Abstract
Introduction
We tested the latent variable “δ” (for “dementia”)'s ability to predict conversion to “mild cognitive impairment” (MCI) and Alzheimer's disease (AD).
Methods
An ethnicity equivalent d homolog (“dEQ”) was constructed in n = 1113 Mexican- American (MA) and n = 1958 non-Hispanic white (NHW) participants in the Texas Alzheimer's Research and Care Consortium. “Normal Controls” (NC) (n = 1276) and MCI cases (n = 611) were followed annually for up to 6 years [m = 4.7(0.6)].
Results
22.0% (n = 281) of NC converted to “MCI” or “AD”. 17.3%( n = 106) of MCI converted to “AD.” Independently of covariates, each quintile increase in the dEQ scores of NC increased the odds of conversion by 52%. Each quintile increase in the dEQ scores of MCI cases increased the odds of conversion to AD almost three-fold.
Discussion
Baseline δ scores predict MCI and AD conversions from nondemented states in MA and NHW.
Keywords: Aging, Cognition, Dementia, Functional status, g, Intelligence, MCI
1. Introduction
We have proposed a latent variable phenotype for dementia itself, as distinct from impairment(s) in domain-specific cognitive performance [1]. Our intent has been to identify dementia's essential cognitive features and to distinguish them from an illness' nondementing cognitive changes. Our approach has been to explicitly extract the fraction of variance that is shared between cognitive performance and measures of functional status, that is, in a structural equation model framework. We accomplish this by a novel confirmatory bifactor model. Both factors are indicated by all observed cognitive measures. However, only one, “δ” (for “dementia”), is allowed to also be indicated by one or more functional status “target” indicators. The resulting latent δ variable is relatively free of measurement error, continuously distributed, strongly related to instrumental activities of daily living (IADL) [1], agnostic to dementia etiology [2], and “indifferent” to its cognitive indicators.
δ's indifference to its cognitive indicators suggests that it can be modeled in almost any cognitive battery that also includes a measure of IADL. Thus, we further distinguish between δ, that is, “the cognitive correlates of functional status,” and “d,” that is, δ's operationalization in a specific cognitive battery or analysis. d's extraction from multiple batteries results in a set of δ homologs, all of which appear to share similar psychometric properties.
δ homologs have been constructed in multiple data sets. They accurately diagnose dementia [1], [3] and have been associated with atrophy in the default mode network [4], Alzheimer's disease (AD) neuropathology [5], AD-specific cerebrospinal fluid (CSF) biomarkers [6], certain serum inflammatory proteins [7], hippocampal atrophy [6], prospective change, and future clinical dementia rating (CDR) scores [2], [8]. Here, we examine δ's ability to predict prospective conversion to mild cognitive impairment (MCI) and AD from nondemented states using a homolog [i.e., “dEQ”] that exhibits factor equivalence across ethnicity [3].
2. Methods
2.1. Subjects
Subjects included N = 3072 Texas Alzheimer's Research and Care Consortium (TARCC) participants. TARCC is a longitudinally followed convenience sample of elderly persons with Alzheimer's disease (AD; n = 1182), MCI (n = 611), or normal controls (NC; n = 1276) and three “others” recruited from five Texas medical schools. Each participant underwent a standardized annual examination that included a medical evaluation, neuropsychological testing, and clinical interview. Categorical clinical diagnoses of “AD,” “MCI,” and “NC” were established through consensus. The diagnosis of AD was based on National Institute for Neurological Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria [9]. The diagnosis of MCI was based on site-specific consensus-based clinical diagnoses derived from all available information but without reliance on specific neurocognitive tests and/or cut scores. “All available information” included the results of TARCC's entire neuropsychological battery, clinical evaluations, informant interviews, and any available outside medical records. We could not easily use cut scores because Mexican-American (MA) norms are not available for many measures. Institutional review board approval was obtained at each site, and written informed consent was obtained for all participants.
2.2. Transitions
Categorical clinical diagnoses were updated annually. Transitions across categorical boundaries were determined by incident conversion at any visit relative to the modeled nondemented state(s) at visit 1. Only 10 individuals converted to AD from baseline control status. Because this is too small to model, we combined them with MCI conversions.
2.3. Clinical variables
The Clinical Dementia Rating Scale sum of boxes (CDR-SB) [10]: The CDR is used to evaluate dementia severity. The information necessary to those ratings is collected during an interview with the patient and their caregiver. Each CDR domain is rated on a scale of 0.0–3.0. A total CDR-SB score is calculated as the sum of all six domains.
The Geriatric Depression Rating Scale (GDS) [11], [12]: GDS scores range from 0–30. Higher scores are worse. The GDS is valid in demented persons [13].
The Mini–Mental Status Examination (MMSE) [14]: The MMSE is a well known and widely used test for screening cognitive impairment. Scores range from 0 to 30.
