Abstract
Pancreatic cancer is the 10th most commonly diagnosed cancer in the UK and the fifth most common cause of cancer death. It remains one of the most aggressive cancers with over 95% of patients affected dying of their disease. Often presenting at an advanced stage of disease progression, there is currently no simple screening test available. Therefore a high clinical suspicion and prompt appropriate investigation is required from physicians when dealing with patients with symptoms in keeping with pancreatic cancer. The gastroenterology 2010 curriculum states that trainees should learn the presentation and multidisciplinary management of patients with pancreatic tumours. In this article we discuss the typical clinical presentations of common and less common pancreatic tumours followed by the investigation, staging and management required.
Keywords: Pancreas, Pancreatic Cancer, Pancreatic Endocrine Tumour, Pancreatic Tumours, Pancreatic Disease
Introduction
The section of the gastroenterology 2010 curriculum corresponding to this article is shown in box 1. Pancreatic ductal adenocarcinoma (PDAC) is the most common malignant tumour arising from the pancreas originating from the pancreatic head in 80–90% of cases. It remains a therapeutic challenge due to its late presentation, aggressive behaviour and lack of effective therapeutic modalities.
Box 1. Gastroenterology 2010 curriculum section f: pancreatic and biliary diseases.
Pancreatic tumours
To learn the presentation and multidisciplinary management of patients with pancreatic tumours
Knows the presentation, investigation and staging of pancreatic cancer
Recognises the importance of considering, and being able to identify, uncommon pancreatic tumours (such as neuroendocrine or intrapapillary mucinous tumours)
Knows the range of potential therapies and recognises the factors that make such tumours potentially operable or inoperable
Knows the prevalence and natural history of benign cysts/serous cystadenoma and potentially malignant cystic lesions
Knows the options for palliative treatment
Shows ability to sequence investigations appropriately
Understands value of MDT. Recognises the importance of considering possibility that the tumour is unusual
Communicates effectively within the MDT and with the patient and their family
Patients presenting with potentially resectable disease are in the minority (10–20%). Overall median survival from PDAC is 6 months, and 5-year survival for all stages combined is low at approximately 5%.1 Over 8000 people a year in the UK are diagnosed with pancreatic cancer, the majority being aged over 60 years. The incidence and mortality of pancreatic cancer have unfortunately remained static over the past two decades; the age-standardised incidence rate in the UK is 9.3 per 100 000 population.
Cigarette smoking is firmly associated with pancreatic cancer and is thought to account for 25–30% of cases.2 Other risk factors for PDAC are shown in box 2.
Box 2. Risk factors and genetic predisposition for PDAC.
Cigarette smoking
Male sex
Diet (high fat and protein, low fruit and vegetable)
Increased body mass index
Chronic pancreatitis
Adult onset diabetes less than 2 years duration
Alcohol excess
Family history of pancreatic cancer
Hereditary pancreatitis (PRSS1 mutations)
BRCA2/PALB2 mutations
Peutz–Jeghers syndrome (STK11 mutation)
Hereditary non-polyposis colorectal carcinoma
Familial adenomatous polyposis
Familial atypical multiple mole melanoma
The majority of PDAC evolve from precursor lesions known as pancreatic intraepithelial neoplasia but can also arise from intraductal papillary mucinous neoplasms (IPMN) or mucinous cystic neoplasms (MCN), which are described in further detail later.
Clinical presentation
Pancreatic cancer is usually clinically silent in the early stages and as a result patients with pancreatic cancer often present late. Jaundice is the commonest presenting symptom and usually implies the presence of a tumour in the head of the pancreas/ampullary region; however, patients with tumours in the body or tail of the pancreas can develop jaundice in the setting of hepatic metastases or hilar lymphadenopathy. Other symptoms of pancreatic cancer are shown in box 3.
Box 3. Clinical presentation of pancreatic cancer.
