Abstract
Background
Ulcerative colitis is a lifelong, chronic, relapsing-remitting disease.
Objective
To assess the relationship between ulcerative colitis disease status and patient quality of life, and to determine the impact of ulcerative colitis on healthcare costs and work productivity, in the UK.
Methods
Clinicians assessed 173 adult patients’ current disease status at a single study visit using the partial Mayo (pMayo) instrument. Patients completed the Euro Quality of Life 5-dimension, 5-level (EQ-5D-5L) questionnaire, the Work Productivity and Activity Impairment (WPAI) questionnaire. Healthcare resource use was determined from questionnaires and from patients’ medical charts.
Results
Patients in remission had a significantly higher EQ-5D-5L scores (mean (SD) 0.86 (0.15)) than patients with active disease (0.71 (0.20); p<0.001). Patients with mild disease had significantly higher mean (SD) EQ-5D-5L scores than patients with moderate/severe disease: 0.77 (0.11) and 0.66 (0.24), respectively (p<0.001). The mean percent productivity impairment was greater for patients with active disease than for patients in remission on all items of the WPAI questionnaire: 24.6% vs 1.8% for work time missed, 34.1% vs 12.9% for impairment while working, 40.8% vs 14.4% for overall work impairment and 42.7% vs 13.0% for activity impairment (p<0.001 for all comparisons). The mean (SD) total cost of healthcare for ulcerative colitis in the prior 3 months was £1211 (1588).
Conclusions
When compared with patients in remission, patients with active ulcerative colitis have significantly worse quality of life and significantly more work impairment. The healthcare costs of ulcerative colitis are considerable.
Keywords: ULCERATIVE COLITIS, HEALTH SERVICE RESEARCH, QUALITY OF LIFE
Introduction
Ulcerative colitis is a lifelong, chronic, relapsing-remitting disease whose primary symptoms are frequent bloody diarrhoea, rectal urgency and tenesmus.1 2 Ulcerative colitis typically requires prolonged drug treatment to minimise disease activity during relapses and to maintain symptom remission.3 In 20–30% of patients, colectomy is required.4 The most recently reported value for the prevalence of ulcerative colitis in the UK is 243 per 100 000 people.5
The severity of symptoms and the uncertainty about when a relapse may occur can significantly decrease the health-related quality of life (HRQOL) of patients with ulcerative colitis.6 Observational studies have shown that disease activity correlates with lower HRQOL7 and higher costs.8 There is, however, little information about the relationship between disease severity and patient HRQOL and about the healthcare costs of ulcerative colitis in the UK.9 10 Such information would be of value to bodies producing national guidelines for the treatment of ulcerative colitis based on clinical outcomes, quality of life and cost-effectiveness, such as the National Institute for Health and Clinical Excellence.
This study was designed to examine the relationship between disease activity, HRQOL and work productivity impairment in patients with ulcerative colitis in the UK. In addition, we also assessed the healthcare resource use and healthcare costs associated with ulcerative colitis.
Methods
Study design and data source
We conducted a cross-sectional observational study between October 2011 and March 2012 at nine sites throughout the UK (St Mark's Hospital-London, Glasgow Royal Infirmary, Royal Hampshire County Hospital, Winchester; Gloucester Royal Hospital, Bristol Royal Infirmary, University College Hospital, London; Western General Hospital, Edinburgh; Hull Royal Infirmary and Derriford Hospital, Plymouth). Ulcerative colitis patients with all levels of disease severity were recruited during routine outpatient visits to a gastroenterologist. Each study site defined minimum and maximum enrolment thresholds via purposive sampling. The total enrolment of patients in remission was capped at 100 to ensure sufficient representation of patients with active disease. Cross-sectional data were obtained via clinician assessments and patient questionnaires during a single study visit and retrospective data by contemporaneous review of medical charts. Institutional Review Board approval was received at each of the sites.
Study population
All men or women ≥18 years of age with a confirmed diagnosis of ulcerative colitis from a gastroenterologist were eligible for the study. Patients were excluded if they had undergone a colectomy, had participated in any clinical trial within the past 12 months or, in the opinion of the recruiting physician, had a current acute or chronic comorbidity or life event (unrelated to ulcerative colitis) that was likely to impact their quality of life.
