) at 5 minutes and 10 minutes after imposition of an HUT.
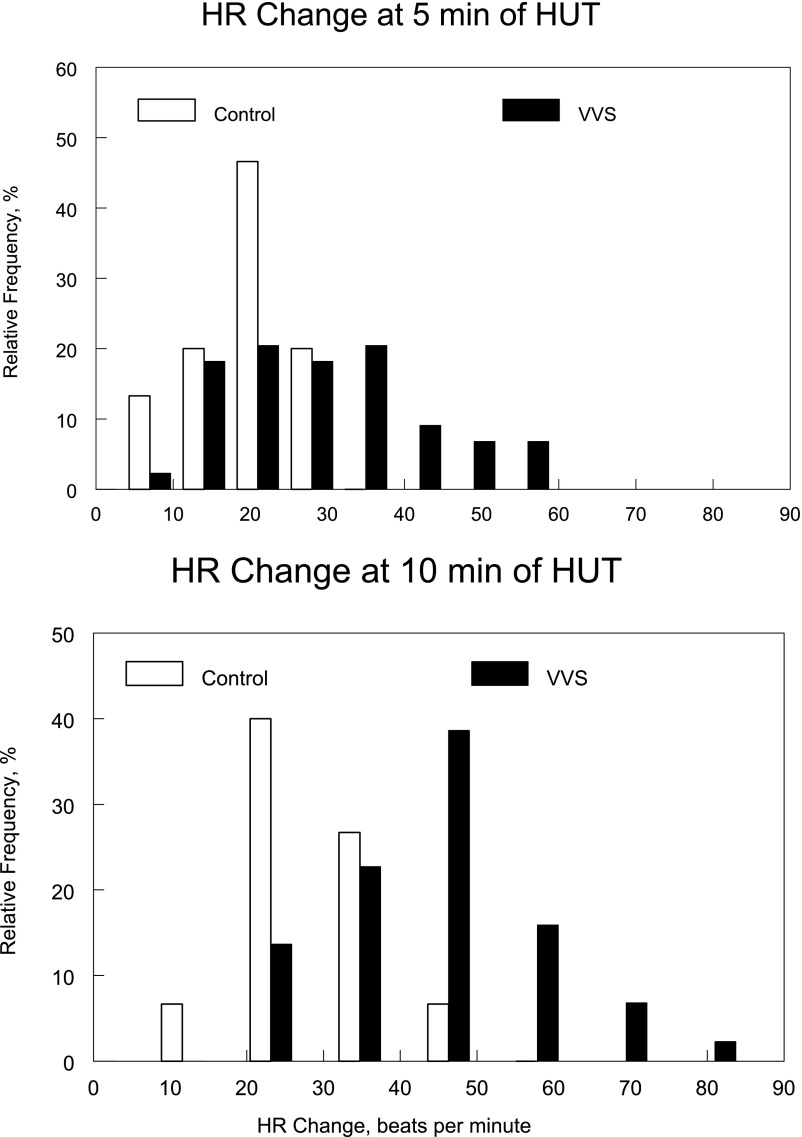
Figure 3 reveals HR changes at 5 and 10 minutes after HUT binned, to show the trend of HR increase. This figure reveals that although the responses at 5 and 10 minutes are somewhat similar for controls where the majority of subjects exhibited a change of HR of ∼20, patients with VVS exhibited a “shift to the left” indicating greatly increased HR increases at 10 minutes. This occurred in a significantly greater percentage (P < .05) of patients with VVS in whom 20 had HR increases of >40 beats per minute after HUT.
FIGURE 3.
Changes in HR (in beats per minute) binned, at 5 minutes (upper panel) and 10 minutes (lower panel) after the imposition of an HUT. HR changes are depicted as their relative frequency (%) of occurrence.
Discussion
Our findings reveal that HR increases, due to an orthostatic challenge, in young patients with VVS fall into the range associated with POTS in the absence of POTS. Although syncope may be due to cardiac diseases in older patients,2 the majority of syncope in the young is due to common postural faint, denoted postural VVS.17,18 The vasovagal response, the association of vasodilatation with vagal-induced bradycardia, causes hypotension and a transient LOC.
In our cohort of patients with VVS, TPR was reduced during orthostasis, likely due to impaired vasoconstriction. Accordingly, this led to a decrease of BP by 10 minutes and a reflexive HR increase; that is, a compensatory tachycardia resulted.28
Two common causes of OI in younger patients are VVS and POTS.11,12 The diagnosis of postural VVS connotes a diagnosis of transient upright hypotension with rapid recovery. What we have shown is that a large percentage of young patients with postural VVS and characteristic terminal hypotension also have a sinus tachycardia in response to upright positioning similar to that observed in POTS during tilt table testing. However, patients with postural VVS do not have POTS. And, because the range of HR changes with upright tilt is unknown in young patients with VVS, increases in HR elicited by tilt often result in a misdiagnosis of POTS.24,25
The diagnosis of VVS is largely on the basis of clinical history that includes characteristic prodromal symptoms, physical signs such as sweating, pallor, and hypotension, LOC, and rapid recovery times.10,19,23 Prodromal symptoms with orthostasis can include progressive diaphoresis, flushing, sensation of warmth, nausea, abdominal pain, visual blurring/loss, followed by temporary LOC resulting from hypotension and a characteristic pallor. Postdromal fatigue is frequent and may last for minutes to hours. Most importantly, VVS usually occurs without day-to-day symptoms.10
In contrast, POTS is historically chronic, not episodic, and there is no orthostatic LOC because there is no hypotension. However, many patients with POTS experience VVS, but presyncope is much more common.10 If there is hypotension and LOC with orthostasis, then syncope rather than POTS should be diagnosed by such an episode, provided there is rapid onset of the episode; it is brief and recovery occurs spontaneously.11,13
In the current study, on the basis of clinical criteria, all of our patients had VVS with hypotension and transient LOC, none had chronic OI, and none had day to day symptoms. Therefore, they did not have POTS. Our patients with VVS had significant increases in HR compared with supine when upright at both 5 and 10 minutes. In fact, >40% had increases of more than 40 beats per minute, and in 2, their HR increased by >70 beats per minute. And all of these HR increases in patients with VVS were recorded before the onset of sudden hypotension and bradycardia. Although none of these patients had POTS and all changes in HR were recorded before they became syncopal, it is important to note that some 30% of subjects with POTS also experience syncope.29,30
Thus, the excessive tachycardia preceding hypotension and bradycardia is often part of the vasovagal response in young patients, as described here in our cohort of patients with VVS. Therefore, a significant increase in HR and symptoms with orthostasis alone does not confer a diagnosis of POTS.
