Abstract
Objectives
The British Society of Gastroenterology (BSG) Cancer Group designed a survey to determine how we might understand and improve the service for patients at elevated risk of hereditary colorectal cancer (CRC).
Design and Setting
United Kingdom (UK) gastroenterologists, colorectal surgeons, and oncologists were invited by email to complete a 10 point questionnaire. This was cascaded to 1,793 members of the Royal College of Radiologists (RCR), Association of Cancer Physicians (ACP), the Association of Coloproctology of Great Britain and Ireland (ACPGBI), as well as BSG members.
Results
Three hundred and eighty-two members responded to the survey, an overall response rate of 21.3%. Although 69% of respondents felt there was an adequate service for these higher risk patients, 64% believed that another clinician was undertaking this work. There was no apparent formal patient pathway in 52% of centres, and only 33% of centres maintain a registry of these patients. Tumour block testing for Lynch Syndrome is not usual practice. Many appeared to be unaware of the BSG/ACPGBI UK guidelines for the management of these patients.
Conclusions
There is wide variability in local management and in subsequent clinical pathways for hereditary CRC patients. There is a perception that they are being managed by 'another', unspecified clinician. National guidelines are not adhered to. We therefore recommend improved education, well defined pathways and cyclical audit in order to improve care of patients with hereditary CRC risk.
Keywords: Cancer Genetics, Cancer Syndromes, Colorectal Cancer Genes, Hnpcc Syndrome, Genetic Testing
Introduction
Colorectal cancer (CRC) is diagnosed in approximately 35 000 people in the UK each year, with a lifetime risk of approximately 5.5%.1 With over 16 000 deaths annually it is the second most common cause of cancer death. More than 95% of CRCs are adenocarcinomas arising from benign precursor adenomas or other polyps. Endoscopic surveillance with polyp removal is an effective tool for making CRC one of the most preventable cancers. Heritable factors contribute about 35% of all CRC risk,2 so identification of individuals at high genetic risk provides a unique opportunity to prevent CRC and improve survival. The management of inherited CRC and other gastrointestinal diseases therefore plays an increasingly important role in daily clinical activity in UK hospitals.
Highly penetrant Mendelian genetic syndromes account for 5–10% of CRC cases.3 These include well characterised conditions such as familial adenomatous polyposis (FAP) and Lynch syndrome (LS) (also known as hereditary non-polyposis colorectal cancer (HNPCC)). LS accounts for 1–4% of CRCs and is characterised by cancers with DNA microsatellite instability and loss of expression of one of four proteins easily detectable by immunohistochemistry, due to underlying mutations in DNA repair genes MLH1, MSH2, MSH6 or PMS2. If tumour genotype testing with microsatellite instability/immunohistochemistry analysis is suggestive of underlying LS this may determine which families should undergo colonoscopic screening and/or germline genetic testing. The results of the Colorectal Adenoma/Carcinoma Prevention Programme study indicated a significant reduction in cancer incidence in patients with LS taking aspirin.4 In addition there are patients with other familial CRC syndromes, such as familial CRC-type X, where the predisposing genes have yet to be identified, for whom empirical screening based on family history is effective in the prevention of CRC.
Resources are unfortunately not available to perform regular colonoscopic screening effectively on whole populations from an early age. Identifying individuals at high risk due to genetic or environmental factors allows us to undertake screening and other preventative measures. Fortunately there is good evidence that endoscopic surveillance of patients with high-risk genetic predisposition prevents cancer.5 6 The British Society of Gastroenterology (BSG) and Association of Coloproctology of Great Britain and Ireland (ACPGBI) released updated guidelines in 2010 for the management of patients with a family history of CRC.7 Past assessments of UK practice suggested that adherence to guidelines for family history of patients with bowel cancer is highly variable for endoscopic screening and genetic testing of individuals for inherited conditions such as LS.8 9 We therefore designed a survey the aims of which were to understand better what should be done to improve the service for patients with inherited gastrointestinal disease, and also to raise awareness of this issue among clinicians.
Design and setting
A 10-point questionnaire was devised following consultation within the BSG Cancer Group. Approval for the survey was obtained from the BSG, ACPGBI, Royal College of Radiologists and Association of Cancer Physicians prior to its distribution via email to 1793 members of these organisations representing gastroenterologists, colorectal surgeons, and clinical and medical oncologists. The online survey package SurveyMonkey was used to collect and collate responses (http://www.surveymonkey.com/). Respondents remained anonymous, although they were asked for their institution. The 10 questions in this survey were
Are you clear about which patients need to be referred to a clinical genetics service from gastroenterology clinics?