Study outcomes and definition of variables
Clinicians evaluated patients’ current disease activity and severity using the three-item partial Mayo (pMayo) score (range 0–9), which combines assessments of stool frequency and rectal bleeding with the physician's rating.11 Disease activity was categorised as remission (pMayo score 0–2) or active disease (pMayo score ≥3). Within active disease, disease severity subgroups were assigned as follows: mild disease (pMayo score 3–4) or moderate/severe disease (pMayo score ≥5).
Patient questionnaires provided demographic information, disease characteristics and HRQOL data. HRQOL was measured by the Euro Quality of Life 5-dimension, 5-level (EQ-5D-5L) utility instrument12 and the Inflammatory Bowel Disease Questionnaire (IBDQ).13 The EQ-5D-5L rates patient HRQOL on the day of administration in 5D: mobility, self-care, usual-activities, pain/discomfort and anxiety/depression. Index scores ranging from 0 to 1 (with a higher score indicating better quality of life) were calculated using the EQ-5D-5L Crosswalk Index Value Calculator provided by the EuroQol Group.14 The IBDQ measured HRQOL over the previous week and consists of 32 items divided into 4 subdomains: digestive symptoms, systemic symptoms, emotional function and social function. Composite scores range from 32 to 224.
Work impairment over the past 7 days was assessed using the Work Productivity and Activity Impairment (WPAI) questionnaire.15 The WPAI comprises six questions measuring the amounts of absenteeism (work time lost) and presenteeism (lost on-the-job productivity), as well as impairment in daily activities attributable to ulcerative colitis. Scores were calculated according to the WPAI manual.16 The scores were multiplied by 100 to produce percentages of work time missed, impairment while working, overall work impairment (a composite of the previous two) and impairment in regular daily activities.
Data on use of healthcare resources were derived from both patient questionnaires and the retrospective medical chart review. Patient questionnaires provided the number of general practitioner visits, allied health visits (dieticians, occupational therapists, naturopaths or osteopaths, and psychologists/counsellors) and offsite (ie, at a hospital other than where the questionnaire was administered) emergency department (ED) visits without hospital admission in the prior 12 months, and a listing of current over-the-counter (OTC) medications. The medical chart review determined ulcerative colitis-related healthcare resource use in the form of consultations with gastroenterologists or other specialists (other than the study visit), nursing care, ‘other’ tests (ie, blood tests, radiology) and onsite (ie, at the hospital where the questionnaire was administered) ED visits in the prior 3 months. Single-day and multiple-day hospitalisations were extracted over the prior 12 months. Current prescription medication use was extracted for 5-aminosalicylic acids, immunomodulators, corticosteroids, antitumor necrosis factor agents and symptomatic therapy (stool softeners, antidiarrheals, calcium/vitamin D supplements). The pooled number of ED visits over the prior 12 months was calculated by adding the patient-reported number of offsite visits to the chart-derived number of onsite visits, after conversion to the 12-month time period by multiplying by 4.
Resource use recorded in the medical chart was used to determine the costs associated with ulcerative colitis. Costs in the prior 3 months were calculated by multiplying the price of each resource by the frequency of its use. Costs for single-day and multiple-day hospitalisations were converted to the 3-month time period by dividing the 12-month cost by 4. Unit resource costs were derived from the United Kingdom's National Schedule of Reference Costs 2010–2011 for National Health Service Trusts,17 and from Unit Costs of Health and Social 2011, compiled by the Personal Social Services Research Unit.18 Prescription drug costs were derived from the British National Formulary, March 2012 issue.19 OTC medication costs were derived from online pharmacy prices to consumers and were available for a limited number of products. The 3-month cost of current prescription medication use was estimated by extrapolating the reported dose and frequency to 90 days.