Jaundice
Abdominal pain
Back pain
Weight loss
Late-onset diabetes mellitus within preceding 2 years
Acute pancreatitis (rare)
Migratory thrombophlebitis
Several studies have demonstrated an association between new-onset diabetes and the development of pancreatic cancer.3 4 Recent onset of diabetes mellitus in adulthood without predisposing factors (such as obesity or family history) or the presence of ketosis or rapid requirement for insulin in adult-onset diabetes should all be considered as possible presentations of pancreatic cancer.5
Investigation
Making the diagnosis
No specific diagnostic blood tests exist for pancreatic cancer, and liver function tests are not always abnormal. CA19-9 should be used with caution in the initial diagnosis as although it has a sensitivity of 79–81% and specificity of 82–90% it has a low positive predictive value of 0.5–0.9% and is not expressed in up to 10% of the population6 Elevated levels of CA19-9 can predict unresectability, survival and disease recurrence.6
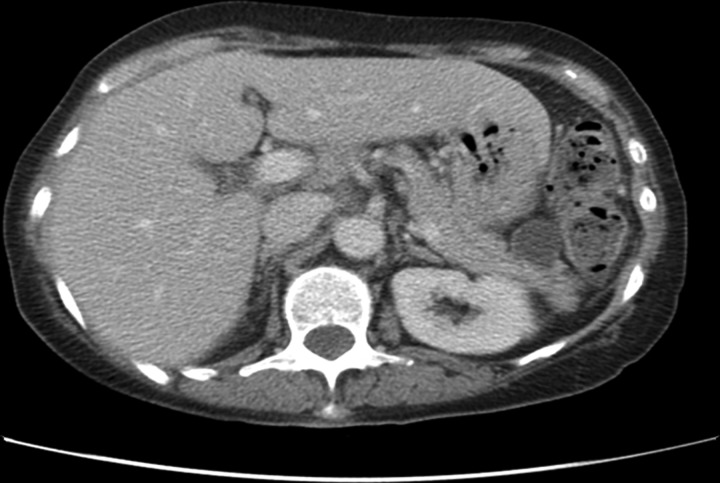
Abdominal ultrasonography is useful in identifying biliary tree dilation in patients presenting with jaundice but has poor sensitivity in evaluation of the pancreas as images are often compromised by bowel gas or body habitus, and for this reason in patients with a clinical suspicion of pancreatic cancer ultrasound should not be used as a first-line investigation as it may in fact delay diagnosis. For patients with suspected pancreatic cancer a triphasic pancreatic protocol CT or MRI scan should be performed.7 8 Figure 1 shows an example of a suspicious mass within the head of the pancreas associated with dilation of the pancreatic duct. In the case of ongoing suspicion of pancreatic cancer with normal imaging then endoscopic ultrasound (EUS) should be performed as EUS has a higher sensitivity for small tumours than CT or MRI scan.9 10
Figure 1.
CT scan showing mass within the head of pancreas (pancreatic ductal adenocarcinoma) with associated dilated pancreatic duct.
The ‘double duct sign’ is simultaneous dilation of the common bile duct and pancreatic duct visible on radiological imaging such as ultrasound, endoscopic retrograde cholangiopancreatography (ERCP), CT or MRI and is most frequently associated with a periampullary malignancy. Less common benign causes include chronic pancreatitis and benign ampullary stenosis. The ‘double duct sign’ is present in 60–80% of patients with pancreatic cancer but the absence of this sign does not exclude the diagnosis.11 12
In cases in which imaging suggests resectable cancer it is not necessary to obtain cytology before surgery;7 8 however, cytology is recommended if there is a suspicion of benign disease, which can mimic pancreatic cancer clinically and radiologically such as autoimmune pancreatitis (AIP)13 (described in further detail below). Obtaining a tissue diagnosis is recommended in all patients to confirm malignancy before chemotherapy. While CT-guided fine needle aspiration (FNA) is possible, cytology samples are ideally obtained via EUS FNA, which has a sensitivity of approximately 80%.14 The yield of cells from endoscopic brushings of the bile duct or pancreatic duct at ERCP is low at approximately 20%,13 although there is ongoing research into molecular techniques to enhance the diagnostic yield of both brush and FNA cytology.
Staging
All patients with a radiologically or clinically suspected pancreatic cancer should be referred for discussion at a specialised pancreatic multidisciplinary team (MDT) meeting. Treatment options, as with most other cancers, are dependent on accurate staging of disease. CT or MRI imaging is the primary means by which the radiological stage and potential resectability of the tumour is determined. High quality pancreatic-protocol CT scans have reduced the need for staging laparoscopy, especially as pancreatic cancer rarely generates peritoneal metastatic deposits in the absence of other criteria of irresectability. EUS is highly accurate for the diagnosis of pancreatic cancers and may provide additional staging information, particularly with regard to venous involvement. Positron emission tomography (PET)-CT is not currently routinely used in the staging of pancreatic cancers but is being assessed in clinical trials. Following radiological review, clinical staging can be used to divide patients into those with resectable disease, borderline resectable disease and unresectable metastatic disease (see tables 1 and 2).7
Table 1.
