Abstract
Objectives
The understanding of changes in comorbidity might improve the management of upper gastrointestinal bleeding (UGIB); such changes might not be detectable in short-term studies. We aimed to study UGIB mortality as adjusted for comorbidity and the trends in risk scores over a 14-year period.
Methods
Patients presenting with UGIB to a single institution, 1996–2010, were assessed. Those with multiple comorbidities were managed in a multi-disciplinary care unit since 2000. Trends with time were assessed using logistic regression, including those for Charlson comorbidity score, the complete Rockall score and 30-day mortality.
Results
2669 patients were included. The Charlson comorbidity score increased significantly with time: the odds of a high (3+) score increasing at a relative rate of 4.4% a year (OR 1.044; p<0.001). The overall 30-day mortality was 4.9% and inpatient mortality was 7.1%; these showed no relationship with time. When adjusted for the increasing comorbidity, the odds of death decreased significantly at a relative rate of 4.5% per year (p=0.038). After the introduction of multi-disciplinary care, the raw mortality OR was 0.680 (p=0.08), and adjusted for comorbidity it was 0.566 (p=0.013).
Conclusions
30-day mortality decreased when adjusted for the rising comorbidity in UGIB; whether this is related to the introduction of multi-disciplinary care needs to be considered.
Keywords: Bleeding, Bleeding Peptic Ulcer, Endoscopy, Epidemiology
Introduction
The past 50 years have proven that upper gastrointestinal bleeding (UGIB) is here to stay and its impact continues to be felt in terms of mortality and cost of care.1 National and international guidelines continue to be released, almost at regular intervals, in an attempt to reduce the incidence and improve the management and outcomes of UGIB, with varying degrees of success.2–5 Recent studies have shown that UGIB incidence, its rate of hospitalisation and related costs have all decreased, with the use of gastro-protective agents being considered as a key factor.6–8 National audits carried out in the UK showed that mortality following UGIB has fallen from 14% in 1993 to 10% in 2007.9 10 Promising as they seem, these observations indicate that fresh thinking is still needed to further minimise such mortality. One strategy is to focus more on coexisting conditions in patients with UGIB, and not to continue to consider the pathogenesis of UGIB as a purely gastrointestinal phenomenon.11 This is because UGIB remains a dynamic event that reflects changes in disease patterns and treatments of conditions that might even originate outside the gastrointestinal system. Examples of these include rheumatological diseases treated with non-steroidal anti-inflammatory drugs and cardiovascular diseases treated with antithrombotic drugs, both of which are associated with high incidence of UGIB.12 13 The stability or otherwise of these and similar extra-intestinal conditions frequently pose significant challenges to both their management and to that of UGIB and may impact on clinical outcomes in such patients.2–5 11 Given the potential impact of coexisting conditions, we aimed to measure UGIB mortality with adjustment for the level of comorbidity over a 14-year period.
Methods
Design
This is a retrospective observational analysis of trends in 30-day mortality adjusted for comorbidity as a primary objective, and of trends in clinical details, risk scores and drug use as secondary objective in subjects presenting with UGIB to University Hospital Crosshouse in southwest Scotland and affiliated to the University of Glasgow, 1996–2010. Another secondary objective is to assess mortality trends following the introduction, in 2000, of multi-disciplinary care for patients with UGIB.
Sources of data
The clinical details of all patients presenting with UGIB to our institution were recorded every third year until 2005, and annually since then. To ensure complete capture of all cases, hospital records of out-patients or inpatients developing variceal or non-variceal UGIB were additionally searched using diagnostic codes that were consistent with the International Classification of Diseases (ICD-10) for bleeding upper gastrointestinal disorders. Our institution has a geographically well-defined catchment area in which the primary care physicians refer all patients with UGIB for assessment, regardless of UGIB risk score.
The work is part of an ongoing programme that assesses the epidemiology, aetiology and outcomes of UGIB.8 13 14
Definitions
Patients with UGIB were included in this analysis if they were adults, 18 years of age or older, and regardless of their Charlson score, used as a measure of their comorbidity.15 The Charlson score has been thoroughly validated by us and others to grade comorbidity in a range of conditions including UGIB.14 15 The conditions covered by Charlson and their weights are shown in table 1. These are entered into the Charlson Calculator in order to obtain the scores.14 15
Table 1.