Statistical analysis
All statistical analyses were carried out using SAS 9.2. Descriptive statistics were used to characterise patient demographics and disease characteristics, as well as healthcare use and cost. Continuous variables were reported as means and SDs or medians and IQRs, and categorical variables as numbers and percentages. Missing data were not imputed. Statistical comparisons were made using χ2 tests and t tests as appropriate. EQ-5D-5L scores were compared across disease activity and disease severity subgroups using Kruskal–Wallis tests for non-normally distributed data. A p value of ≤0.05 was considered statistically significant.
Results
Patient demographics
The median age of the 173 ulcerative colitis patients eligible for this study was 47 years (table 1). Most (90.8%) were born in the UK, 55.5% were female and a majority were employed either full time (42.8%) or part time (13.3%; table 1). There were no differences in demographic characteristics by disease activity or disease severity.
Table 1.
Patient demographics by disease status*
| All patients (N=173) | Remission (N=100) | Active disease† (N=73) | p Value‡ | Mild disease (N=31) | Moderate/severe disease (N=42) | p Value§ | |
|---|---|---|---|---|---|---|---|
| Age (median years, IQR) | 47 (29) | 47.5 (29) | 44 (30) | 0.479 | 48 (29) | 40.5 (30) | 0.749 |
| Female | 96 (55.5) | 55 (55.0) | 41 (56.2) | 0.937 | 16 (51.6) | 25 (59.5) | 0.879 |
| Country of birth | 0.564 | 0.296 | |||||
| UK | 157 (90.8) | 91 (91.0) | 66 (90.4) | 26 (83.9) | 40 (95.2) | ||
| Other | 14 (8.1) | 7 (7.0) | 7 (9.6) | 5 (16.1) | 2 (4.8) | ||
| Current work status | 0.593 | 0.436 | |||||
| Full time | 74 (42.8) | 43 (43.0) | 31 (42.5) | 15 (48.4) | 16 (38.1) | ||
| Part time | 23 (13.3) | 11 (11.0) | 12 (16.4) | 3 (9.7) | 9 (21.4) | ||
| Unemployed¶ | 11 (5.8) | 6 (6.0) | 5 (6.8) | 3 (9.7) | 2 (4.8) | ||
| Working in the home/home duties | 5 (2.9) | 2 (2.0) | 3 (4.1) | 0 (0.0) | 3 (7.1) | ||
| Retired | 42 (24.3) | 24 (24.0) | 18 (24.7) | 8 (25.8) | 10 (23.8) | ||
| Student | 10 (5.8) | 7 (7.0) | 3 (4.1) | 2 (6.5) | 1 (2.4) | ||
| Other | 6 (3.5) | 5 (5.0) | 1 (1.4) | 0 (0.0) | 1 (2.4) | ||
| Adjusted household income (median £, IQR) | 17 640 (20 160) | 17 640 (17 640) | 17 640 (19 425) | 0.918 | 12 600 (23 520) | 18 270 (13 230) | 0.937 |
*Values are presented as N (%) unless otherwise indicated. Percentages may not sum to 100% because of missing data (n=1 for gender, n=2 for country of birth, n=2 for current work status).
†‘Active disease’ is mild, moderate and/or severe UC.
‡p Values for the comparison of remission and active disease.
§p Values for the comparison of mild and moderate/severe disease.
¶Unemployed, seeking work or unable to seek work due to UC.
UC, ulcerative colitis.
The median pMayo score for all patients was 2, and 57.8% of patients were in remission (table 2). The rest had disease activity that was either mild (17.9%) or moderate/severe (24.3%). The median time since diagnosis was 5 years. Most patients had had three or fewer acute exacerbations in the previous 12 months (25.4% had 2–3, 38.7% had 0–1; table 2). Significant differences in the number of acute exacerbations and level of health were observed between disease status groups (table 2). A majority of patients (84.4%) had a current prescription for a medication for ulcerative colitis (not shown)—primarily 5-aminosalicylates (67.1%), immunomodulators (37.0%) and corticosteroids (20.8%)—and a few used OTC medications (12.1%).
Table 2.