The American Joint Committee of Cancer tumour–node–metastasis system for pancreatic cancer
| T categories | |
| TX | Main tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| Tis | Carcinoma in situ (includes PanIN 3 classification) |
| T1 | Tumour within pancreas and <2 cm max diameter |
| T2 | Tumour within pancreas and >2 cm max diameter |
| T3 | Tumour spread beyond pancreas but not to coeliac axis or superior mesenteric artery |
| T4 | Tumour invading coeliac axis or superior mesenteric artery |
| N categories | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Regional lymph node metastases |
| M categories | |
| MX | Spread to distant organs cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
PanIN, pancreatic intraepithelial neoplasia.
Table 2.
Staging of pancreatic cancer
| Local or resectable | |
| Stage 0 | Tis, N0, M0 |
| Stage IA | T1, N0, M0 |
| Stage IB | T2, N0, M0 |
| Stage IIA | T3, N0, M0 |
| Stage IIB | T1, N1, M0; T2, N1, M0; T3, N1, M0 |
| Borderline resectable | |
| Stage III | T4, any N, M0 |
| Tumour abutment or ≤180° circumference of SMA or CA, or short segment HA or SMV | |
| Locally advanced, unresectable | |
| Stage III | T4, any N, M0 |
| Tumour encasement >180° circumference of SMA or CA | |
| Metastatic disease | |
| Stage IV | Any T, any N, M1 |
CA, coeliac arteries; HA, hepatic artery; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Management
Management of patients with pancreatic cancer should be coordinated by the MDT, which usually consists of surgeons, oncologists, radiologists, gastroenterologists, pathologists, dieticians and specialist nurses.
Surgery
Approximatley15% of patients diagnosed with PDAC will have localised disease, with cardiovascular fitness sufficient for them to be considered candidates for resection with curative intent.15 For tumours of the pancreatic head a classic pancreatico-duodenoectomy (Whipple procedure) or alternatively a pylorus-preserving pancreatoduodenectomy can be performed. Both procedures are acceptable and have similar survival rates.16 The hypothesis that a pylorus-preserving pancreatoduodenectomy procedure may improve gastric emptying, and post-surgical nutritional status has not been consistently proved in studies. For localised tumours of the pancreatic body or tail a distal resection and splenectomy may be performed. Pancreatic resections should ideally be performed in tertiary referral centres, and there is a clear association between surgical volume and both short and long-term outcomes.17 Patients with borderline resectable disease (stage III, see table 1) should be considered for neoadjuvant chemotherapy.
Stenting the jaundiced patient
Preoperative biliary drainage should be performed for all patients with cholangitis; however, routine preoperative biliary drainage for jaundice is less clear. In a prospective multicentre randomised trial a 35% reduction in the risk of serious complications was demonstrated by avoiding preoperative drainage in patients with serum bilirubin levels of 40–250 µmol/L and absence of ongoing cholangitis.18 The complications were associated with the preoperative drainage procedure itself; there was no significant difference in mortality or the length of hospital stay between patients treated with preoperative drainage and patients undergoing early surgery.
Obstructive jaundice should be corrected before neoadjuvant treatment in patients with borderline resectable disease. A plastic stent or covered metallic stent can be inserted via ERCP in these patients. There has been debate over the use of plastic or metal stents in these circumstances. Plastic stents have been associated with an increase in stent blockage and cholangitis when compared with self-expandable metal stents;19 20 although it has been postulated that metal stents may complicate subsequent surgery this has not been proved in the literature.20 Prospective randomised trial evidence comparing metal and plastic stents for preoperative biliary drainage is lacking. Percutaneous transhepatic cholangiography, while an option in these patients when ERCP fails, carries a risk of tumour seeding.
In jaundiced patients with non-resectable, metastatic disease then either a plastic stent or an expandable metal stent should be inserted at ERCP (or percutaneous transhepatic cholangiography if ERCP is unsuccessful) for symptomatic purposes, and can be combined with brush cytology.
Adjuvant chemotherapy
Several randomised controlled trials, including the European ESPAC-121 and ESPAC-322 trials have shown the benefit of adjuvant chemotherapy, and this is now standard care for all patients following pancreatic resection with curative intent. A baseline CT scan and serum CA19-9 is performed before treatment. Within the UK and Europe gemcitabine is the standard therapeutic agent, although clinical trials involving other agents are under investigation. Adjuvant chemoradiotherapy is used in some centres, particularly in the USA.
