Abstract
Background
The dissociation between specific IgE and skin prick test reactivity to aeroallergens, a common finding in populations living in low and middle-income countries, has important implications for the diagnosis and treatment of allergic diseases. Few studies have investigated the determinants of this dissociation. In the present study, we explored potential factors explaining this dissociation in children living in an urban area of Northeast Brazil, focusing in particular on factors associated with poor hygiene.
Methods
Of 1445 children from low income communities, investigated for risk factors of allergies, we studied 481 with specific IgE antibodies to any of Blomia tropicalis, Dermatophagoides pteronyssinus, Periplaneta americana and Blatella germanica allergens. Data on demographic, environmental and social exposures were collected by questionnaire; serum IgG and stool examinations were done to detect current or past infections with viral, bacterial, protozoan and intestinal helminth pathogens. We measured atopy by skin prick testing (SPT) and specific IgE (sIgE) to aerollergens in serum (by ImmunoCAP). SIgE reactivity to B. tropicalis extract depleted of carbohydrates was measured by an in-house ELISA. Total IgE was measured by in house capture ELISA. SNPs were typed using Illumina Omni 2.5.
Results
Negative skin prick tests in the presence of specific IgE antibodies were frequent. Factors independently associated with a reduced frequency of positive skin prick tests were large number of siblings, the presence of IgG to herpes simplex virus, Ascaris lumbricoides and Trichuris trichiura infections, living in neighborhoods with infrequent garbage collection, presence of rodents and cats in the household and sIgE reactivity to glycosylated B. tropicalis allergens. Also, SNP on IGHE (rs61737468) was negatively associated with SPT reactivity.
Conclusions
A variety of factors were found to be associated with decreased frequency of SPT such as unhygienic living conditions, infections, total IgE, IgE response to glycosylated allergens and genetic polymorphisms, indicating that multiple mechanisms may be involved. Our data, showing that exposures to an unhygienic environment and childhood infections modulate immediate allergen skin test reactivity, provide support for the “hygiene hypothesis”.
Background
Atopy can be defined by either a positive skin prick test (SPT) or the presence of allergen-specific IgE in serum (sIgE) and, generally in high-income countries, both measurements tend to be consistent in the same individual [1]. It is expected, therefore, that an individual with a detectable sIgE would also have a positive SPT to the same allergen, and vice-versa, but for practical reasons SPT has been more frequently used in clinical practice and research. However, there is growing evidence for a dissociation between SPT positivity and sIgE, particularly in marginalized populations living in low and middle-income countries. The dissociation has been observed either as a negative SPT in subjects with detectable sIgE or, less frequently, as an absence of sIgE in a SPT-positive individual. The ISAAC phase II study involving large samples from affluent and non-affluent populations observed a dissociation between detectable sIgE and negative SPT in four non-affluent study centers ranging 31.1–78.9% [1]. Other studies, conducted in rural children of Europe [2] or Africa [3,4], have shown also large proportions of children with positive sIgE to be SPT negative for the same allergens. A possible explanation for this dissociation is the down-regulation of allergic effector responses in skin, such that individuals with measurable levels of sIgE fail to develop immediate hypersensitivity responses. Some environmental exposures, in particular helminths [3–5] and other childhood infections [2–6], have been associated with a suppression of in vivo skin immediate (Type I) hypersensitivity responses.
IgE reactive to carbohydrate epitopes present in both helminth antigens and allergens have been described–such IgE seems to have a lower affinity for the high affinity IgE receptor (FCɛRI) present in the cells that mediate Type I hypersensitivity reactions [7] such as mast cells and basophils, leading to a failure of their activation and degranulation. In addition, polymorphisms (SNPs) of IgE and FCɛRI-encoding genes may regulate IgE levels [8] or interfere with mast cell degranulation either by altering the IgE molecule itself or its affinity for FCɛRI.
To examine the hypothesis that poor hygiene causes the dissociation between SPT and sIgE in among non-affluent populations living in poor regions of low and middle-income countries, we studied children living in poor neighborhoods in a large Brazilian city. We analyzed data associated with poor environmental hygiene and childhood infections and also genetic factors to identify potential determinants of a reduced SPT reactivity among children with detectable levels of sIgE for the same allergens.