Disease characteristics by disease status*
| All patients (N=173) | Remission (N=97) | Active disease† (N=73) | p Value‡ | Mild disease (N=31) | Moderate/ severe disease (N=42) | p Value§ | |
|---|---|---|---|---|---|---|---|
| Partial Mayo score (median, IQR) | 2 (4) | 0 (1) | 5 (3) | <0.001 | 3 (1) | 7 (1) | <0.001 |
| Time since UC diagnosis (median years, IQR) | 5 (13) | 5 (13) | 7 (15) | 0.944 | 6 (11) | 8 (16) | 0.057 |
| Pension/benefit | 0.497 | 0.763 | |||||
| Yes | 12 (6.9) | 8 (8.2) | 4 (5.5) | 2 (6.5) | 2 (4.8) | ||
| No | 159 (91.9) | 90 (91.8) | 69 (94.5) | 29 (93.5) | 40 (95.2) | ||
| Level of health | 0.006 | <0.001 | |||||
| Excellent | 11 (6.4) | 8 (8.2) | 3 (4.1) | 1 93.2) | 2 (4.8) | ||
| Very good | 32 (18.5) | 22 (22.5) | 10 (13.7) | 2 (6.5) | 8 (19.1) | ||
| Good | 56 (32.4) | 39 (39.8) | 17 (23.3) | 12 (38.7) | 5 (11.9) | ||
| Fair | 42 (24.3) | 17 (17.4) | 25 (34.3) | 13 (41.9) | 12 (28.6) | ||
| Poor | 23 (13.3) | 11 (11.2) | 12 (16.4) | 3 (9.7) | 9 (21.4) | ||
| Very poor | 4 (2.3) | 0 (0) | 4 (5.5) | 0 (0) | 4 (9.5) | ||
| Extremely poor | 3 (1.7) | 1 (1.0) | 2 (2.8) | 0 (0) | 2 (4.8) | ||
| Number of acute exacerbations in the prior12 months | <0.001 | <0.001 | |||||
| All the time | 21 (12.1) | 3 (3.1) | 18 (24.7) | 3 (9.7) | 15 (35.7) | ||
| >6 | 17 (9.8) | 7 (7.2) | 10 (13.7) | 5 (16.1) | 5 (11.9) | ||
| 4–6 | 21 (12.1) | 10 (10.3) | 11 (15.1) | 5 (16.1) | 6 (14.3) | ||
| 2–3 | 44 (25.4) | 22 (22.7) | 22 (30.1) | 14 (45.2) | 8 (19.1) | ||
| 0–1 | 67 (38.7) | 55 (56.7) | 12 (16.4) | 4 (12.9) | 8 (19.1) |
*Values are presented as N (%) unless otherwise indicated. Percentages may not sum to 100% because of missing data (n=2 for pension/benefit, n=2 for level of health, n=3 for number of acute exacerbations).
†‘Active disease’ is mild, moderate, and/or severe UC.
‡p Values for the comparison of remission and active disease.
§p Values for the comparison of mild and moderate/severe disease.
IBDQ, inflammatory bowel disease questionnaire; UC, ulcerative colitis.
Relationship between disease severity and quality of life and work impairment
The mean EQ-5D-5L score was significantly higher in patients in remission (0.86) versus those with active disease (0.71; p<0.001; table 3). A significant difference was also observed in the mean EQ-5D-5L scores of patients with mild versus moderate/severe disease (0.77 vs 0.66; p<0.001). IBDQ scores for patients in remission (180.42) were significantly higher than patients with active disease (130.36; p < 0.001; table 3). Similarly, statistically significant differences were observed between patients with mild and moderate/severe disease (148.81 vs 116.41; p < 0.001)
Table 3.
EQ-5D-5Land IBDQ scores by disease status
| Remission (N=97) | Active disease* (N=73) | p Value† | Mild disease (N=31) | Moderate/severe disease (N=42) | p Value‡ | |
|---|---|---|---|---|---|---|
| EQ-5D-5L score | <0.001 | <0.001 | ||||
| Mean | 0.86 | 0.71 | 0.77 | 0.66 | ||
| SD | 0.15 | 0.20 | 0.11 | 0.24 | ||
| Median | 0.84 | 0.74 | 0.77 | 0.72 | ||
| IQR | 0.23 | 0.20 | 0.14 | 0.29 | ||
| IBDQ score | <0.001 | <0.001 | ||||
| Mean | 180.42 | 130.36 | 148.81 | 116.41 | ||
| SD | 31.82 | 37.51 | 32.56 | 35.20 | ||
| Median | 187.0 | 127.5 | 156.0 | 116.0 | ||
| IQR | 39.0 | 58.0 | 62.0 | 47.0 |
*‘Active disease’ is mild or moderate/severe UC.