Neoadjuvant chemo(radio)therapy
Neoadjuvant therapy for resectable disease is not used in current UK practice; however, its use remains highly topical in the treatment of PDAC. The rationale behind giving neoadjuvant therapy is that the period of neoadjuvant therapy provides a window during which some patients with aggressive disease rapidly progress and are thus spared the significant morbidity associated with non-curative surgery.23 Neoadjuvant therapy treats any micrometastatic disease earlier, and increases the likelihood that patients complete multimodal therapy as a proportion of patients struggle to complete adjuvant therapy due to postoperative morbidity.
Neoadjuvant therapy has been shown to downstage approximately 30% of patients with borderline resectable disease before resection.24 The anatomical criteria for borderline resectable disease are shown in table 2; these tumours are likely to have positive margins if surgery is performed as the initial treatment.25 Following neoadjuvant therapy, patients should be restaged before surgical resection is considered.
Data from the MD Anderson Cancer Centre have demonstrated that patients with resectable disease treated with neoadjuvant therapy whose disease did not progress had a lower incidence of positive resection margins and increased survival when compared with historical data.26 27 Internationally, clinical trials with neoadjuvant therapy are ongoing and the ideal neoadjuvant regimen is yet to be determined.
Palliative chemotherapy
Palliative chemotherapy has been shown to improve median survival for selected patients with adequate performance status. Gemcitabine is the standard agent; however, in patients with good performance status the FOLFIRINOX regimen (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) provides a significant survival advantage over gemcitabine monotherapy (median survival of 11.8 vs 8.8 months; p<0.001), although it does have greater toxicity.28
High stromal and low epithelial expression of secreted protein acidic and rich in cysteine (SPARC) is a poor prognostic biomarker in pancreatic cancer,29 and due to its role as an albumin-binding protein it was selected as a therapeutic target for nab-paclitaxel (Abraxane) to enable ‘stromal depletion’ and, in turn, improve drug delivery. A phase I/II study of gemcitabine plus nab-paclitaxel demonstrated SPARC expression in the stroma, but not in the epithelium, co-segregated with improved survival, demonstrating it to be a possible predictive biomarker for nab-paclitaxel responsiveness.30 This led to the phase III Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) randomised controlled trial comparing gemcitabine versus gemcitabine plus Abraxane. It demonstrated that the addition of Abraxane conferred significant survival benefit over gemcitabine alone in patients with metastatic pancreatic cancer (median overall survival 8.5 vs 6.7 months; p<0.001);31 however, data concerning SPARC as a predictive biomarker are not currently available. Although the relationship between SPARC expression and nab-paclitaxel responsiveness is still evolving in pancreatic cancer, these proof-of-concept results show that it may become a useful biomarker that can potentially better select patients for nab-paclitaxel treatment.
Palliation
Unfortunately, for many patients with PDAC neither surgery nor chemotherapy is appropriate due to the advanced stage of disease, and symptom palliation is paramount. Pain is a common symptom of pancreatic cancer and should be managed with the assistance of palliative care specialists if severe. Analgesia should be prescribed as per the World Health Organization analgesic ladder. In some cases a CT or EUS-guided coeliac plexus nerve block, in which the coeliac ganglia are ablated with ethanol or phenol, can be performed. This can produce effective palliation of pain in about 70% of cases.32 Thorascopic division of splanchnic nerves has also been described as an effective technique. Palliative radiotherapy may be used for pain refractory to coeliac plexus blockade. Patients may develop gastric outlet obstruction and could benefit from duodenal stenting or surgical gastroenterostomy.
Patients should have access to a cancer specialist nurse, palliative care team and patient support groups when available.