Material and methods
Study population and data collection
The study was a post hoc analysis of data collected during a survey of 1,445 children aged 4–11 years and living in 24 poor neighborhoods in the city of Salvador, Northeast Brazil. The study was done in 2005 as part of a cohort study to investigate risk factors for asthma and allergy and is described in detail elsewhere [9]. Neighborhoods and children were selected for a previous study to measure the impact of sanitation on diarrhea [10]. Here, we have analyzed data from those children with detectable sIgE (≥0.70 kU/L) to at least one of four tested allergens and for whom complete data for other relevant variables were available. Data on asthma symptoms were collected using a Portuguese-adapted ISAAC Phase II questionnaire. The following measurements were performed for each child: anthropometric measurements, SPT testing and serum sIgE to four aeroallergens, circulating IgG against six pathogens, stool examination for intestinal helminthic infections, and dust samples from children´s beds to measure relevant aeroallergens. In a subgroup of this population, sera from Blomia tropicalis-sensitized children were tested for IgE to B. tropicalis extract that had either been treated or not treated with methaperiodate to deplete carbohydrates. Data were collected also on the presence of cockroaches, rodents, cats or dogs in the household, and the proportion of households linked to a sewage system and with daily garbage collection. Children were genotyped for polymorphisms on FCER1A, IGHE and ancestry informative markers (AIMs) as described previously [11].
Allergen SPTs
SPTs were done by two trained technicians using a standardized protocol using extracts of D. pteronyssinus, B. tropicalis, B. germanica, P. americana, dog and cat epithelia, and a fungal allergen mix (ALK-Abelló, São Paulo, Brazil). Extracts, saline and histamine controls were pricked onto the forearm skin using a disposable lancet (ALK-lancet®; ALK-Abelló, São Paulo, Brazil). Reactions were read after 15 minutes and a reaction was considered positive if the mean diameter of the wheal was 3 mm or larger than the saline control. Frequencies of positive skin test reactions to dog and cat epithelia and a fungal allergen mix were low (<4%) and were excluded from further analysis.
Detection of intestinal helminth ova in fecal samples
Two faecal samples were collected two days apart and analyzed using the Hoffman sedimentation method and Kato-Katz thick-smear technique [12] for the presence of helminth parasites (Trichuris trichiura, Ascaris lumbricoides, hookworms and Schistosoma mansoni). Hookworms and S. mansoni infections were rare (<1%) and were not considered further.
Dust
Dust was collected using a 1,200W vacuum cleaner (Electrolux, São Paulo, Brazil) with a 25 μm polystyrene filter from an area of 1m2 from the upper part of the child’s mattress for 2 minutes. The concentrations of the allergens Blo t 5, Der p 1, Bla g 2, Can f 1 and Fel d 1 were measured using commercial kits (Indoor Biotechnologies, Virginia, USA) following the manufacturer’s instructions.
Serum immunoassay for IgG to bacteria, protozoa, and viruses
Serum IgG antibodies to Helicobacter pylori, Toxoplasma gondii, herpes simplex virus (HSV), herpes zoster virus (HZV), Epstein-Barr virus (EBV) were measured using commercial ELISA kits (Diamedix, Miami, Florida, USA; Adaltis, Toronto, Canada). For hepatitis A virus (HAV), kits from ADALTIS were used (Toronto, Canada). Assays were performed following the manufacturers’ instructions.
Serum immunoassays for detection of total and allergen specific IgE
Total IgE was measured by in-house capture ELISA as described [13]. For the present analysis total IgE results were stratified into tertiles, corresponding to the following concentrations: <0.04, 0.04 - <0.1, and 0.1> ng/mL for 1st, 2nd, and 3rd tertiles, respectively.
sIgE to Dermatophagoides pteronyssinus, Blomia tropicalis, Blatella germanica and Periplaneta americana was measured in sera using the Immunocap System (Pharmacia AB, Uppsala, Sweden) according to the manufacturer’s instructions. Sera with ≥0.70 kU IgE to any of the four allergens were considered positive. This cut-off we have used previously [13–15] to minimize positive reactions associated with high levels of low-affinity and cross-reactive IgE in helminth-infected populations.