Which member of your colorectal multidisciplinary team has responsibility for managing patients with CRC with an elevated inherited risk?
Do you have a family history of bowel cancer clinic in your hospital?
Is this clinic led by a gastroenterologist, colorectal surgeon, clinical geneticist, nurse specialist or other specialist?
Do you keep a screening registry for patients at higher risk of hereditary CRC?
Do you arrange tumour testing for LS in patients with CRC diagnosed under the age of 50 years?
Do you feel that you have an adequate clinical genetics service for your patients?
What kind of support would you like to help manage patients with inherited gastrointestinal disease?
What is the name of your hospital?
Are you a gastroenterologist, colorectal surgeon, oncologist or other specialist?
The survey was advertised on the websites and via the bulletins of these organisations, and was accessible from March 2012 to April 2012.
Results
Response rate: There were 382 respondents, a response rate of 21.3%. Gastroenterologists were the largest single group, comprising 41.9% of the total (table 1).
Table 1.
Responses to the survey questions 1–7 and 10
| Question | Total | Options | Responses | % |
|---|---|---|---|---|
| 1. Are you clear about which patients need to be referred to a clinical genetics service? | 382 | Yes | 240 | 63 |
| No | 62 | 16 | ||
| Sometimes | 74 | 2 | ||
| Other | 6 | |||
| 2. Which MDT member has responsibility for managing patients with CRC with an elevated inherited risk? | 380 | Gastroenterologist | 85 | 22 |
| Colorectal surgeon | 144 | 38 | ||
| Oncologist | 20 | 5 | ||
| Clinical geneticist | 62 | 16 | ||
| Other | 69 | 18 | ||
| 3. Do you have a family history of bowel cancer clinic? | 380 | Yes | 159 | 42 |
| No | 221 | 58 | ||
| 4. Who leads this clinic? | 156 | Gastroenterologist | 24 | 15 |
| Colorectal surgeon | 28 | 18 | ||
| Nurse specialist | 18 | 12 | ||
| Clinical geneticist | 84 | 54 | ||
| Other | 2 | 1 | ||
| 5. Do you keep a screening registry for high risk patients? | 312 | Yes | 210 | 67 |
| No | 42 | 14 | ||
| Unaware | 60 | 19 | ||
| 6. Do you arrange tumour testing for Lynch syndrome in young patients (aged <50 years)? | 378 | Always | 122 | 32 |
| Usually | 56 | 15 | ||
| Rarely | 52 | 14 | ||
| Never | 72 | 19 | ||
| Unaware | 76 | 20 | ||
| 7. Do you feel that you have an adequate clinical genetics service? | 380 | Yes | 262 | 69 |
| No | 92 | 24 | ||
| Other | 26 | 7 | ||
| 10. Are you a …? | 382 | Gastroenterologist | 163 | 43 |
| Colorectal surgeon | 144 | 38 | ||
| Oncologist | 58 | 15 | ||
| Other | 17 | 4 |
Some questions are paraphrased for presentation reasons, please refer to the ‘design and setting’ section for the full versions.
CRC, colorectal cancer; MDT, multidisciplinary team.
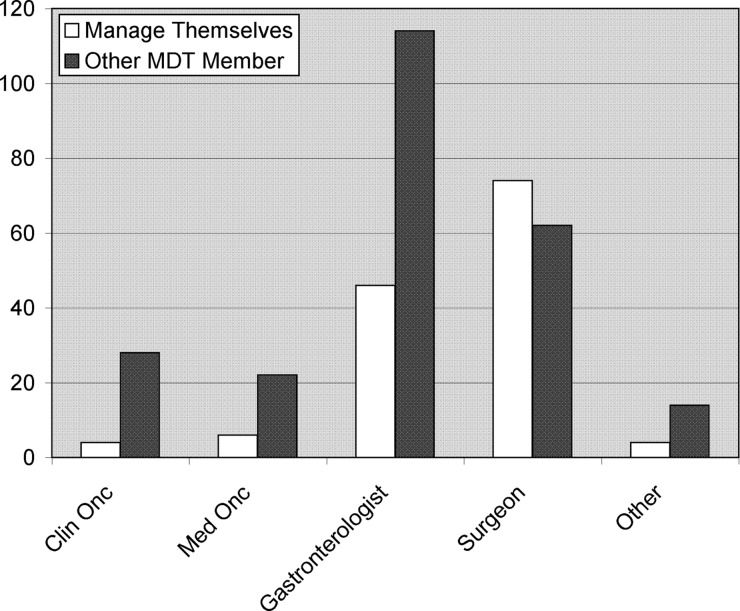
Multidisciplinary care: Most respondents felt confident they knew when to refer their patients to a clinical genetics service. However, there was wide variation in opinion as to who should take responsibility for these patients. When responses were collated (questions 2 and 10; figure 1), over 64% of respondents felt these patients should be managed by another specialist (rather than within their own specialty). This finding was consistent across all respondents other than colorectal surgeons. Of those who entered ‘other specialist’ as a reply, 65% claimed that there was no one specialist with specific responsibility, and the remainder responded that this responsibility was shared between specialists. There was no consensus as to who the nominated specialist should be.