Nutrition
Patients with pancreatic cancer develop malnutrition due a combination of factors: pain, nausea, anorexia, pancreatic enzyme insufficiency as a result of pancreatic duct obstruction and gastric outlet obstruction. Cancer-associated metabolic effects also contribute to cachexia and loss of muscle mass. Weight loss and malabsorption are present in 80–90% of patients at the time of diagnosis, and it has been demonstrated that the amount of weight loss is an independent determinant of survival in patients with pancreatic cancer.33 34 Early dietetic involvement alongside pancreatic enzyme supplementation can slow and even prevent weight loss. Pancreatic enzyme supplementation has been demonstrated to improve quality of life and symptom scores in palliative patients.35
Future advances
Screening
Several single and multicentre studies have evaluated the benefit of screening individuals at high risk of pancreatic cancer. Cystic lesions are the most commonly detected lesions in screened individuals. International consensus guidelines36 suggest screening patients with an estimated lifetime risk of over 5%. This included individuals with a significant family history of pancreatic cancer (individuals with two affected blood relatives and at least one first-degree relative) and individuals with genetic mutations associated with pancreatic cancer (BRCA2, PALB2, STK11, PRSS1, p16, STK11, ATM and Lynch syndrome). There was no consensus on what age to start screening. For patients with familial pancreatic cancer the average age at diagnosis is 68 years; however, patients with PRSS1 mutations develop pancreatic cancer at a younger age and screening should probably be started earlier in these patients. EUS and MRI were considered the most accurate tools for screening, with the benefit of not involving ionising radiation. There is limited evidence to support a definite time interval for ongoing surveillance imaging of these individuals, although 12 months has been suggested for those with no pancreatic abnormalities at baseline screening.
Currently within Europe, the European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC) is a collaborative study screening individuals who have been assessed and deemed to be at high risk of pancreatic cancer with a combination of EUS, CA19-9 analysis, CT scanning and ERCP with analysis of pancreatic juice DNA.
Screening does, however, bring with it the risk of overtreatment. To date there is no randomised trial evidence demonstrating that screening for pancreatic cancer reduces mortality.
Targeted therapeutic agents
The identification of cancers with germline mutations of genes involved in the Fanconi DNA repair pathway (BRCA2, PALB2, FANCC, FANCG) has therapeutic implications as these cancers have been shown to be highly sensitive to poly-ADP ribose polymerase inhibitors and alkylating agents such as cisplatin. Trials involving the use of poly-ADP ribose polymerase inhibitors for selected patients are underway.
MicroRNA are short non-coding RNA sequences that regulate the expression of genes involved in cell proliferation, differentiation and apoptosis. Alterations of microRNA expression have been linked to development and progression in various cancers including chronic lymphocytic leukaemia, colorectal and pancreatic cancer. MicroRNA profiling of FNA samples may have clinical future applications in diagnosis, prognosis and predicting chemotherapeutic response. The identification of specific expression signatures may in the future provide further therapeutic targets.
Other therapeutic strategies include targeting the pancreatic stroma surrounding the tumour epithelium, thus improving drug delivery of standard chemotherapeutic agents. Currently under evaluation are inhibitors of the hedgehog pathway, which is thought to enhance the desmoplastic reaction. Other targeted therapies include the inhibitor of the RAS/RAF signalling pathway sorafenib and agents targeting insulin-like growth factor 1. The possibility of endoscopically delivered treatments such as radiofrequency ablation, photodynamic therapy and chemotherapy is also under investigation.
Differential diagnosis of pancreatic lesions
Cystic lesions
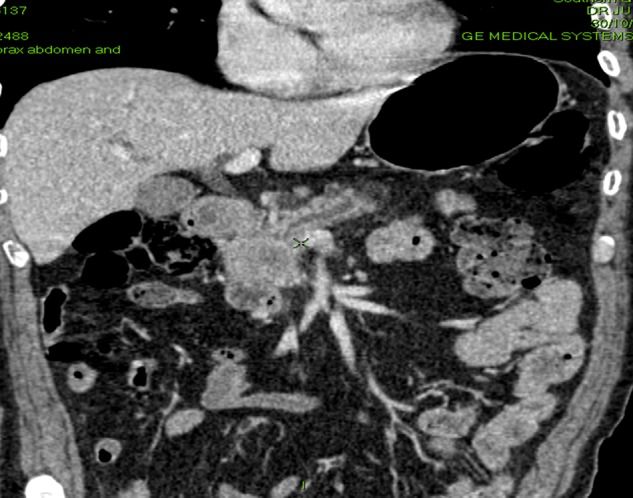
The incidence of pancreatic cystic lesions is increasing in frequency as the widespread use of cross-sectional imaging has led to an increased detection of these usually asymptomatic lesions. Appropriate investigation and follow-up of these lesions is important as some have a significant malignant potential.37 Figure 2 shows an example of a cystic lesion of the pancreas that was picked up incidentally.
Figure 2.
Incidental finding of a cystic lesion arising from tail of pancreas on CT scan.