†p Value for comparison of remission and active disease.
‡p Value for comparison of remission and mild and moderate/severe disease.
EQ-5D-5L, Euro Quality of Life 5-dimension, 5-level; IBDQ, Inflammatory Bowel Disease; Questionnaire; UC, ulcerative colitis.
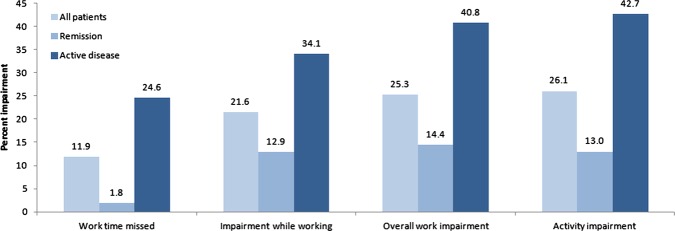
Ulcerative colitis patients reported missing an average of 11.9% of their work time in the prior 7 days and having 21.6% impairment while working (figure 1). Impairment in their general activities was 26.1%. The scores for all WPAI items were higher in patients with active disease compared with those in remission (p<0.001 for all comparisons).
Figure 1.
Mean Work Productivity and Activity Impairment questionnaire scores by disease status. All comparisons between remission (N=100) and active disease (N=73) were significant at p < 0.001.
Healthcare resource use
Many ulcerative colitis patients visited a general practitioner in the 12 months prior to the study visit: 69.5% had one or more visits (see online supplementary figure S1). Few patients (2.9–8.1%) visited dieticians, occupational therapists, osteopaths or psychologists. Most ulcerative colitis patients (88.4%) had visited a gastroenterologist (in addition to the study visit) at least once in the 3 months prior to the study (see online supplementary figure S2). A substantial minority (30.7%) had consulted with an IBD or home nurse regarding their ulcerative colitis. A total of 42.7% of ulcerative colitis patients had one or more single-day hospitalisations in the 12 months prior to the study visit, and 13.3% had one or more multiple-day hospitalisations (see online supplementary figure S3). In that same time period, 8.2% had one or more colitis-related ambulatory visits to an onsite or offsite ED.
Costs associated with ulcerative colitis
The total mean (SD) and median per-patient cost associated with ulcerative colitis over a 3-month period was £1211 (£1588) and 782.61 (see online supplementary table S1). The largest components of this cost were hospitalisations (32.9%), prescription medications (27.7%) and ‘other’ tests (blood tests, radiology; 21.8%).
Discussion
In this population of UK residents with ulcerative colitis, we observed that HRQOL decreased with increasing disease activity and severity. This trend has been reported by many previous studies,7 but few of them used a whole-of-health, preference-based utility instrument such as the EQ-5D-5L to measure HRQOL. Where the EQ-5D was applied, reported scores (or score differentials) were similar to those in the current study. For example, in a cohort of ulcerative colitis patients in Spain, Casellas et al20 reported a median (IQR) EQ-5D score of 1.0 (0.8–1.0) in patients in clinical and endoscopic remission and scores of 0.7 (0.5–0.8) and 0.5 (0.5–0.7) in patients with mild and moderate/severe disease, respectively. In a German population, mean (SD) EQ-5D index scores were 0.96 (0.08) for patients in remission and 0.84 (0.15) for those with active disease.21
The only previous UK-based study to examine the relationship between disease activity and HRQOL used the disease-specific IBDQ to assess quality of life.9 In that cohort of 111 ulcerative colitis patients attending a hospital as an inpatient or outpatient, disease activity was predictive of the total IBDQ score in three different regression models and accounted for 59% of the variation in total IBDQ score. In the current study, we observed mean IBDQ scores of 180.42, 148.81 and 116.41 for patients in remission or with mild or moderate/severe disease, respectively (p<0.001).