2.4. dEQ's construction and validation
dEQ's construction and validation in this sample has been previously described [3]. Briefly, dEQ's cognitive indicators included the Controlled Oral Word Association [15], Logical Memory II [16], the Digit Span Test [16], and Wechsler Memory Scale Visual Reproduction I [16]. dEQ's target indicator was IADL [17]: IADLs were assessed using caregiver ratings. The resulting latent variables g′ and dEQ were adjusted for age, education, and gender before reification as composite scores. This dEQ homolog has previously been reported to have acceptable factor determinacy by Grice's method [18], to be factor equivalent across ethnicity in this sample with respect to all indicator loadings and several residuals, to be strongly associated with its IADL target (r = 0.89, P < .001), and to correlate strongly with CDR-SB (r = 0.99, P < .001). The dEQ composite accurately distinguished NC from AD (area under the receiver operating characteristic curve [AUC] = 0.95; 95% confidence interval [CI] = 0.94–0.96) [3].
2.5. Statistical analyses
2.5.1. Analysis sequence
As previously described [3], this analysis was performed using Analysis of Moment Structures software [19]. The maximum likelihood estimator was chosen for these models. All observed indicators were adjusted for age, education, gender, GDS, and apolipoprotein E (APOE) ε4 burden. Covariances between the residuals were allowed to be estimated if they were significant and improved model fit.
We used multivariate logistic regression to test baseline dEQ and g′ as independent predictors of prospective conversion over up to six annual waves. NC conversion to MCI or AD and MCI conversion to AD were modeled separately. Baseline dEQ and g′ scores were divided into quintiles. Age, education, GDS scores, gender, and APOE ε4 burden were dichotomized and added to these models as additional independent predictors. Age was split at ±80 years, education at ±12 years, GDS scores at ±10/30, and APOE at ± any ε4 allele.
2.5.2. Missing data
We used the newest instance of TARCC's data set (circa 2015). The entire data set was used. Clinical diagnoses were available at visit 1 for 3072 subjects, 2113 of whom had complete data for δ's cognitive indicators and covariates. The logistic regression and receiver operating characteristic (ROC) analyses were performed in Statistical Package for the Social Sciences (SPSS) [20] and therefore limited to complete cases (N = 2113).
2.5.3. ROC curves
The diagnostic performance or accuracy of a test to discriminate diseased from normal cases can be evaluated using ROC curve analysis [21], [22]. Briefly, the true positive rate (sensitivity) is plotted as a function of the false positive rate (100 − specificity) for different cutoff points of a parameter. Each point of the ROC curve represents a sensitivity/specificity pairing corresponding to a particular decision threshold. The AUC is a measure of how well a parameter can distinguish between two diagnostic groups (diseased/normal). The ROC analysis was performed in SPSS.
3. Results
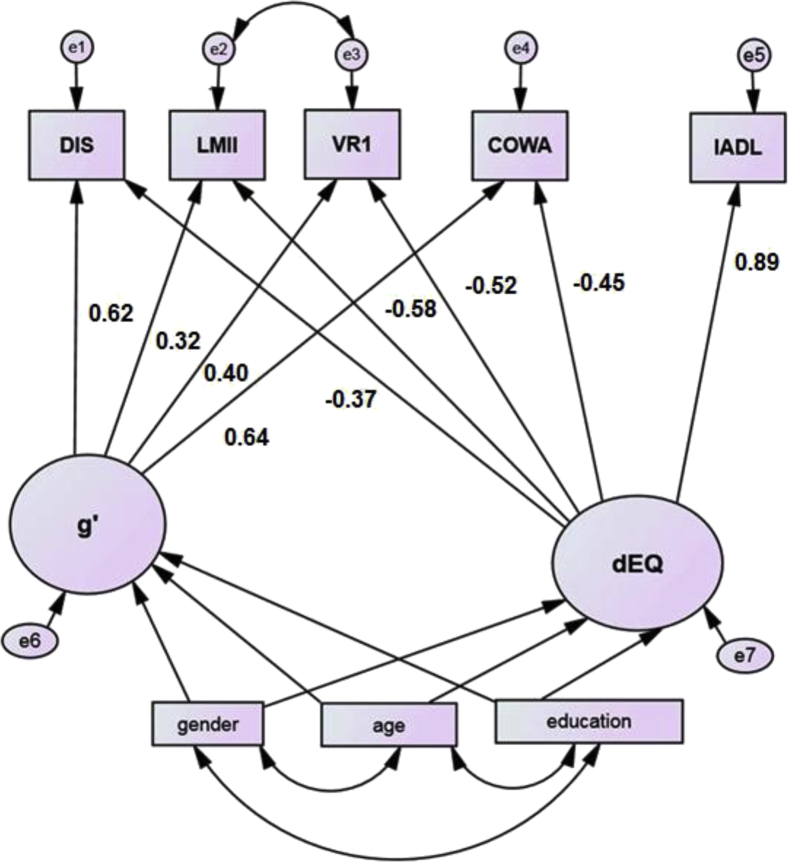
Descriptive statistics are presented in Table 1. dEQ's factor weights have been previously reported [3] and are presented in Fig. 1. Our approach results in two latent variables, dEQ, and “g′,” which is δ's residual in Spearman's g (Fig. 1; [1]).
Table 1.