All of the data were collected during HUT-table testing, which can result in syncope in up to 15% of healthy subjects with no previous history of fainting31; however, none of our control subjects fainted. Findings in patients who faint recurrently may not be generalizable to those who faint less frequently, and to subjects with competitive athletic training and recent long-term bed rest.
Glossary
- BP
blood pressure
- CO
cardiac output
- HR
heart rate
- HUT
head-up tilt
- LOC
loss of consciousness
- MAP
mean arterial pressure
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- POTS
postural tachycardia syndrome
- SBP
systolic blood pressure
- TPR
total peripheral resistance
- VVS
vasovagal syncope
Footnotes
Drs Medow and Stewart were responsible for the design of the experiments, analysis, and interpretation of the data, and drafting the original manuscript; Dr Merchant, Ms Suggs, and Dr O’Donnell-Smith were responsible for collection, assembly, and interpretation of the data; Mrs Terilli was responsible for subject recruitment, collection, assembly, and interpretation of the data; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funding was provided by grants RO1 HL 112736 and RO1 HL 074873 from the National Heart, Lung, and Blood Institute, and R21 NS 094644 from the National Institute of Neurologic Disorders and Stroke. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2016-4161.
References
- 1.Sheriff DD, Nådland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol (1985). 2010;108(3):523–532 [DOI] [PubMed] [Google Scholar]
- 2.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone–epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol. 2010;95(1):80–92 [DOI] [PubMed] [Google Scholar]
- 3.Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol (Oxf). 2011;203(1):37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raczak G, La Rovere MT, Mortara A, et al. . Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J Heart Lung Transplant. 1999;18(5):399–406 [DOI] [PubMed] [Google Scholar]
- 5.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61(1):62–97 [DOI] [PubMed] [Google Scholar]
- 6.Von Euler US. Identification of the sympathomimetic ergone in adrenergic nerves of cattle (sympathin N) with levonoradrenaline. Acta Physiol Scand. 1948;16:63–74 [Google Scholar]
- 7.Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol. 1982;243(5):H676–H681 [DOI] [PubMed] [Google Scholar]
- 8.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol. 1980;49(5):809–814 [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Opfer-Gehrking TL, McPhee BR, et al. . Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc. 1995;70(7):617–622 [DOI] [PubMed] [Google Scholar]
- 10.Sheldon RS, Grubb BP II, Olshansky B, et al. . 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. 2013;131(5):968–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008;16(1):4–20 [DOI] [PubMed] [Google Scholar]
- 13.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr. 2004;145(6):725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43(1):132–137 [DOI] [PubMed] [Google Scholar]
- 15.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr. 2012;160(2):222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowers WR. A lecture on vagal and vasovagal attacks. Lancet. 1907;173:716–724 [Google Scholar]
- 17.Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003;91(8):1006–1008, A8 [DOI] [PubMed] [Google Scholar]
- 18.Moya A, Sutton R, Ammirati F, et al. ; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS) . Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brignole M, Alboni P, Benditt D, et al. ; Task Force on Syncope, European Society of Cardiology . Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace. 2001;3(4):253–260 [DOI] [PubMed] [Google Scholar]
- 20.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond). 2007;112(3):157–165 [DOI] [PubMed] [Google Scholar]
- 21.Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol (1985). 2012;113(10):1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardine DL, Melton IC, Crozier IG, et al. . Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282(5):H1804–H1809 [DOI] [PubMed] [Google Scholar]
- 23.Hainsworth R. Pathophysiology of syncope. Clin Auton Res. 2004;14(suppl 1):18–24 [DOI] [PubMed] [Google Scholar]
- 24.Nwazue VC, Raj SR. Confounders of vasovagal syncope: postural tachycardia syndrome. Cardiol Clin. 2013;31(1):101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart JM. Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr. 2009;154(4):481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart JM, Suggs M, Merchant S, et al. . Postsynaptic α1-adrenergic vasoconstriction is impaired in young patients with vasovagal syncope and is corrected by nitric oxide synthase inhibition. Circ Arrhythm Electrophysiol. 2016;9(8):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation. 2004;110(17):2575–2581 [DOI] [PubMed] [Google Scholar]
- 28.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol. 2008;295(1):H372–H381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R. Postural tachycardia syndrome--current experience and concepts. Nat Rev Neurol. 2011;8(1):22–34 [DOI] [PubMed] [Google Scholar]
- 31.Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R. The normal response to prolonged passive head up tilt testing. Heart. 2000;84(5):509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]