Pancreatic cysts can be neoplastic or non-neoplastic, with more than 50% being the former even in patients with a previous history of pancreatitis. Non-neoplastic cysts, such as pseudocysts and retention cysts only require treatment if symptomatic and are not discussed in this article. There are four main subtypes of pancreatic cystic neoplasms: IPMN; serous cystadenomas; MCN; and solid pseudopapillary neoplasms, which have varying properties and degrees of malignant potential summarised in box 4.
Box 4. Pancreatic cystic neoplasms.
IPMN
Mucin producing
Arises from pancreatic ductal system (main or side branch)
Men=women
Age over 50 years
Malignant potential
Serous cystadenoma
Often multicystic with central scar
Men=women
Age over 50 years
Abdominal pain due to mass effect
Negligible malignant potential
MCN
Mucin producing
Exclusively women
Young to middle age
Usually a single septated cyst lesion
Malignant potential
Commonly in tail of pancreas
No communication with pancreatic duct
Solid pseudopapillary neoplasms
Mixed solid cystic lesions
Young women
Malignant potential
Imaging and investigation of cystic lesions
Cystic lesions of the pancreas can be evaluated by CT scan, MRI or MRCP and by EUS, which has largely replaced ERCP in this regard. At the time of EUS, mucin may be seen extruding from the papilla in IPMN or MCN. EUS appearances can further evaluate the lesion by detecting communication with the pancreatic duct and detecting the presence of intramural nodules. Cystic fluid can be sampled by FNA; cytology is often limited due to the paucicellular nature of fluid obtained, although it may demonstrate mucin-containing cells. When compared with histological findings of resected cystic lesions cytology has a sensitivity and specificity of 35% and 83%, respectively for detecting mucinous lesions.38 Elevated cyst fluid levels of carcinoembryonic antigen (CEA) are associated with mucinous lesions, although the optimal cut-off varies in studies.
A meta-analysis of pooled data from 12 centres suggested that a CEA level of over 800 ng/mL is 98% specific for a mucinous cysts and less than 5 ng/mL is 98% specific for a serous cystadenoma.39 Cyst amylase levels can help to differentiate between pseudocysts, in which the amylase level is typically in the thousands, and neoplastic cysts. Cyst fluid DNA can also be examined for molecular markers such as the KRAS mutation in an attempt to differentiate mucinous from non-mucinous lesions and to predict malignancy, and this may be useful in combination with CEA levels and cytology.40 41
Clinical management
IPMN, the most frequently occurring of the pancreatic cystic neoplasms, arise from the pancreatic duct and can be further classified as main duct IPMN or side branch duct IPMN. Main duct IPMN have a greater malignant potential and should be resected in all patients with a good performance status. The revised 2012 Sendai consensus guidelines42 were developed to guide the management of suspected side branch duct IPMN or MCN. Surgical resection is recommended if the following high-risk features are present following radiological imaging:
Jaundice in the presence of a pancreatic head cyst.
Enhancing solid components/definite mural nodules within the cyst.
Dilation of the main pancreatic duct greater than 10 mm.
Cysts with ‘worrisome features’ on radiological imaging require further evaluation and consideration of surgery. These features include:
Cyst size greater than 3 cm.
Thickened enhancing cyst walls.
Main pancreatic duct size of 5–9 mm.
Non-enhancing mural nodules.
An abrupt change in calibre of the pancreatic duct with distal pancreatic atropy.
Lymphadenopathy.
Serous cyst adenomas are benign neoplasms and only require surgical resection if symptomatic or rapidly growing. Solid pseudopapillary neoplasms have a low but recognised malignant potential and should be considered for resection in most cases given that the patent is often a young woman.
All patients with pancreatic cystic lesions should be referred to a local pancreatic MDT for further assessment and management.
Autoimmune pancreatitis
AIP is a rare form of pancreatic disease first recognised in Japan in 1995, and is most commonly diagnosed in men over the age of 50 years. It can manifest as a pancreatic mass mimicking pancreatic cancer, mild recurrent acute pancreatitis or biliary and pancreatic duct strictures resembling primary sclerosing cholangitis. Histology shows a lymphoplasmacytic infiltrate with fibrosis often with the presence of IgG4-positive plasma cells. Patients can have disease affecting other organs, commonly the salivary glands, lungs, kidneys and thyroid, which has led to the concept of systemic IgG4 disease.43 A serum IgG4 of 2 or greater times the upper limit of normal is highly suggestive of AIP, but it should be noted that serum IgG4 levels may also be elevated in pancreatic cancer. One series has shown that a serum IgG4 concentration of over 140 mg/dL had a sensitivity of 76% and specificity of 93% for the diagnosis of AIP.44 Radiologically, AIP can appear as diffuse ‘sausage-like’ pancreatic enlargement, pancreatic duct strictures or a focal pancreatic mass mimicking pancreatic cancer. The Mayo Clinic has introduced the HI-SORt criteria for diagnosing AIP: histology, imaging, serology, other organ involvement and response to corticosteroid therapy.45 Treatment is usually with oral corticosteroids for 4–6 weeks, with repeat imaging and serial IgG4 levels, although there are no randomised trial data for the treatment of patients with IgG4 disease. In patients who relapse on reduction or discontinuation of corticosteroids, oral immunosuppression with agents such as azathioprine can be used.