We measured work impairment by a series of questions that quantified the degree of productivity loss per patient. This method differs from that used by other European studies of work productivity in ulcerative colitis, which simply report the percentage of patients with work impairment (ie, time missed or lower productivity at work). A single UK study assessed work impairment by surveying ulcerative (and indeterminate) colitis patients receiving secondary care at a university hospital in 2000.10 The authors found that 32% had missed work days in the previous 6 months. In a survey conducted in 2005 by the European Crohn's and Ulcerative Colitis Associations, 27.6% of 2333 ulcerative colitis patients in 7 European countries (including the UK) reported a change in jobs or altered job responsibilities due to their symptoms.22 A majority (65.6%) reported that their symptoms affected their ability to perform job functions.
We found that the average 3-month per-patient cost of ulcerative colitis-related healthcare in the UK in 2010 was £1211. The 6-month cost of ulcerative colitis in 2000–2001 was reported by Bassi et al10 to be £1256,—an amount equivalent to £1476 in 2010 after accounting for inflation18—from which we tentatively infer that UK patients are now accumulating approximately the same costs in half the time. (An alternative conclusion is that the costs are higher because of a potential bias toward active cases in the study design; we note, however, that nearly 60% of the enrolled patients were in remission.) In 2000–2001, direct medical costs were distributed approximately as follows: half for inpatient and surgery costs, one quarter for medications and one quarter for tests and outpatient services.10 According to our results, hospitalisations and prescription medicines continue to dominate the distribution of costs for ulcerative colitis in the UK.
Although this study contributes the most up-to-date information on the relationship between ulcerative colitis disease activity and patients’ HRQOL in the UK, estimates of the disease burden are subject to several limitations. With respect to the source data, tests and treatments entered in the medical charts were formatted as free text, rather than selected from a predefined list, and there was consequently a great deal of variation in how tests and treatments could be interpreted by the analysts. In addition, the partial Mayo score, with its subjective rating of disease activity, and by extension the relationship of disease activity to quality of life, is vulnerable to physician bias. With respect to the design of the study, the study population was geographically representative of England and Scotland, but, due to the location of centres and inclusion criteria it may not be representative of the total ulcerative colitis population in the UK. The implication of this for the cost data is that it does not reflect the total cost in patients requiring surgery. The questionnaires required patients to report on resource use for the previous year and were thus subject to recall bias. Patient-reported healthcare resource use during the previous 12 months was recorded using ranges of visit numbers. As a result, these visits could not be used to determine ulcerative colitis-related costs. Because the relapsing-remitting nature of ulcerative colitis precludes the assumption that disease activity will be constant over a period of months, we could not assess costs and resource use (measured over 3–12 months) in relation to disease activity (assessed on a single day). Finally, the 3-month total costs did not include the cost of offsite ED visits, which made up nearly 75% of the pooled total number of ED visits, and are therefore underestimated.
In conclusion, we found that, compared with patients in remission, patients with active ulcerative colitis had significantly worse quality of life as measured by the EQ-5D-5L and significantly more work impairment as measured by the WPAI. The healthcare costs of ulcerative colitis in the UK in 2011–2012 were considerable, and future studies should investigate the extent to which these costs fluctuate with disease activity.
What is already known on this topic.
Ulcerative colitis is a chronic, debilitating disease typically requiring prolonged drug usage. Intermittent hospitalisation is often required with up to 30% of patients having a colectomy. Disease activity correlates with reduced quality of life using disease specific assessment tools and with an increase in the cost of treatments.
What this study adds.
This UK specific study provides new information on the impact of ulcerative colitis using a whole-of-health rather than a disease specific quality of life instrument. It further provides up to date healthcare costs and work impairment figures for this relapsing-remitting disease.
How might it impact on clinical practice in the foreseeable future.
This information may be of values to healthcare bodies in the production of treatment guidelines.