Figure 1.
Respondents’ specialty (white columns, question 10); and opinion about which specialists they feel should manage these patients (black columns; question 2). The majority of respondents felt that someone else should be managing their patients at high risk of hereditary colorectal cancer. Y-axis=Numbers of respondents.
Clinic assessment and clinical pathways: Forty-two per cent of hospitals in the survey have a clinic for the management of these patients, with 6.8% referring to a Clinical Genetics service elsewhere. However 52% of centres apparently do not have a referral pathway for these patients to such a service. There was wide variation regarding which specialist clinic reviewed these patients in practice, with a perception that clinical geneticists are reviewing 54% of these patients. In addition, few centres maintain a register of such patients (33%).
Lynch syndrome: Tumour genotype testing for patients diagnosed with cancer under 50 years of age is recommended in BSG guidelines,7 but was performed ‘usually’ or ‘always’ in under half of the centres surveyed (47%). Twenty per cent of respondents were unaware of this guideline.
Service requirements: Sixty-nine per cent of clinicians felt they had an adequate service for patients with hereditary CRC. However when asked what they would like to augment the service they provide, 41% of all respondents requested ‘clear guidelines’, ‘pathways’ and dedicated support networks (figure 2). Many also appeared to be unaware of the BSG/ACPGBI guidelines for the management of these patients, specifically requesting development of the guidelines which already exist.
Figure 2.
Word cloud derived from responses to question 8: What kind of support would you like to help manage patients with inherited gastrointestinal disease?
Discussion
The concordance rates of cancer in monozygous and dizygous twins suggest that about a third of the variation in cancer risk might typically be ascribed to genetic factors.2 If highly penetrant genetic syndromes account for 10% of CRC cases, approximately 3500 cases annually are due to these heritable factors, with LS alone possibly accounting for up to 500–1000 cases per year.
In view of this, the BSG and ACPGBI devised guidelines for the management of these high risk individuals. For example, all incident cases of CRC diagnosed under 50 years of age should have tumour testing for molecular changes suggestive of LS, and patients with a history suggestive of a highly penetrant genetic syndrome should be referred to a clinical genetics service. Despite BSG guidelines, just under half of respondents (47%) regularly test people under 50 years of age for the molecular features suggestive of LS. Therefore, many patients with potential LS will have an opportunity for improved diagnosis of this condition by increasing awareness among clinicians. Systematic approaches such as universal testing of all patients with CRC has been suggested. Nevertheless there is good evidence that a more selective approach such as testing patients with CRC diagnosed under 50 years of age is cost-effective.10 We propose that UK data on this practice is included in the national bowel cancer audit programme and implemented as a quality standard.
There is considerable evidence, internationally,11 and within the UK,12 13 that adherence to guidelines for screening patients with a familial risk of CRC is highly variable. This can be related to a poor understanding among clinicians of how the risk of CRC varies with history of the disease in the patient's family. One might assume that the consensus among health professionals, which has led to the publication of the BSG guidelines, had improved consistency in screening by clinicians. However, the responses to this survey suggest a poor understanding of the current guidelines and variable pathways for these patients.
There is a perception that these patients are well managed by their local centres, and that 46% of these patients are seen by clinical geneticists. Currently in the UK there are 106 whole-time equivalent consultants according to workforce assessment (http://www.clingensoc.org/information-education/websites-downloads/), and considerable expansion would be required to manage all patients with familial CRC nationally. Although the role of genetic centres is defined by the BSG guidelines in that they see patients with a history suggestive of hereditary syndromes such as LS or FAP, the vast majority of patients with familial risk are not presenting with a known syndrome such as LS or FAP. These are the so-called ‘moderate’ risk population who require empirical risk assessment and screening protocols. It is these patients who most typically present to general gastroenterology and/or colorectal surgical clinics in district general as well as teaching hospitals throughout the UK, rather than tertiary clinical genetics centres. These moderate-risk patients are usually managed locally by the clinicians who will also perform endoscopic surveillance. They account for approximately 100 colonoscopies annually in a district general hospital with a population of 300 000.6 However the possibility of higher risk syndromes such as LS need to be part of a routine active assessment of these patients and this should be provided by a local service.