Charlson's weighted index of comorbidity
| Condition | Assigned weight |
|---|---|
| Myocardial infarction | 1 |
| Congestive heart failure | 1 |
| Peripheral vascular disease | 1 |
| Cerebrovascular disease | 1 |
| Dementia | 1 |
| Chronic pulmonary disease | 1 |
| Connective tissue disease | 1 |
| Ulcer disease | 1 |
| Liver disease mild | 1 |
| Diabetes | 1 |
| Hemiplegia | 2 |
| Renal disease moderate or severe | 2 |
| Diabetes with end organ damage | 2 |
| Any malignancy | 2 |
| Leukaemia | 2 |
| Malignant lymphoma | 2 |
| Liver disease. moderate or severe | 3 |
| Metastatic solid malignancy | 6 |
| AIDS | 6 |
UGIB was defined as previously described and included haematemesis, melaena or both.8 13 14 Haematemesis meant the vomiting of fresh or altered (coffee-ground) blood as confirmed by clinical testing at primary care or in hospital. This definition excluded anaemia without overt UGIB and melaena due to proximal colonic lesions detected by colonoscopy or barium studies. Melaena, confirmed by clinical testing, and frequently associated with a rise in blood urea level, was considered of upper gastrointestinal origin, particularly in the presence of upper gastrointestinal endoscopic abnormalities and in the absence of colonic lesions.8 13 14 The complete Rockall risk scoring system for acute UGIB was also calculated and comprised both clinical and endoscopic findings and stigmata of recent bleeding.9 16 In the UK, the Rockall score is recommended by the National Guidelines for the management of UGIB.3 5 It also uses endoscopic findings to predict mortality and has been validated in many countries.4 14 17
Multi-disciplinary care of UGIB patients, formalised at our unit since 2000, involves the combined assessment and management delivered by nurses experienced in high dependency care, gastroenterology physicians and surgeons, and one or more of other clinicians with special interest in the comorbid conditions of UGIB patients, such as cardiology, endocrinology, nephrology and respiratory medicine. The team is led by the clinician who has specialist knowledge in the management of the most unstable condition at any one phase of the patient's presentation; this does not necessarily need to be the gastroenterologist all the time. Besides observing the national recommendations for the management of UGIB,3 5 this approach involves the following understanding: patients with risk scores equivalent to Charlson and/or Rockall of three or more are triaged into this level of care by the emergency physicians and/or gastroenterologists; patients with variceal bleeding and relatively stable non-variceal bleeding are looked after by the gastroenterology team; the surgical team is involved early in the monitoring of unstable non-variceal bleeding; patients developing UGIB while in a specialist unit for a given condition (such as the coronary care, renal or intensive care units) continue to be cared for by the relevant specialists with the help of gastroenterology input; likewise, patients admitted with UGIB can still be transferred to such specialist units, if needed, while maintaining dual gastroenterology and other relevant specialist care; and, if at all possible, endoscopy should be performed only after stabilising/correcting coexisting conditions with the help of the relevant specialists (such as arrhythmias, electrolyte imbalance, glycaemic state, pyrexia, hypoxia, coagulopathies) even if this means delaying the procedure to beyond the desirable 24-h limit and in the absence of active UGIB.
Verification of data
As mentioned above, patients’ details, including the clinical components of the various scoring systems, were collected and tabulated on a regular basis while protecting patients’ identifiable details. Before being tabulated by members of the research team, the clinical components or measurements, such as pulse rate, blood pressure and routine blood test results, had already been taken and documented by non-research staff caring for the patients. In this analysis, both the Charlson and the complete Rockall scores were recalculated for all patients, reviewed and revised by two investigators (CMcC and TC) who standardised definitions and compilations of the relevant components, thus keeping inter-observer variation to a minimum.
Death as an outcome was documented from hospital or primary care records. The causes of death within 30 days were ascertained, reviewed and verified by a committee of clinicians.