Detection of anti-B. tropicalis IgE using total or carbohydrate depleted extract
An in-house indirect ELISA was done to measure IgE to B. tropicalis before and after treatment with sodium metaperiodate (VETEC, Rio de Janeiro, Brazil). Briefly, delipidated B. tropicalis were added to microassay plates, some of which were treated with 10 mM sodium metaperiodate in 50 mM acetate buffer, pH 4.5, for 1 hour at room temperature in the dark, so that carbohydrates were oxidized to aldehyde. The assays was run as described previously [16]. Percentage of carbohydrate determinant-reactive anti-B. tropicalis IgE (BtE), in relation to total anti-B. tropicalis IgE (BtE), was calculated in accordance with the formula: % of carbohydrate-reactive to BtE = [1.0 - (mean OD of methaperiodate/boro-hydride-treated wells/mean OD of untreated wells).
DNA extraction and genotyping
DNA was extracted from peripheral blood using a commercial kit (Gentra® Puregene® Blood Kit (Qiagen). Subjects were genotyped using the 2.5 HumanOmni Beadchip from Illumina (San Diego, California, USA) for ten single nucleotide polymorphisms (SNPs) (rs2494262, rs2511214, rs2427837, rs2247584, rs16841987, rs2427825, rs7548864, rs2427827, rs12119226, rs2252226) on FCER1A and three SNPs (rs61737468, rs12884681, rs74091262) on IGHE. Individual genetic ancestry estimates were based on 370,539 SNPs shared by samples from the HapMap Project, the Human Genome Diversity Project (HGDP) in the SCAALA population. We used the ADMIXTURE software [17] to estimate the contribution from Africans, Europeans and Native Americans to the SCAALA individuals. All analyzed SNPs were in Hardy-Weinberg equilibrium. Non-template negative controls and genotyping-positive controls were included in each genotyping plate. Automatic calling was performed with a quality value above 99%.
Statistical analysis
The aim of the analysis was to evaluate the effects of a number of exposures on frequency of SPT positivity among children with detectable sIgE. Analysis for all exposures was performed using a parsimonious procedure. First, we assessed the association between each exposure in isolation and SPT reactivity among children with detectable sIgE by univariate logistic regression. Second, we built a multivariable model that included all potential associations with P<0.20 from univariate analyses. Using backwards stepwise selection and likelihood ratio tests, all environmental and infectious exposures variable with P<0.10 were left in the model. A priori confounders of age, gender and parental asthma were kept in the multivariate model. Initial analyses were done using logistic regression models with random effects to adjust for neighbourhood clustering, but given that standard errors without controlling for clustering gave similar results to ‘unclustered’ models, we present here only results of the latter. The following variables were used in the analysis: excreta disposal classified as “good” (sewage system), “intermediate” (septic tank) and “bad” (hole in the ground or open air); number of other children living at home; presence of cats or dog; infestation of households by cockroaches and rodents; infections with T. trichiura and A. lumbricoides; positive IgG to T. gondii, H. pylori, herpes simplex, varicella zoster, and Epstein-Barr viruses; and two neighborhood-level variables (the proportion of neighbourhoods linked to a sewage system and the proportion of neighbourhoods with daily garbage collection). Differences in sIgE levels from the indirect ELISA between SPT positives and negatives were evaluated using Mann-Whitney test while those between levels of anti-B. tropicalis IgE for glycosylated versus non-glycosylated extracts were done (only samples positive to the raw extract using a cut-off optical density of 0.250) using the Wilcoxon signed rank test for matched pairs. Genetic data were analyzed using logistic regression adjusted for sex, age, helminth infection and individual genetic ancestry markers using PLINK software. Analyses were done using STATA (version 8, StataCorp, TX, USA,) and SPSS (version 16, SPSS, Inc, Chicago, Ill, US.).
Ethical considerations
Ethical approval was obtained from the Brazilian National Ethical Committee. Written, informed consent detailing all procedures to be carried out on the children was signed by the child’s parent or legal guardian.
Results
1,355 of 1,445 children enrolled had both SPT and sIgE measured. Detection of serum sIgE equal or above 0.70 kU/L for at least one of four measured allergens (D. pteronyssinus, B. tropicalis, B. germanica and P. americana) or a positive SPT for at least one of the same four allergens was observed in 510 (37.6%) and 401 (29.4%) of the children, respectively. Among all tested children, 2.7%, 2.4%, 4.3% and 8.1%, had positive SPT but were negative for sIgE anti-B. tropicalis D. pteronyssinus B. germanica and P. americana respectively (data not shown).