Descriptive statistics
| Variable | N | Mean (SD) |
|---|---|---|
| Age (observed) | 3072 | 70.92 (9.66) |
| (1 = ≥80 years, n = 644) | 3072 | 0.21 (0.41) |
| APOE alleles (observed) | 2881 | |
| (1 = e4+, n = 1133) | 2877 | 0.39 (0.48) |
| CDR (Sum of Boxes) | 3066 | 2.5 (3.4) |
| COWA | 2982 | 8.4 (3.6) |
| DIS | 2915 | 8.8 (3.1) |
| EDUC (observed) | 3072 | 13.19 (4.32) |
| (1 = >12, n = 1767) | 3072 | 0.58 (0.49) |
| Ethnicity (1 = MA, n = 1113) | 3071 | 0.36 (0.48) |
| GDS30 (observed) | 2765 | 5.62 (5.26) |
| (1 ≥10, n = 500) | 2765 | 0.18 (0.38) |
| Gender (♂ = 1, n = 1196) | 3067 | 0.39 (0.48) |
| IADL (Summed) | 2556 | 10.1 (4.8) |
| MMSE | 3071 | 25.4 (4.9) |
| WMS LM II | 2529 | 8.2 (4.6) |
| WMS VR I | 2480 | 8.1 (4.0) |
| Complete cases | 2113 |
Abbreviations: SD, standard deviation; APOE, apolipoprotein E; CDR, clinical dementia rating; COWA, Controlled Oral Word Association Test; DIS, Digit Span Test; GDS, Geriatric Depression Scale; IADL, instrumental activities of daily living; MMSE, Mini–Mental State Examination; WMS LM II, Wechsler Memory Scale Delayed Logical Memory; WMS VR I, Wechsler Memory Scale Immediate Visual Reproduction.
Fig. 1.
The latent variable dEQ's construction. All loadings are significant at P < .001. Abbreviations: COWA, Controlled Oral Word Association Test; dEQ, ethnicity equivalent δ homolog; DIS, Digit Span Test; IADL, instrumental activities of daily living; LM II, Delayed Logical Memory; VR I, Immediate Visual Reproduction.
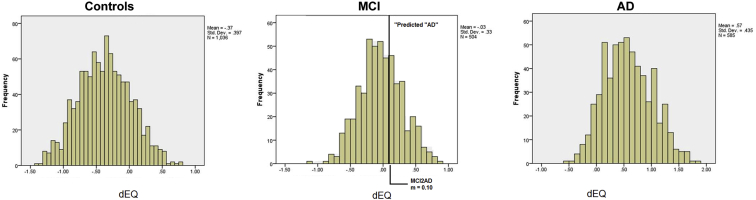
Fig. 2 presents the dEQ distributions by categorical diagnoses. dEQ scores in Fig. 2 were referenced to the entire cohort's standardized mean (i.e., dEQ = 0.0). The optimal threshold for AD's discrimination from NC was found to be 0.088 (sensitivity = 0.872, specificity = 0.870), that is, very close to the mean for MCI cases.
Fig. 2.
dEQ histograms by wave 1 diagnosis*. *dEQ scores are referenced to the entire cohort's standardized mean (i.e., dEQ = 0.0). Abbreviations: AD, Alzheimer's disease; dEQ, ethnicity equivalent δ homolog; MCI, mild cognitive impairment.
Visual inspection reveals substantial overlap in the dEQ scores of MCI cases and both AD and NC, in contrast to the distinct distributions of the latter two groups. This is consistent with dEQ's relatively weak AUCs for both NC versus MCI, and MCI versus AD [(NC vs. MCI = 0.74; 95% CI = 0.72–0.77); (MCI vs. AD = 0.86; 95% CI = 0.84–0.88)].
Table 2 presents results of the logistic regressions. Over 6 years [ṁ = 4.7(0.6)], n = 281 (22.0%) of NC converted to “MCI” or “AD.” n = 106 (17.4%) of MCI converted to “AD.” dEQ, APOE ε4 burden, age, education, and ethnicity contributed independently to NC conversion to a more advanced state (i.e., MCI or AD). Education, gender, and g′ did not enter. Independently of the covariates, each quintile increase in the dEQ scores of NC increased the odds of conversion by 52% [OR = 1.52 (1.13–2.04); Table 2].
Table 2.
Conversion predictors
| Parameter | Estimate | SE | χ2 (df) | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| NC Conversion to “MCI” or “AD” | ||||||
| Intercept | −11.57 | 1.26 | 83.11 | <.001 | ||
| dEQ | 0.42 | 0.15 | 7.76 | .005 | 1.52 | 1.13–2.04 |
| g′ | 0.09 | 0.11 | 0.63 | .42 | 1.10 | 0.87–1.38 |
| APOE ε4 | 0.69 | 0.22 | 9.52 | .002 | 2.00 | 1.28–3.11 |
| GDS | −0.57 | 0.34 | 2.83 | .09 | 0.56 | 0.28–1.09 |
| Age | 0.11 | 0.01 | 69.21 | <.001 | 1.12 | 1.08–1.14 |
| Gender | 0.25 | 0.20 | 1.50 | .22 | 1.28 | 0.86–1.92 |
| Education | 0.64 | 0.25 | 6.35 | .01 | 1.89 | 1.15–3.12 |
| Ethnicity | 1.60 | 0.27 | 34.51 | <.001 | 4.98 | 2.91–8.51 |
| MCI conversion to “AD” | ||||||
| Intercept | −11.65 | 1.70 | 46.61 | <.0001 | ||
| dEQ | 1.01 | 0.16 | 35.68 | <.0001 | 2.75 | 1.97–3.83 |
| g′ | 0.40 | 0.14 | 7.92 | .01 | 1.50 | 1.13–1.98 |
| APOE ε4 | 0.60 | 0.29 | 4.12 | .04 | 1.83 | 1.02–3.28 |
| GDS | 0.17 | 0.32 | 0.27 | .59 | 1.19 | 0.62–2.61 |
| Age | 0.07 | 0.01 | 17.09 | <.0001 | 1.08 | 1.03–1.11 |
| Gender | 0.62 | 0.27 | 5.06 | .02 | 1.87 | 1.08–3.26 |
| Education | 0.22 | 0.31 | 0.47 | .49 | 1.24 | 0.66–2.32 |
| Ethnicity | −0.69 | 0.33 | 4.33 | .03 | 0.50 | 0.26–0.96 |
Abbreviations: SE, standard error; OR, odds ratio; CI, confidence interval; MCI, mild cognitive impairment; AD, Alzheimer's disease; dEQ, ethnicity equivalent δ homolog; APOE, apolipoprotein E; GDS, Geriatric Depression Rating Scale.