Statistical analyses
Trends with time were assessed using logistic regression analysis with year of presentation as a continuous predictor variable. Regression coefficients were expressed as OR with 95% CI, representing the relative change in odds of death or other binary dependent variables over a time interval of 1 year. Multivariate logistic regression was used to assess independent predictors of mortality and to adjust temporal trends in mortality for comorbidity and other covariates.
In a secondary analysis of the possible effects on mortality of the introduction of multi-disciplinary care of UIGB patients in the year 2000, data from all years before and after that date respectively were pooled and compared using similar methods.
All clinical tests and treatments were in line with standard medical care at our institution and no randomisation or allocation to treatment groups took place, but patients’ identities were concealed by using code numbers. No formal ethical approval was required, and the work was approved and supported by the institutional Clinical Effectiveness and Governance Team of NHS Ayrshire and Arran, Scotland.
Results
Trends in risk factors
Figure 1 demonstrates the trends in risk factors over the study period. By logistic regression analysis, the odds of a high Charlson comorbidity score (≥ 3) increased significantly with time by 4.4% per year (OR 1.044, (95% CI 1.022 to 1.065); p<0.001). There was a weaker increasing trend in the proportion of older patients which failed to reach significance (OR 1.016 (0.997 to 1.036); p=0.09), while trends in Rockall score (p=0.94) and haemoglobin (p=0.47) were wholly non-significant.
Figure 1.
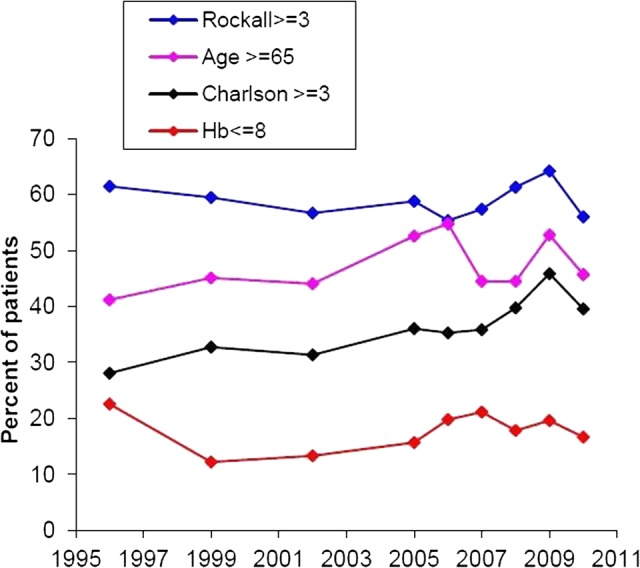
Trends in risk factors over the study period. By logistic regression analysis, the odds of a high Charlson comorbidity score increased significantly with time by 4.4% per year (OR 95% CI 1.044 (1.022 to 1.065); p<0.001). There was a weaker increasing trend in the proportion of older patients which failed to reach significance (1.016 (0.997 to 1.036); p=0.09), while trends in Rockall score (p=0.94) and haemoglobin (p=0.47) were wholly non-significant.
Also, 2.1% of patients required surgical intervention. There was a highly significant decreasing trend with time in the odds of requiring surgery (OR 0.884 (0.834 to 0.937); p<0.001).
Trends in 30-day mortality
As shown in table 2, the overall raw 30-day mortality was 4.9%, and, by logistic regression analysis, there was no significant trend with time (OR 0.977 (0.936 to 1.019); p=0.28). Inpatient mortality was 7.1% and this also showed no trend with time (p=0.99).
Table 2.