AIP can be classified into two types. In type I other organs are involved in keeping with a diagnosis of IgG4 systemic disease and this type meets the HI-SORt criteria. Type II AIP does not have evidence of IgG4-positive cells on histology and there is no systemic involvement. Type I patients are more likely to relapse off steroids. Type II AIP has been associated with inflammatory bowel disease.
Neuroendocrine tumours
Pancreatic neuroendocrine tumours (NET) are rare solid tumours that account for approximately 5% of primary pancreatic neoplasms,46 and occur most often in the fourth to sixth decades of life. NET produce a variety of peptides such as chromogranin A and pancreatic polypeptide, which can be detected in serum. Chromogranin A levels correlate with disease burden and serve as a biomarker of disease activity. Pancreatic NET may also produce a variety of hormones such as gastrin, insulin, vasoactive intestinal peptide and glucagon, which cause a hormonal clinical syndrome. The majority of pancreatic NET, however, are non-functioning (not associated with a hormonal syndrome).47
Pancreatic NET are usually sporadic but can occur as part of multiple endocrine neoplasia type 1, von Hippel Lindau syndrome, neurofibromatosis type 1 and tuberous sclerosis. Functioning NET present with symptoms associated with their excreted hormone. Non-functioning NET may be picked up incidentally on imaging or present with clinical symptoms due to local compression or metastatic disease such as abdominal pain, weight loss, jaundice or a palpable mass.
NET are highly vascular and show early enhancement during the arterial phase and washout during the portal venous imaging phase of a CT scan. On MRI images pancreatic NET have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Most NET express high levels of somatostatin receptors and can be imaged using somatostatin-receptor scintigraphy (OctreoScan), which can identify both primary tumour and metastases. EUS has a higher sensitivity than CT or MRI for detecting pancreatic NET,48 which appear as hypoechoic, well circumscribed hypervascular tumours. EUS has the added advantage of allowing FNA of the lesion. Histologically well-differentiated tumours with a lower Ki-67 proliferation index have a lower risk of disease progression and a better prognosis than those with a high Ki-67 proliferation index and poor differentiation.48
Surgery is used for resectable tumours and also debulking in certain circumstances. Pancreatic NET tend to be chemosensitive and there are newer agents, such as the tyrosine kinase inhibitor sunitinib, which have been shown to improve progression-free survival in patients with functional or non-functional well-differentiated pancreatic NET. Somatostatin analogues such as octreotide can be used for symptom control in patients with functional tumours.
Footnotes
Contributors: LAS wrote the manuscript with guidance and contributions from NBJ and CJM.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cancer Research UK. http://www.cancerresearchuk.org/cancer-info/cancerstats (accessed Jul 2013).
- 2.Pancreatic Section of the BSG, Pancreatic Society of GB and Ireland, Association of Upper Gastrointestinal Surgeons of GB and Ireland, Royal College of Pathologists, Special Interest Group for Gastro-intestinal Radiology. Guidelines for the management of patients with pancreatic cancer, periampullary and ampullary carcinomas. Gut 2005;54:v1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA 1995;273:1605–160. [PubMed] [Google Scholar]
- 5.Lee JH, Kim SA, Park HY, et al. New-onset diabetes patients need pancreatic cancer screening? J Clin Gastroenterol 2012;46:e58–61. [DOI] [PubMed] [Google Scholar]
- 6.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 2012;3:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Pancreatic adenocarcinoma NCCN guidelines version 2.2012. http://www.nccn.org (accessed Oct 2012).