The present analysis was restricted to 481 children with sIgE (≥0.70kU/L) to at least one of the four aeroallergens. No statistically significant differences were seen for outcomes and variables between children included and children excluded from the analysis (data not shown).
Table 1 shows the frequency of SPT positivity in children with a sIgE ≥ 0.70kU/L for at least one allergen and for each individual allergen. Only 65.6% of children with detectable sIgE for at least one of the four specific allergens had a positive SPT positive for at least one of the same allergens. When individual allergens were considered, frequencies of positive SPTs varied from 74.2% (P. americana) to 33.5% (B. germanica).
Table 1. Frequencies of positive skin prick tests (SPT) in 481 children with aeroallergen-specific IgE (sIgE).
| Specific IgE | N | STP ≥ 3mm | |
|---|---|---|---|
| positivity (≥0.70 kU/L) | N | % | |
| At least one allergen | 491 | 322 | 65.6 |
| B. tropicalis | 437 | 244 | 55.8 |
| D. pteronyssinus | 273 | 168 | 61.5 |
| B. germanica | 182 | 61 | 33.5 |
| P. americana | 120 | 89 | 74.2 |
Table 2 shows crude and adjusted analyses for statistically significant associations between SPT reactivity to at least one allergen or B. tropicalis or D. pteronyssinus allergens and demographic, environmental, social variables and infection markers among children with positive sIgE. Associations for non-significant variables are provided in S1 Table (presence of cockroach, dog at home; presence of Blo t 5, Der p 1, Bla g 2, Can f 1 allergens in bed dust samples; seropositivity to Hepatitis A, Herpes zoster viruses, Toxoplama gondii and Helicobacter pylori; A. lumbricoides and T. trichiura and total serum IgE for one, two or all tested outcomes). In multivariable analyses (Table 2), we observed negative associations between SPT to at least one allergen and number of siblings, intestinal helminth infections, seropositivity to Epstein-Barr and Herpes simplex viruses, rodent infestation, household cats, and low neighborhood coverage for garbage collection. SPT to B. tropicalis was negatively associated with number of siblings and household cats. SPT to D. pteronyssinus allergens was negatively associated with low neighborhood coverage for garbage collection and with H. simplex infection. P. americana or B. germanica skin reactivity was not associated with any of the variables among children with sIgE for each of these allergens (data not shown).
Table 2. Crude and adjusted odds-ratio (OR) of statistically significant associations of possible determinants with skin prick test (SPT) to at least one allergen or B. tropicalis or D. pteronyssinus in 481 children with specific IgE to the respective allergens.
| Possible determinants | Positive SPT for at least one allergen in children with specific IgE | ||