Numbers (%) of patients who were alive or dead (30-day mortality) year by year (excluding a total of 31 patients with uncertain survival data)
| Year | Alive | Dead | Total |
|---|---|---|---|
| 1996 | 191 (94.6%) | 11 (5.4%) | 202 (100%) |
| 1999 | 207 (92.4%) | 17 (7.6%) | 224 (100%) |
| 2002 | 244 (96.8%) | 8 (3.2%) | 252 (100%) |
| 2005 | 350 (94.1%) | 22 (5.9%) | 372 (100%) |
| 2006 | 345 (95.8%) | 15 (4.2%) | 360 (100%) |
| 2007 | 348 (96.1%) | 14 (3.9%) | 362 (100%) |
| 2008 | 337 (94.7%) | 19 (5.3%) | 356 (100%) |
| 2009 | 238 (95.2%) | 12 (4.8%) | 250 (100%) |
| 2010 | 249 (95.8%) | 11 (4.2%) | 260 (100%) |
| Total | 2509 (95.1%) | 129 (4.9%) | 2638 (100%) |
The trends in 30-day mortality were tested in patients grouped by Charlson comorbidity score, as illustrated in figure 2. The fitted lines were derived from a logistic regression model incorporating time and comorbidity as non-interacting predictor variables. In this analysis, the odds of death in both comorbidity subgroups, that is, Charlson score < 3 and ≥ 3, decreased significantly with time by 4.5% per year (OR 0.955 (0.914 to 0.997); p=0.038).
Figure 2.
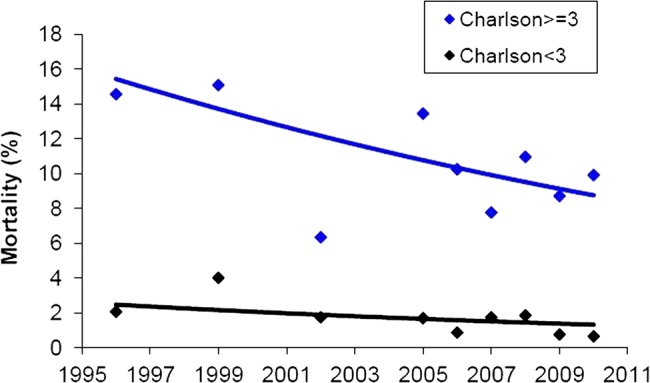
Trends in 30-day mortality in patients grouped by Charlson comorbidity score. The fitted lines are derived from a logistic regression model incorporating time and comorbidity as non-interacting predictor variables. In this analysis, the odds of death in both comorbidity groups decreased significantly with time by 4.5% per year (OR 95% CI 0.955 (0.914 to 0.997); p=0.038).
The preceding analysis assumes temporal changes in mortality occurred continuously at a constant relative rate. To avoid this assumption, we also tested whether mortality changed following the introduction of formal multi-disciplinary care in 2000 by a simple binary comparison of pooled data from before and after that date. In the years 1996 and 1999 combined, raw 30-day mortality was 6.6% (28/426), and over the period 2002–2010 it was 4.6% (101/2212), which is not a significant change (p=0.09, Fisher's exact test). After adjusting for comorbidity in the same manner as above, the reduction in mortality was enhanced and became significant. For raw mortality, the OR was 0.680 ((0.441 to 1.048); p=0.08), and adjusted for comorbidity it was 0.566 ((0.362 to 0.885); p=0.013), where the ORs here represent an overall (post-2000 vs pre-2000) rather than annual change in odds of death.
Over the study period, and in a total of 174 patients, UGIB was considered to be due to oesophageal and/or gastric varices, and portal hypertensive gastropathy complicating alcohol liver disease. The overall mortality in this subgroup of patients was 16.1% and this had no trend with year (p=0.95).
Trends in drug use by patients with UGIB
As shown in table 3, the use of potentially damaging drugs, including both low-dose aspirin and other antithrombotic drugs, has significantly increased, but that of non-steroidal anti-inflammatory drugs has not. Also, more was used of the specific serotonin reuptake inhibitors. At the same time, more patients were using potentially protective drugs, predominantly proton pump inhibitors.
Table 3.