- 8.Cascinu S, Jelic S, on behalf of the ESMO Guidelines Working Group. Pancreatic cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:iv37–40. [DOI] [PubMed] [Google Scholar]
- 9.Muller MF, Meyenberg C, Bertschinger Pet al. Pancreatic tumors: evaluation with endoscopic US, CT and MR imaging. Radiology 1994;190:745–51. [DOI] [PubMed] [Google Scholar]
- 10.Deerenberg EB, Poley JW, Hermans JJ. Role for endoscopic ultrasonography in patients suspected of pancreatic cancer with negative helical MDCT scan. Dig Surg 2011;28:398–403. [DOI] [PubMed] [Google Scholar]
- 11.Fulcher AS, Turner MA. MR pancreatography: a useful tool for evaluating pancreatic disorders. RadioGraphics 1999;19:5–24. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim MJ, Chung JJ, et al. Differential diagnosis of periampullary carcinomas at MR imaging. RadioGraphics 2002;22:1335–52. doi:10.1148/rg.226025060 [DOI] [PubMed] [Google Scholar]
- 13.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 2002;97:1386–91. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049–57. [DOI] [PubMed] [Google Scholar]
- 16.Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg 1999;86:603–7. [DOI] [PubMed] [Google Scholar]
- 17.Bilmoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg 2008;207:510–19. [DOI] [PubMed] [Google Scholar]
- 18.Van der Gaag NA, Rauws EAJ, van Eijck CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129–37. [DOI] [PubMed] [Google Scholar]
- 19.Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992;340:1488–92. [DOI] [PubMed] [Google Scholar]
- 20.Mullen JT, Lee JH, Gomez HF, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg 2005;9:1094–104. [DOI] [PubMed] [Google Scholar]
- 21.Neoptolemos JP, Stocken DD, Friess H, et al. A randomised trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Eng J Med 2004;350:1200–10. [DOI] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection; a randomised controlled trial. JAMA 2010;304:1073–81. [DOI] [PubMed] [Google Scholar]
- 23.Abbott DE, Tzeng CW, Merkow RP, et al. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol Feb 10 2013. PMID 23397153 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Gillen S, Schuster T, Buschenfelde CMZ, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management and role of preoperative therapy. Ann Surg Oncol 2006;13:1035–46. [DOI] [PubMed] [Google Scholar]
- 26.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496–502. [DOI] [PubMed] [Google Scholar]
- 27.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survivial in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:268–77. [DOI] [PubMed] [Google Scholar]
- 28.Conroy T, Desseigne F, Ychou M, et al. Groupe tumeurs digestives of unicancer; PRODIGE intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 29.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol 2007;25:319–25. [DOI] [PubMed] [Google Scholar]
- 30.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Hoff DD, Ervin TJ, Arena FP, et al. Results of a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas with PET and CA19-9 correlates. J Clin Oncol (meeting abstracts) 2013;13(15):suppl ab4005.
- 32.Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: influence on pain relief. Anesthesiology 2000;92:347–54. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann J, Büchler MW, Friess H, et al. Cachexia in patients with chronic pancreatitis and pancreatic cancer: impact on survival and outcome. Nutr Cancer 2013;65:827–33. [DOI] [PubMed] [Google Scholar]
- 34.Davidson W, Ash S, Capra S, et al. ; Cancer Cachexia Study Group. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr 2004;23:239–47. [DOI] [PubMed] [Google Scholar]
- 35.Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cnacer of the pancreatic head region. Gut 1998;42:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canto MI, Harinck F, Hruban RH, et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2012;62:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49. [DOI] [PubMed] [Google Scholar]
- 38.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the copperative pancreatic cyst study. Gastroenterology 2004;126:1330. [DOI] [PubMed] [Google Scholar]
- 39.Van der Waaji LA, van Dulleman HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc 2005;62:383–9. [DOI] [PubMed] [Google Scholar]
- 40.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009;69:1095–102. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Brugge WR, Dimaio CJ, et al. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer 2009;117:217–27. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97. [DOI] [PubMed] [Google Scholar]
- 43.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982–4. [DOI] [PubMed] [Google Scholar]
- 44.Ghazale A, Suresh TC, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007;102:1646–53. [DOI] [PubMed] [Google Scholar]
- 45.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4:1010–6. [DOI] [PubMed] [Google Scholar]
- 46.Ramage JK, Davies AHG, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005;54:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine Tumors. Version 1. 2013. http://www.ncn.org/professional/physician_gls/pdf/neuroendocrine.pdf (accessed Aug 2013).
- 48.Pelosi G, Bresaola E, Bogina G, et al. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol 1996;27:1124–34. [DOI] [PubMed] [Google Scholar]