Supplementary Material
Acknowledgments
The authors thank Lauren Weisenfluh and Melissa Stauffer, PhD, in collaboration with SCRIBCO, for medical writing assistance.
Footnotes
Contributors: Study design and interpretation of data: CJV, PRG, RJN, ARW, TF and CMB. Clinical advisory role: CJV, PRG and TF. Study site supervision: DRG, SS, IS, SL, SB, JNG, AB, IA and SC. Data collection: DRG, SS, IS, SL, SB, JNG, AB, IA and SC. Data analysis: CMB. Revisions of the manuscript for intellectual content: CJV, PRG, RJN, ARW, TF, CMB, DRG, SS, IS, SL, SB, JNG, AM, IA and SC. All authors have approved the final version of the article and the authorship list.
Funding: The study was funded in full by Merck & Co., Inc. Writing support was provided by Lauren Weisenfluh and Melissa Stauffer of SCRIBCO and funded by Merck & Co., Inc.
Competing interests: CMB and TF are employees of Merck & Co., Inc. JNG has served on an advisory board for Merck Sharp & Dohme. DRG and IS have received funds for advisory roles with Merck Sharp & Dohme and for speaking at Merck Sharp & Dohme-sponsored events.
Ethics approval: University College London Hospitals NHS Foundation Trust, Manchester Royal Infirmary, Glasgow Royal Infirmary, Royal Hampshire Hospital, St Marks Hospital, Gloucester Royal Hospital, Bristol Royal Infirmary, Western General Hospital St Marks Hospital, Departmenmt of Surgery, Hull Royal Infirmary, Derriford Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Langan RC, Gotsch PB, Krafczyk MA, et al. Ulcerative colitis: diagnosis and treatment. Am Fam Physician 2007;76:1323–30. [PubMed] [Google Scholar]
- 2.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- 3.Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol 2011;17:3204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biondi A, Zoccali M, Costa S, et al. Surgical treatment of ulcerative colitis in the biologic therapy era. World J Gastroenterol 2012;18:1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone MA, Mayberry JF, Baker R. Prevalence and management of inflammatory bowel disease: a cross-sectional study from central England. Eur J Gastroenterol Hepatol 2003;15: 1275–80. [DOI] [PubMed] [Google Scholar]
- 6.Dudley-Brown S, Baker K. Ulcerative colitis from patients’ viewpoint: a review of two Internet surveys. Gastroenterol Nurs 2012;35:54–63. [DOI] [PubMed] [Google Scholar]
- 7.Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis 2008;14: 554–65. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther 2010;31:693–707. [DOI] [PubMed] [Google Scholar]
- 9.Han SW, McColl E, Barton JR, et al. Predictors of quality of life in ulcerative colitis: the importance of symptoms and illness representations. Inflamm Bowel Dis 2005;11:24–34. [DOI] [PubMed] [Google Scholar]
- 10.Bassi A, Dodd S, Williamson P, et al. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004;53:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JD, Chuai S, Nessel L, et al. Use of the Noninvasive Components of the Mayo Score to Assess Clinical Response in Ulcerative Colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804–10. [PubMed] [Google Scholar]
- 14.EuroQol Group. EQ-5D-5L Index Value Calculator. Rotterdam, The Netherlands, 2012. [Google Scholar]
- 15.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- 16.Margaret Reilly Associates. Work Productivity Activity Impairment (WPAI) Scoring. New York, 2002. [Google Scholar]
- 17.United Kingdom Department of Health. National Schedule of Reference Costs 2010–11 for National Health Service Trusts. London, UK, 2011. [Google Scholar]
- 18.Curtis L. Unit Costs of Health and Social Care 2011. Canterbury, UK: Personal Social Services Research Unit, 2011. [Google Scholar]
- 19.British Medical Association, Royal Pharmaceutical Society. British National Formulary. March 2012 Issue 63 edn. Pharmaceutical Press, 2012. [Google Scholar]
- 20.Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis 2005;11:488–96. [DOI] [PubMed] [Google Scholar]
- 21.Stark RG, Reitmeir P, Leidl R, et al. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis 2010;16:42–51. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis 2007;1:10–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.