Trends in the prevalence of drug use by patients with upper gastrointestinal bleeding
| Drug | OR (95% CI) for presence by year | p Value |
|---|---|---|
| Aspirin (75–325 mg/day) | 1.025 (1.002 to 1.048) | 0.030 |
| Other antithrombotic drugs* | 1.089 (1.057 to 1.122) | <0.001 |
| Non-steroidal anti-inflammatory drugs | 1.014 (0.985 to 1.043) | 0.35 |
| Selective serotonin reuptake inhibitors | 1.048 (1.009 to 1.088) | 0.015 |
| β-Blockers | 1.086 (1.054 to 1.118) | <0.001 |
| ACE inhibitors | 1.136 (1.100 to 1.173) | <0.001 |
| Diuretics | 0.999 (0.976 to 1.021) | 0.91 |
| Statins | 1.168 (1.131 to 1.207) | <0.001 |
| Proton pump inhibitors | 1.126 (1.102 to 1.152) | <0.001 |
| Histamine-2 receptor antagonists | 0.877 (0.850 to 0.905) | <0.001 |
*Other antithrombotic drugs include clopidogrel, dipyridamole and warfarin.
Causes and predictors of death
While some patients (33 of the 129 total deaths, 26%; or 1.3% of all patients) died primarily of their UGIB, the main causes of death included other systemic conditions including cardiovascular, respiratory and malignant diseases, as shown in table 4. Apart from four patients with peptic ulcer disease, all those who died (N=129) had other serious comorbid conditions that had mainly caused or contributed to the 30-day mortality.
Table 4.
Comorbidity in all patients who died (N=129) following upper gastrointestinal bleeding
| Comorbid cause of death | Bleeding as main cause of death* | Total number of deaths |
|---|---|---|
| Liver disease | 15 (12%) | 33 (26%) |
| Vascular disease | 4 (3%) | 23 (18%) |
| Malignancy | 2 (2%) | 24 (19%) |
| Pneumonia | 1 (1%) | 20 (16%) |
| Chronic lung disease | 3 (2%) | 5 (4%) |
| Peptic ulcer disease | 4 (3%) | 4 (3%) |
| Miscellaneous | 4 (3%) | 20 (16%) |
| Total | 33 (26%) | 129 (100%) |
*Patients still had other comorbid conditions contributing to their death.
In univariate analysis, factors found to significantly predict 30-day mortality included age, Charlson score, Rockall score, units of blood transfused, haemoglobin level, urea level, prothrombin time, and pulse and blood pressure measured at time of initial presentation with UGIB, but a number of these factors were inter-dependent.
In multivariate analysis, the following continuous variables were found to be independent predictors of 30-day mortality: age (OR 1.020 (1.007 to 1.034); p=0.003); Charlson score (1.291 (1.192 to 1.398); p<0.001); complete Rockall score (1.274 (1.149 to 1.413); p<0.001); and units of blood transfused (1.085 (1.049 to 1.123); p<0.001).
Of the drugs used, only diuretics were found to be associated with higher mortality: they were used by 34.1% of 129 patients who died versus 22.5% of 2509 who survived at 30 days (p=0.004; Fisher's exact test), although there was no significant trend in their use over the 14-year period of the study, as shown in table 3.
Discussion
Our analysis has shown that, over a 14-year period, 30-day mortality has decreased when adjusted for the rising comorbidity in patients with UGIB; the possibility of this being related to the introduction of multi-disciplinary care needs to be considered.
Our work is limited by being observational in nature while its strength stems from being long-term and conducted in a single centre with the local availability of patients’ detailed clinical characteristics, results of investigations and outcomes. Our methods were also standardised, the data verified and we believe that our findings might have clinical implications.
The trend analysis showed no significant change in patients’ age, Rockall scores or haemoglobin level. However, the Charlson comorbidity score has been steadily rising: this in turn explains why the overall raw 30-day mortality remained unchanged but when adjustment was made for comorbidity a significant decrease in mortality was demonstrated. It could be argued that the 14-year duration of our work was not long enough to demonstrate a significant rise in the age of patients with UGIB. However, it is widely accepted that clinicians in general are now dealing with an ageing population thanks to the more successful treatment of general medical conditions using drugs that might cause gastrointestinal damage. This in turn would explain the rising Charlson scores in our patients.