Each quintile increase in the dEQ scores of MCI cases increased the odds of conversion to AD almost three-fold [OR = 2.75 (1.97–3.83)], independently of g′, APOE, age, gender, and ethnicity (Table 2). Education and GDS scores did not enter. Each quintile increase in the g′ scores of MCI increased the odds of conversion to AD by 50% [OR = 1.50 (1.13–1.98)]. MA ethnicity had a protective effect, independent of dEQ and g′ [OR = 0.50 (0.26–0.96)].
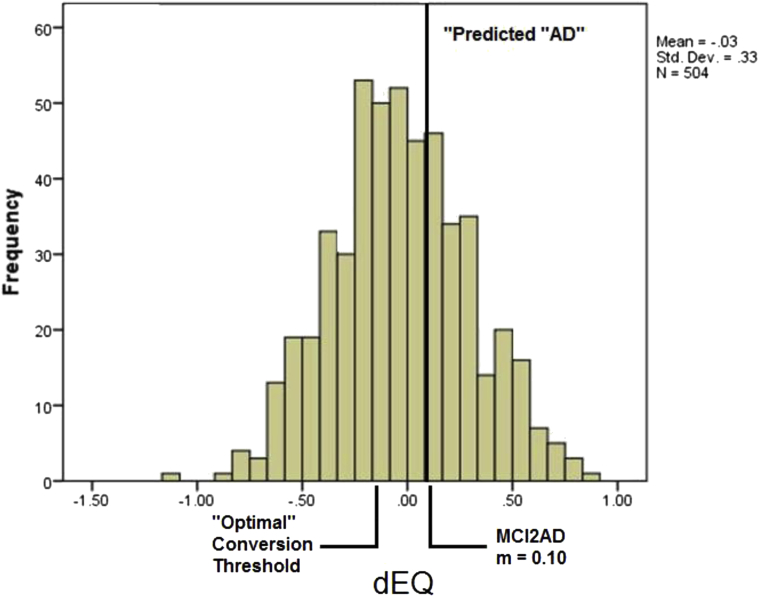
The baseline dEQ scores of MCI cases had an AUC = 0.86 for the prediction of prospective conversion to AD (95% CI = 0.819–0.891). The mean dEQ score of AD converters is 0.100. N.b. this is very near the optimal dEQ threshold for AD's discrimination from NC in TARCC (i.e., 0.088). The optimal dEQ threshold to predict conversion was −0.14 (sensitivity = 0.75, specificity = 0.76) (Fig. 3).
Fig. 3.
Mean dEQ score for MCI2AD converters and “optimal” dEQ threshold for prediction of conversion*. *The optimal threshold of −0.14 has an AUC = 0.86 for MCI2AD. The mean dEQ score of MCI2AD converters (0.10) is near the optimal threshold for AD versus NC (0.09; AUC = 0.953). Abbreviations: AD, Alzheimer's disease; AUC, area under the receiver operating characteristic curve; dEQ, ethnicity equivalent δ homolog.
4. Discussion
Change in δ scores is strongly associated with change in dementia severity, as measured by CDR [2], [3]. Additionally, Koppara et al. [6] have shown that δ homologs outperform the Alzheimer's Disease Cooperative Study ADAS–Cognitive (ADAS-COG) [23] and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) [24] as predictors of MCI's conversion to AD over 3 years. This analysis builds on their work by demonstrating that δ predicts MCI's conversion in a second cohort, in both non-Hispanic white (NHW) and MA, with a larger sample size, and a longer period of follow-up. Moreover, we have extended this finding to NCs' conversion to MCI or AD, at an even earlier stage of cognitive decline.
TARCC does not collect autopsy or biomarker data needed to confirm its clinical diagnoses. However, TARCC is a highly selected convenience sample enriched with clinical AD. In similar highly selected samples, δ is associated with AD pathology [5] and outperforms the CERAD and the ADAS-COG as a predictor of both hippocampal volume and AD-specific CSF biomarkers [6].