Previous studies showed wide variation in systematic UK practice.8 9 12–14 In our survey, 52% of centres had no defined pathway leading to an assessment in a hereditary CRC genetics clinic. Two-thirds of centres in this study do not keep a registry or other record of patients with familial cancer. Maintenance of an accurate registry is a highly effective method of ensuring delivery of these services to the UK population, facilitating transparency through auditable data and providing a potential research tool for our population. Importantly, a registry facilitates appropriate endoscopic surveillance and other CRC prevention measures.
The responsibility for the management of these patients is performed by different members of the colorectal multidisciplinary team in different institutions. However, rather than taking personal responsibility, the widespread perception among clinicians is that these patients are managed by somebody else. This may explain the wide variation in care and low adherence to guidelines.
Although this survey may be limited by ascertainment bias, it was designed to capture clinicians’ perspectives and opinions of their local service provision. The results indicate that although the respondents feel these patients are well managed, this is not actually the case. The framework for the patient pathways and adherence to guidelines is poor. We recommend the development of clear structures through national audit, development of quality standards and education of physicians and surgeons in the UK. Only in this way will this ad hoc approach to the management of hereditary CRC be improved.
What is already known on this topic.
Heritable factors contribute about 35% of all colorectal cancer (CRC) risk.
There is good evidence that correct management of patients with an elevated hereditary risk, by screening according to guidelines, is a highly effective method of preventing CRC.
However in some studies there is evidence of an inconsistent approach to the management of those patients, with low risk patients being screened too often, and high risk patients not frequently enough. There is also a low referral rate to genetic services for high risk patients.
What this study adds.
Responses to this national survey suggest a poor understanding of the current guidelines amongst clinicians and variable clinical pathways for patients.
There is also a perception that another unspecified clinician is undertaking this work.
This may explain the wide variation in care and low adherence to guidelines in the United Kingdom (UK).
How might it impact on clinical practice in the foreseeable future .
We recommend the development of clear structures through national audit, development of quality standards and education of physicians and surgeons in the UK.
Each hospital should develop a lead clinician for the delivery of these services.
Only in this way will this ad hoc approach to the management of hereditary CRC be improved.
Acknowledgments
The authors thank the BSG Cancer Group and Dr Sam Pannick for proof reading this report.
Footnotes
Collaborators: BSG Cancer Group.
Contributors: KJM and SKC developed the survey and cowrote this report. KJM collated the responses to the survey.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Office for National Statistics. Cancer Statistics Registrations, England (Series MB1), No. 41, 2010.
- 2.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- 3.Cannon-Albright LA, Skolnick MH, Bishop DT, et al. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl JMed 1988;319:533–7. [DOI] [PubMed] [Google Scholar]
- 4.Burn J, Gerdes AM. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829–34. [DOI] [PubMed] [Google Scholar]
- 6.Dove-Edwin I, Sasieni P, Adams J, et al. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer. BMJ 2005;331:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns DR, Scholefield JH, Steel RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups, British Society of Gastroenterology (May 2010 update from 2002). Gut 2010;59:666–89. [DOI] [PubMed] [Google Scholar]
- 8.Adelson M, Pannick S, Dawson P, et al. The unknown unknowns: UK colorectal cancer patients are inadequately assessed for Lynch syndrome. Frontline Gastroenterol 2014;5:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chivers KC, Basnyat PS, Taffinder N. How compliant do we want to be with the colonoscopy surveillance guidelines? Colorectal Dis 2007;9:830–3. [DOI] [PubMed] [Google Scholar]
- 10.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin OS, Gluck M, Nguyen M, et al. Screening patterns in patients with a family history of colorectal cancer often do not adhere to national guidelines. Dig Dis Sci 2013;58:1841–8. [DOI] [PubMed] [Google Scholar]
- 12.Sergot L, Stevens S, Turkes F, et al. A Dedicated colorectal cancer genetics service improves adherence with molecular testing for Lynch Syndrome. Gut 2013;62(Suppl 1):A200. [Google Scholar]
- 13.John SKP, George S, Primrose JN, et al. Symptoms and signs in patients with colorectal cancer. Colorectal Dis 2011;13:17–25. [DOI] [PubMed] [Google Scholar]
- 14.Clark SK, Carpenter S, Broughton CIM, et al. Surveillance of individuals at intermediate risk of colorectal cancer–the impact of new guidelines. Colorectal Dis 2003;5:582–4. [DOI] [PubMed] [Google Scholar]