The causes of death in our patients illustrate the key relevance of comorbid conditions. These are increasingly present in almost all patients dying following UGIB.10 14 We have also found that the Charlson and Rockall scores were strong independent predictors of death. This emphasises the recommendation that management of UGIB, besides the usual resuscitation and endoscopic therapy, will have to include stabilising and treating coexisting conditions.1–5 It is interesting to find that of the drugs used, the intake of diuretics was associated with higher mortality. This is a marker of the conditions they are usually prescribed for, namely, hypertension and cardiac failure: cardiovascular diseases were important causes of death in our patients with UGIB.
The need for surgery has steadily decreased in our unit and the overall rate (2.1%) is comparable with that reported in the last UK National Audit (1.9%).10 Likewise, the mortality rate of our patients with variceal bleeding (16.1%) is similar to that of the same Audit (15%).10 The main explanation for this might be the fact that patients who survive their bleeding episode still die of their liver disease particularly as active alcohol aetiology excludes such patients from further consideration for transplantation in most, if not all, units in the UK.
The decrease in UGIB mortality, shown in the previous audits,9 10 was attributed to the wider use of therapeutic endoscopy and proton pump inhibitors.1 10 Our complete Rockall scores, which include stigmata of recent bleeding that might require therapeutic endoscopy, have not significantly increased over the study period, while the use of proton pump inhibitors had.8 While accepting the benefits of these agents in UGIB prevention and management, our overall 30-day mortality (4.9%) is still less than that reported in the last national Audit (10%), although the latter assessed mortality per an admission episode which in most patients with UGIB is shorter than 30 days and might detect a lower mortality than that at 30 days.10 These points are further illustrated in another study that covered the hospitals in England, 1999–2007.18 This was a matched case-control study using data bases linking primary and secondary care data. Like our study, it used the Charlson score to measure comorbidity and corrected for potential risk factors. It found that the overall 28-day mortality following non-variceal haemorrhage was reduced from 14.7% to 13.1%, and following variceal haemorrhage it was reduced from 24.6% to 20.9%. Adjustments for age and comorbidity partly accounted for the observed trends in mortality.18 These rates, even after the observed reductions, are still relatively higher than our raw mortality data. Perhaps more striking is our inpatient mortality (7.1%) which is also lower than that of the UK Audit (26%).10 These apparent differences again highlight the possible clinical advantages of our multi-disciplinary care approach to managing patients with UGIB.
While there has been some recognition of the need to take comorbidity into account,1 2–5 little clarity has been provided on how best to implement this principle in the management of UGIB. Over the years, efforts have, instead, focused on immediate resuscitation measures in specialised areas including high dependency units,19 specialist gastroenterology services,20 intensive care units21 22 or, more recently, in dedicated upper-gastrointestinal haemorrhage units.23 The long-term outcomes of care in such facilities are not clear. With the exception of the gastroenterology units, the other initiatives have not been widely followed, and their use has not been endorsed by national guidelines.3 However, given the increasing comorbidity scores, it would seem appropriate that a multi-disciplinary approach is considered in the management of UGIB and this seems to be associated with favourable outcome, as demonstrated in our study.
What is already known on this topic.
Despite some improvement, mortality remains significant in patients with upper gastrointestinal bleeding (UGIB) and fresh thinking is still required.
The understanding of changes in comorbidity might improve the management of UGIB.
Such changes and the impact of response to them might not be detectable in short-term studies.
What this study adds.
The Charlson comorbidity score increased significantly with time, 1996–2010.
Thirty-day mortality decreased when adjusted for the rising comorbidity in UGIB.
The introduction of multi-disciplinary care seems to have helped in reducing mortality.
How might it impact on clinical practice in the foreseeable future.
Besides the gastroenterology skills, a multi-disciplinary approach now needs to be considered in the management of UGIB.
Dedicated UGIB units should be supported with multi-disciplinary expertise.
Footnotes
Contributors: AST planned the study and wrote the draft manuscript; all authors helped with data collection and interpretation; WJA carried out the statistical analysis; all authors read and approved the final version of the manuscript.
Competing interests: None.