Regardless, δ has also been shown to be “agnostic” to dementia's etiology in two recent studies [2], [25]. Both were conducted in the NACC's Unified Dataset (UDS) (N ≅ 26,000). Gavett et al. [2] found δ's AUC = 0.96 for the discrimination between dementia of any etiology versus nondemented cases (MCI and controls). John et al. [25] demonstrated that δ scores are unable to distinguish between any combination of dementing conditions. Instead, only “unique” (i.e., “domain-specific”) variance, residual to δ, was able to make such discriminations.
These findings have important implications for dementia case finding. First, they suggest that the dEQ scores of TARCC participants could have been influenced by any and all dementing processes afflicting TARCC's cohort. Clinical dementia seems increasingly likely to be “overdetermined” by multiple independent and competing processes [26]. Age, the APOE ε4 allele, (subsyndromal) depressive symptoms, and gender are independent predictors of δ in TARCC. They have unique serum protein biomarkers [27]. These risk factors need not contribute to dementia severity through a common AD-specific neurodegeneration. Thus, although TARCC purports to be a study of AD, an estimated 20% of cases diagnosed with clinical “AD” by experienced clinicians may be without beta-amyloid by positron emission tomography [28], [29]. Moreover, δ's biomarkers have been found to differ by ethnicity in TARCC [7]. That also suggests biological heterogeneity within the demented fraction of TARCC participants. Therefore, our demonstration of δ's ability to predict dementia conversion from nondemented states may be but partially explained by AD-specific biological processes. Instead, clinical dementia in TARCC is likely to arise from the sum of independent δ-related processes.
On the other hand, δ is essentially the sole cognitive determinant of dementia severity, both cross-sectionally (e.g., r = 0.99 vs. the CDR-SB in this sample) and longitudinally (e.g., r = 0.94 × ΔCDR-SB) [2], [8]. A δ homolog composite score might therefore be applied to individuals as an omnibus dementia severity metric. The d-score is both highly accurate in distinguishing demented from nondemented persons across diagnoses [2] and is independently associated with well recognized dementia risk factors (e.g., APOE ε4 burden). Correcting any δ-related pathophysiology might improve overall dementia status.
Second, John et al.'s [25] finding suggests that the cognitive differences that distinguish dementing illnesses may be orthogonal to δ and therefore unrelated to dementia severity. This includes domain-specific variance, by definition. Domain-specific variance is thought to be attributable to localized regional structures/networks and the connectivity of those networks. Like domain-specific variance, network connectivity has been shown to distinguish dementing illnesses [30], [31]. However, to the extent that these changes mediate truly domain-specific differences in cognitive performance (i.e., in g/δ-adjusted models), they will be unrelated to (1) g/δ, and therefore (2) IADL, (3) CDR, and (4) categorical dementia. This explains how even successful interventions against domain-specific cognitive performance and its disease-specific biomarkers could have limited impact on dementia severity.
Furthermore, δ's bifactor model's design also ensures that all δ homologs (and similar “paralogs” directed at targets other than IADL) represent subsets of the variance in Spearman's general intelligence factor, g. This is important because it may restrict δ's biomarkers to those of g, and thereby restrict dementia's biology to that of intelligence. Because g contributes to a multiplicity of brain-related measures, this constraint undermines the dementia salience of any regional pathology. Hippocampal volume may be an AD-specific biomarker related to domain-specific memory performance, but it is a relatively poor predictor (vs. δ) of dementia severity [32]. Similarly, δ is unrelated to ischemic pathology (the quintessential regional pathology) in the NACC [5]. The dementing aspect of “vascular cognitive impairment” must therefore be mediated by other mechanisms.
The clinical diagnosis of “MCI” does not ensure dementia conversion in the near term, as evidenced by its relatively low 17.4% 6-year conversion rate in TARCC. In contrast, each quintile increase in the dEQ scores of MCI cases nearly tripled conversion risk. This suggests that the categorization of MCI (or “minor neurodegenerative disorder”) [33] may provide little information about conversion risk, above and beyond the d-score.
Moreover, visual inspection of dEQ's distribution suggests substantial overlap in the δ scores of MCI, NC, and AD (Fig. 2). Although “MCI” cases do occupy the middle ground of TARCC's dEQ distribution, the cross-group overlap in dEQ scores does not support three discriminable entities. That hypothesis could be easily confirmed by a latent class analysis of dEQ scores.
The optimal dEQ threshold for AD's discrimination from NC appears to be near the middle of MCI's distribution and near the mean for MCI to AD converters (MCI2AD). This suggests that a high fraction of MCI cases, and specifically MCI2AD converters, might have already been demented (i.e., misdiagnosed) at their baseline assessment. That is consistent with the high fraction of IADL impaired “MCI” cases in other cohorts [34]. “MCI” cases at the highest risk of AD conversion (i.e., dEQ's top two MCI quintiles) are certain to have been above the optimal dEQ threshold, despite their MCI designations. Conversely, the MCI cases least likely to convert (i.e., in dEQ's bottom two MCI quintiles) are likely to have been below the optimal AD versus NC diagnostic threshold. Such cases may be at no increased near-term conversion risk relative to NC, or at least relative to the fraction of NC presenting above the NC dEQ-score mean.