| N(%) | Crude | Adjusted | |
| OR(95%CI) | OR(95% CI) | ||
| No. of siblings | |||
| 0–1 | 211 (73.3) | 1 | 1 |
| > = 2 | 105 (54.4) | 0.44 [0.30–0.64] | 0.54 [0.35–0.81] |
| *Intestinal helminth infection | |||
| No | 283 (69.9) | 1 | 1 |
| Yes | 33 (43.4) | 0.44 [0.28–0.68] | 0.55 [0.34–0.89] |
| Rodent infestation in household | |||
| No | 158 (72.5) | 1 | 1 |
| Yes | 158 (60.1) | 0.57 [0.39–0.84] | 0.61 [0.40–0.93] |
| Cat in household | |||
| No | 296 (68.1) | 1 | 1 |
| Yes | 20 (43.5) | 0.36 [0.19–0.67] | 0.27 [0.13–0.54] |
| Epstein-Barr virus | |||
| No | 50(78.1) | 1 | 1 |
| Yes | 269(63.4) | 0.48 [0.26–0.91] | 0.49 [0.26–0.93] |
| Herpes simplex virus | |||
| No | 171(73.1) | 1 | 1 |
| Yes | 151(58.8) | 0.52 [0.36–0.77] | 0.50 [0.34–0.74] |
| % of neighborhood with daily garbage collection | |||
| > = 66% | 109 (71.7) | 1 | 1 |
| 33% - 66% | 39 (76.5) | 1.28 [0.61–2.68] | 1.67 [0.75–3.72] |
| 0–33% | 168 (60.4) | 0.60 [0.39–0.92] | 0.56 [0.35–0.89] |
| Possible determinants | Positive SPT to B. tropicalis in children with specific IgE | ||
| Number of siblings | |||
| 0–1 | 164 (62.1) | 1 | 1 |
| > = 2 | 80 (46.2) | 0.52 [0.36–0.77] | 0.58 [0.38–0.88] |
| *Intestinal helminth infections | |||
| No | 217 (58.7) | 1 | 1 |
| Yes | 27 (40.3) | 0.63 [0.39–0.99] | 0.80 [0.49–1.31] |
| Rodent infestation in household | |||
| No | 126 (63.0) | 1 | 1 |
| Yes | 118 (49.8) | 0.58 [0.40–0.85] | 0.58 [0.39–0.87] |
| Cat in household | |||
| No | 231 (58.3) | 1 | 1 |
| Yes | 13 (31.7) | 0.33 [0.17–0.66] | 0.27 [0.13–0.57] |
| Possible determinants | Positive SPT to D. pteronyssinus in children with specific IgE | ||
| % of neighborhood with daily garbage collection | |||
| > = 66% | 56 (66.7) | 1 | 1 |
| 33% - 66% | 24 (70.6) | 0.70 [0.36–1.36] | 0.75 [0.37–1.56] |
| 0–33% | 88 (56.8) | 0.59 [0.39–0.91] | 0.55 [0.35–0.87] |
| Herpes simplex virus infection | |||
| No | 94 (68.6) | 1 | 1 |
| Yes | 74(54.4) | 0.56 [0.33–0.89] | 0.54 [0.32–0.91] |
All adjusted ORs were adjusted for a priori confounders (age, gender and parenteral asthma), and Ascaris lumbricoides and Trichuris trichiura infections.
*Ascaris lumbricoides and Trichuris trichiura infections.
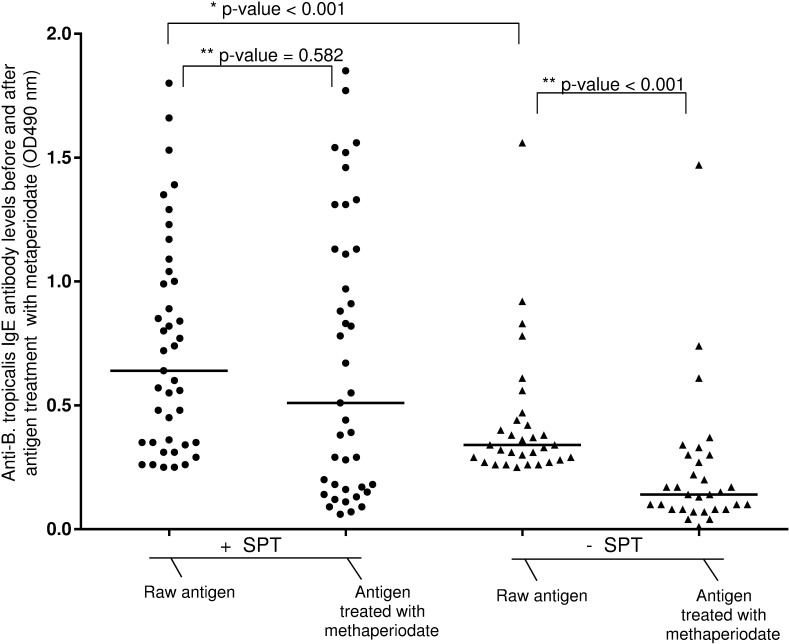
Fig 1 shows that in general, children with positive SPT to B. tropicalis had higher levels of sIgE compared with SPT negatives (untreated, p<0.001). There was a small non-significant decrease in sIgE levels after methaperiodate treatment among SPT positives. However, in the case of SPT negatives, methaperiodate treatment caused a significant reduction in sIgE levels (p<0.001).
Fig 1. Anti-Blomia tropicalis IgE levels in serum samples assayed using as antigens B. tropicalis extract depleted or not of carbohydrate.