Ethics approval: NHS Ayrshire & Arran Clinical Governance Team.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lau JYW, Barkun A, Fan DM, et al. Challenges in the management of acute peptic ulcer bleeding. Lancet 2013;381:2033–43. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox CM, Allison J, Benzully K, et al. Consensus development conference on the use of non-steroidal antiinflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol 2006;4:1082–9. [DOI] [PubMed] [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network (SIGN). National clinical guideline 105. Management of acute upper and lower gastrointestinal bleeding, 2008. http://www.sign.ac.uk/guidelines/fulltext/105/ (accessed 24 Jan 2014).
- 4.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with non-variceal upper gastrointestinal bleeding. Ann Intern Med 2010;152:101–13. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence. NICE clinical guideline 141. Acute upper gastrointestinal bleeding: management, 2012. http://guidance.nice.org.uk/cg141 (accessed 24 Jan 2014).
- 6.Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, et al. The changing face of hospitalization due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther 2011;33:585–91. [DOI] [PubMed] [Google Scholar]
- 7.Abraham NS, Hartman C, Harsche J. Reduced hospitalization cost for upper gastrointestinal events that occur among elderly veterans who are gastroprotected. Clin Gastroenterol Hepatol 2010;8:350–6. [DOI] [PubMed] [Google Scholar]
- 8.Taha AS, Kelly C, McCloskey C, et al. Gastroprotective policy and the incidence of upper gastrointestinal bleeding. Frontline Gastroenterology 2013;4:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockall T, Logan R, Devlin H, et al. Incidence and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. BMJ 1995;311:222–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60:1327–35. [DOI] [PubMed] [Google Scholar]
- 11.Crooks CJ, West J, Card TR. Comorbidities affect risk of nonvariceal upper gastrointestinal bleeding. Gastroenterology 2013;144:1384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langman MJS, Weil J, Wainwright P, et al. Risk of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:1075–8. [DOI] [PubMed] [Google Scholar]
- 13.Taha AS, Angerson WJ, Prasad R, et al. Upper gastrointestinal bleeding and the changing use of COX-2 non-steroidal anti-inflammatory drugs and low-dose aspirin. Aliment Pharmacol Ther 2007;26:1171–8. [DOI] [PubMed] [Google Scholar]
- 14.Taha AS, McCloskey C, Craigen T, et al. Mortality following blood transfusion for non-variceal upper gastrointestinal bleeding. Frontline Gastroenterology 2011;2:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16.Rockall TA, Logan RF, Northfield TC. Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. National Audit of Acute Upper Gastrointestinal Haemorrhage. Lancet 1996;347:1138–40. [DOI] [PubMed] [Google Scholar]
- 17.Camellini L, Merighi A, Pagnini C, et al. Comparison of three different risk scoring systems in non-variceal upper gastrointestinal bleeding. Dig Liver Dis 2004;36:271–7. [DOI] [PubMed] [Google Scholar]
- 18.Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper Gastrointestinal hemorrhage. Gastroenterology 2011;141:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapur K, Green J, Turner R, et al. Auditing mortality from upper gastrointestinal haemorrhage: impact of a high dependency unit. J R Coll Physicians Lond 1998;32:246–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Sandel MH, Kolkman JJ, Kuipers EJ, et al. Nonvariceal upper gastrointestinal bleeding: differences in outcome for patients admitted to internal medicine and gastroenterological services. Am J Gastroenterol 2000;95:2357–62. [DOI] [PubMed] [Google Scholar]
- 21.Hampers MJ, Surgenor SD, Spanjian K, et al. ICU care for patients with gastrointestinal bleeding: Impact on cost and outcome. Clin Intensive Care 2002;13:109–13. [Google Scholar]
- 22.Baradarian R, Ramdhaney S, Chapalamadugu R, et al. Early Intensive Resuscitation of Patients with Upper Gastrointestinal Bleeding Decreases Mortality. Am J Gastroenterol 2004;99:619–22. [DOI] [PubMed] [Google Scholar]
- 23.Sanders DS, Perry MJ, Jones SGW, et al. Effectiveness of an upper-gastrointestinal haemorrhage unit: A prospective analysis of 900 consecutive cases using the Rockall score as a method of risk standardisation. Eur J Gastroenterol Hepatol 2004;16:487–94. [DOI] [PubMed] [Google Scholar]