The optimal dEQ score threshold to predict MCI2AD conversion appeared to be moderately sensitive and specific to that outcome. Its AUC of 0.86 is as good [35] or better than previous psychometric and neuroimaging biomarkers [36], [37], [38]. The combination of psychometrics and biomarkers may have incremental advantages over our approach [39], [40], but ours is obtainable with much less expense and effort. Moreover, δ's accuracy for the prospective prediction of MCI conversion has now been independently replicated in two large language and ethnically diverse samples, and with different sets of psychometric indicators [6]. The prospective ability of this dEQ threshold to predict AD conversion remains to be demonstrated.
Regardless, g′ did contribute independently of dEQ to MCI's AD conversion risk (Table 2). It is possible then that TARCC clinicians may be biased in their diagnostic deliberations by changes in observed cognitive performance that empirically have no functional salience. Even were it to be proven that g′ is mediated by AD-specific neurodegeneration (as δ can be shown to be) [5], a g′-specific diagnostic bias has the potential to undermine the validity of categorical dementia case finding. Clinicians working from observed cognitive performance measures risk to diagnose “dementia” at lesser levels of disability, and/or to attribute functionally trivial cognitive gains as evidence of “dementia's” remediation. This bias also risks to introduce measurement error into studies that associate AD's clinical diagnosis with specific genes or biomarkers. They might identify biomarkers that are merely associated with g′'s clinically trivial variance. In contrast, any change in δ scores would be functionally salient by definition and δ's predictors and biomarkers should offer targets for dementia's specific remediation.
APOE ε4 allele burden, gender, age, and ethnicity predicted MCI conversion independently of dEQ and g′. APOE ε4 allele burden is significantly associated with δ, and AD pathology fully mediates their association in autopsy-proven AD cases [5]. Similarly, age's effect on cognitive performance can be shown to be largely mediated by δ [41]. Each year of increasing age is associated with a 0.02 SD increase in dEQ. 1.0 SD in dEQ scores might be traversed over TARCC's 50-year age range. The current analysis shows that this is not trivial. Even small changes in the dEQ scores of nondemented persons impose a considerable conversion risk. Those findings suggest that APOE ε4 allele burden and age's specific dementia risks may be mediated by δ and therefore also by intelligence.
Finally, MA ethnicity increased the risk of NC to MCI or AD almost fivefold but decreased the risk of MCI2AD conversion, independently of dEQ scores. This cannot be easily attributed to cognitive assessment bias, as the dEQ homolog exhibits factor equivalence across ethnicity. There is no ethnicity effect on the mean δ scores of MCI and AD in TARCC, either cross-sectionally or longitudinally [7], [8].
These findings could reflect an ethnicity bias in dementia's categorical clinical diagnosis. That might arise from the use of inadequate cognitive performance norms among MA, or from clinical obstacles to the recognition of cognitive decline across linguistic, cultural, and/or educational boundaries. The mean δ scores of MA controls in TARCC are nearer to the mean for MCI cases of either ethnicity [7]. Alternatively, MA may be experiencing a modified disease process(es), as evidenced by distinct AD biomarker profiles in MA versus NHW TARCC participants [3], [7], [42], [43], [44]. This finding deserves more investigation.
In summary, baseline δ scores are associated with MCI and AD conversions from nondemented states in MA and NHW. Independent of δ, g′ is also associated with AD conversion risk. This suggests clinician bias in their assessments of functionally salient cognitive changes. The substantial overlap in the δ scores of MCI cases and both AD and NC, in contrast to the distinct distributions of the latter two groups, undermines the validity and utility of categorical “MCI” as a diagnostic entity. d-scores may offer a more parsimonious, transparent, and reproducible way of assessing near-term dementia conversion risk.
Research in context.
-
1.
Systematic review: Cognitive measures and clinical diagnoses are prone to measurement error. Attempts have been made to develop “intermediate phenotypes” for use as outcomes in clinical trials. Recently proposed phenotypes include indices and latent variables constructed from cognitive measures and/or biomarkers. The latent variable “δ” (for dementia) is constructed from cognitive performance and functional status measures by a novel confirmatory bifactor model in a structural equation model framework. Here we show that δ predicts dementia conversion from nondemented states.
-
2.
Interpretation: Each quintile increase in the δ scores of nondemented persons increased dementia conversion risk by 50%. Each quintile in the δ scores of cases with Mild Cognitive Impairment (MCI) increased conversion almost three-fold.
-
3.
Future directions: δ homologs offer a more parsimonious and transparent “omnibus” measure of dementia severity than categorical clinical diagnoses of “MCI”.
Acknowledgments
This project was supported in part by funding provided to the Texas Alzheimer's Research and Care Consortium by the Darrell K Royal Texas Alzheimer's Initiative, directed by the Texas Council on Alzheimer's Disease and Related Disorders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
D.R.R. and R.F.P. have disclosed the results of these analyses to the University of Texas Health Science Center at San Antonio (UTHSCSA), which has filed patent application 2012.039.US1.HSCS and provisional patents 61/603,226 and 61/671,858 relating to the latent variable d's construction and biomarkers.
These data were presented at the 8th Clinical Trials on Alzheimer's Disease (CTAD 2015) Conference, Barcelona Spain, November 5th, 2015.
Texas Alzheimer's Research and Care Consortium List of Investigators: Baylor College of Medicine: Valory Pavlik, PhD, Paul Massman PhD, Eveleen Darby MA/MS, Monica Rodriguear MA, Aisha Khaleeq MD; Texas Tech University Health Sciences Center: John C. DeToledo, MD, Henrick Wilms MD, PhD, Kim Johnson PhD, Victoria Perez, Michelle Hernandez; University of North Texas Health Science Center: Thomas Fairchild PhD, Janice Knebl DO, Sid E. O'Bryant PhD, James R. Hall PhD, Leigh Johnson PhD, Robert C. Barber PhD, Douglas Mains DrPH, Lisa Alvarez, Adriana Gamboa; University of Texas Southwestern Medical Center: Perrie Adams PhD, Munro Cullum PhD, Roger Rosenberg MD, Benjamin Williams MD, PhD, Mary Quiceno MD, Joan Reisch PhD, Linda S. Hynan PhD, Ryan Huebinger PhD, Janet Smith BS, Barb Davis MA, Trung Nguyen MD, PhD; University of Texas Health Science Center – San Antonio: Donald Royall MD, Raymond Palmer PhD, Marsha Polk; Texas A&M University Health Science Center: Alan Stevens PhD, Marcia Ory PhD/MPH; University of Texas at Austin/Dell Medical School: David Paydarfar MD, John Bertelson MD, Martin Woon PhD, Gayle Ayres DO; Alyssa Aguirre LCSW; University of North Carolina: Kirk C. Wilhelmsen MD, PhD, Jeffrey L. Tilson PhD, Scott Chasse, PhD.
D.R.R. designed the study, interpreted the analysis, drafted and revised the article. R.F.P. performed the analysis and revised the article for intellectual content.
References
- 1.Royall D.R., Palmer R.F. Validation of a latent construct for dementia case-finding in Mexican-Americans. J Alzheimer’s Dis. 2013;37:89–97. doi: 10.3233/JAD-130353. [DOI] [PubMed] [Google Scholar]
- 2.Gavett B.E., Vudy V., Jeffrey M., John S.E., Gurnani A., Adams J. The δ latent dementia phenotype in the NACC UDS: cross-validation and extension. Neuropsychology. 2015;29:344–352. doi: 10.1037/neu0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royall D.R., Palmer R.F. Thrombopoietin is associated with δ's intercept, and only in Non-Hispanic Whites. Alzheimer’s Demen Diagn Assess Dis Monit. 2016;3:35–42. doi: 10.1016/j.dadm.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royall D.R., Palmer R.F., Vidoni E.D., Honea R.A., Burns J.M. The Default Mode Network and related right hemisphere structures may be the key substrates of dementia. J Alzheimer’s Dis. 2012;32:467–478. doi: 10.3233/JAD-2012-120424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavett B.E., John S.E., Gurnani A.S., Bussell C.A., Saurman J.L. The role of Alzheimer's and cerebrovascular pathology in mediating the effects of age, race, and apolipoprotein E genotype on dementia severity in pathologically confirmed Alzheimer's disease. J Alzhiemer’s Dis. 2016;49:531–545. doi: 10.3233/JAD-150252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppara A., Wolfsgruber S., Kleineidam L., Schmidtke K., Frölich L., Kurz A. The latent dementia phenotype δ is associated with CSF biomarkers of Alzheimer Disease and predicts conversion to AD dementia in subjects with MCI. J Alzheimer’s Dis. 2016;49:547–560. doi: 10.3233/JAD-150257. [DOI] [PubMed] [Google Scholar]
- 7.Royall D.R., Palmer R.F. Ethnicity moderates dementia's biomarkers. J Alzheimer’s Dis. 2015;43:275–287. doi: 10.3233/JAD-140264. [DOI] [PubMed] [Google Scholar]
- 8.Palmer R.F., Royall D.R. Future dementia status is almost entirely explained by the latent variable δ's intercept and slope. J Alzheimer’s Dis. 2016;49:521–529. doi: 10.3233/JAD-150254. [DOI] [PubMed] [Google Scholar]
- 9.McKhann D., Drockman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 11.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: a preliminary report. J Am Geriatr Soc. 1982;29:164–171. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 12.Maixner S.M., Burke W.J., Roccaforte W.H., Wengel S.P., Potter J.F. A comparison of two depression scales in a geriatric assessment clinic. Am J Geriatr Psychiatry. 1995;3:60–67. doi: 10.1097/00019442-199524310-00008. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell A.J., Bird V., Rizzo M., Meader N. Which version of the Geriatric Depression Scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry. 2010;18:1066–1077. doi: 10.1097/jgp.0b013e3181f60f81. [DOI] [PubMed] [Google Scholar]
- 14.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Benton A., Hamsher K. AJA Associates; Iowa City, Iowa: 1989. Multilingual Aphasia Examination. [Google Scholar]
- 16.Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Memory Scale – Third Edition. [Google Scholar]
- 17.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 18.Grice J.W. Computing and evaluation factor scores. Psychol Methods. 2001;6:430–450. [PubMed] [Google Scholar]
- 19.Arbuckle J.L. SPSS; Chicago: 2006. Analysis of Moment Structures-AMOS (Version 7.0) [Computer Program] [Google Scholar]
- 20.PASW Statistics 18, Release Version 18.0.0. SPSS, Inc.; Chicago, IL: 2009. [Google Scholar]
- 21.Metz C.E. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. Cognitive Impairment. J Intern Med. 1978;256:240–246. [DOI] [PubMed] [Google Scholar]
- 22.Zweig M.H., Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 23.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 24.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 25.John S.E., Gurnani A.S., Bussell C., Saurman J.L., Griffin J.W., Gavett B.E. The effectiveness and unique contribution of neuropsychological tests and the δ latent phenotype in the differential diagnosis of dementia in the uniform data set. Neuropsychology. 2016;30:946–960. doi: 10.1037/neu0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White L.R., Edland S.D., Hemmy L.S., Montine K.S., Zarow C., Sonnen J.A. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86:1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royall D.R., Al-Rubaye S., Bishnoi R., Palmer R.F. Serum protein biomarkers of δ fully mediate multiple AD conversion risks and offer targets for intervention. J Prev Alzheimer’s Dis. 2016;3:283. [Google Scholar]
- 28.Degenhardt E.K., Witte M.M., Case M.G., Yu P., Henley D.B., Hochstetler H.M. Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics. 2016;57:208–216. doi: 10.1016/j.psym.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Witte M.M., Trzepacz P., Case M., Yu P., Hochstetler H., Quinlivan M. Association between clinical measures and florbetapir F18 PET neuroimaging, in mild or moderate Alzheimer's disease dementia. J Neuropsychiatry Clin Neurosciences. 2014;26:214–220. doi: 10.1176/appi.neuropsych.12120402. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dipasquale O., Cercignani M. Network functional connectivity and whole-brain functional connectomics to investigate cognitive decline in neurodegenerative conditions. Funct Neurol. 2016;31:191–203. doi: 10.11138/FNeur/2016.31.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolz R., Heckemann R.A., Aljabar P., Hajnal J.V., Hammers A., Lötjönen J., Alzheimer's Disease Neuroimaging Initiative Measurement of hippocampal atrophy using 4D graph-cut segmentation: application to ADNI. Neuroimage. 2010;52:109–118. doi: 10.1016/j.neuroimage.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association . 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 34.Lindbergh C.A., Dishman R.K., Miller L.S. Functional disability in mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol Rev. 2016;26:129–159. doi: 10.1007/s11065-016-9321-5. [DOI] [PubMed] [Google Scholar]
- 35.Johnson P., Venderwater L., Wilson W., Maruff P., Savage G., Graham P. Genetic algorithm with logistic regression for prediction of progression to Alzheimer's disease. Bioinformatics. 2014;15:S11. doi: 10.1186/1471-2105-15-S16-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozueta A., Rodríguez-Rodríguez E., Vazquez-Higuera J.L., Mateo I., Sánchez-Juan P., González-Perez S. Detection of early Alzheimer’s disease in MCI patients by combination of MMSE and an episodic memory test. BMC Neurol. 2011;11:78. doi: 10.1186/1471-2377-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange C., Suppa P., Frings L., Brenner W., Spies L., Buchert R. Optimization of statistical single subject analysis of brain FDG PET for the prognosis of Mild Cognitive Impairment-to-Alzheimer’s Disease conversion. J Alzheimer’s Dis. 2015;49:945–959. doi: 10.3233/JAD-150814. [DOI] [PubMed] [Google Scholar]
- 38.Collij L.E., Heeman F., Kuijer J.P., Ossenkoppele R., Benedictus M.R., Möller C. Application of machine learning to arterial spin labeling in mild cognitive impairment and Alzheimer disease. Radiology. 2016;281:865–875. doi: 10.1148/radiol.2016152703. [DOI] [PubMed] [Google Scholar]
- 39.Ye J., Farnum M., Yang E., Verbeeck R., Lobanov V., Raghavan N., Alzheimer’s Disease Neuroimaging Initiative Sparse learning and stability selection for predicting MCI to AD conversion using baseline ADNI data. BMC Neurol. 2012;12:46. doi: 10.1186/1471-2377-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ewers M., Brendel M., RIzk-Jackson A., Rominger A., Bartenstein P., Schuff N., Alzheimer’s Disease Neuroimaging Initiative Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. Neuroimage: Clin. 2013;4:45–52. doi: 10.1016/j.nicl.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royall D.R., Palmer R.F. Aging is a weak but relentless determinant of dementia severity. Oncotarget. 2016;7:13307–13318. doi: 10.18632/oncotarget.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Bryant S.E., Johnson L., Edwards M., Soares H., Devous M.D., Ross S., Texas Alzheimer’s Research & Care Consortium The link between C-reactive protein and Alzheimer's disease among Mexican Americans. J Alzheimer’s Dis. 2013;34:701–706. doi: 10.3233/JAD-122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Bryant S.E., Johnson L., Reisch J., Edwards M., Hall J., Barber R. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimer’s Demen. 2013;9:622–631. doi: 10.1016/j.jalz.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Bryant S.E., Xiao G., Edwards M., Devous M., Barber R., Simpkins J., for the Texas Alzheimer’s Research and Care Consortium Biomarkers of Alzheimer's disease among Mexican Americans. J Alzheimer’s Dis. 2013;34:841–849. doi: 10.3233/JAD-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]