Anti-Blomia tropicalis IgE levels were measured by indirect in-house ELISA in individuals positive for this antibody in IMMUNOCAP and were divided in two groups: SPT negative or positive for the same allergen. Antigen was treated with methaperiodate and the sera were assayed against raw and methaperiodate treated antigens. *Difference of sIgE results between SPT positive and SPT negative children (Mann–Whitney test). **Difference of sIgE results before and after antigen treatment with methaperiodate (Wilcoxon test).
Table 3 shows the association between FCER1A and IGHE polymorphisms and SPT. Only one SNP (rs61737468), the T allele, on IGHE was associated with reduced SPT reactivity (OR: 0.29; 95% CI: 0.15–0.58; p<0.001). None of the SNPs on FCER1A were associated with SPT.
Table 3. Associations between FCER1A and IGHE polymorphisms withskin prick test (SPT) reactivity to at least one allergen among children with allergen-serum Ig.
| Gene Polymorphisms | Alleles | MAF | ORa | 95% CI | P value |
|---|---|---|---|---|---|
| FCER1A | |||||
| rs2494262 | C/A | A | 1.10 | 0.76–1.59 | 0.593 |
| rs2511214 | G/T | T | 1.10 | 0.77–1.58 | 0.584 |
| rs2427837 | G/A | A | 1.33 | 0.80–2.22 | 0.268 |
| rs2247584 | T/G | G | 1.10 | 0.75–1.62 | 0.608 |
| rs16841987 | G/A | A | 0.92 | 0.59–1.42 | 0.706 |
| rs2427825 | C/T | T | 0.97 | 0.66–1.44 | 0.909 |
| rs7548864 | G/A | A | 1.20 | 0.82–1.76 | 0.336 |
| rs2427827 | C/T | T | 0.83 | 0.59–1.16 | 0.291 |
| rs12119226 | C/T | T | 1.07 | 0.62–1.84 | 0.805 |
| rs2252226 | T/C | C | 0.84 | 0.60–1.18 | 0.329 |
| IGHE | |||||
| rs61737468 | C/T | T | 0.29 | 0.14–0.58 | <0.001 |
| rs12884681 | A/G | G | 0.94 | 0.67–1.33 | 0.748 |
| rs74091262 | C/T | T | 0.65 | 0.33–1.28 | 0.271 |
We did not observe associations between levels of total IgE and SPT among children with sIgE either for any of the 4 aeroallergens tested or for specific aeroallergens (S1 Table). Then we analyzed also the role of total IgE as an effect modifier of the association between sIgE and SPT in the full study population of 1,355 individuals for which we had data (S2 Table). We observed that the association between sIgE and SPT was smaller in children with total IgE in the 3rd tertile (representing levels >0.1 ng/mL) compared to those with IgE in the 2nd tertile (P = 0.040).
Discussion
In the present study, we explored potential factors that might explain the dissociation between SPT and sIgE to the same allergen that has been observed in marginalized populations living in low and middle-income countries (LMICs) such as Brazil. We analyzed data from children living in poor neighborhoods in the city of Salvador in a tropical coastal region of Brazil. Because previous studies have showed a low prevalence of SPT reactivity among children with childhood infections [4,14] we focused in particular on childhood infections, and factors associated with poorly hygienic living conditions. We observed statistically significant inverse associations between SPT reactivity and living in an unhygienic environment (i.e. greater number of siblings, intestinal helminth infections, having household cat and rodent infestations, and infrequent garbage collection) among children with aeroallergen-specific IgE (sIgE) indicating that such an environment may be an important determinant of this dissociation. The observation that distinct factors affected the SPT-sIgE association for D. pteronyssinus and B. tropicalis supports the idea that the mechanisms linking IgE with SPT for these two mites species may be distinct [18,19], although power for the analysis of individual aeroallergens was limited.
Other studies conducted in LMICs have shown dissociations between the presence of aeroallergen-specific IgE in serum and evidence of in vivo IgE-mediated skin hypersensitivity to the same allergens measured by SPT reactivity [2–4,20]. The environmental factors that may contribute to this effect were not clear [21,22]. Studies have shown a reduced prevalence of atopy measured either as SPT or sIgE among children with fecal-oral or food-borne infections such those caused by hepatitis A virus, H. pylori, T. gondii [6], non-typhoidal Salmonella [23] and helminths [24]. No previous study has investigated the effects of a wide range of social, environmental and infectious exposures on SPT reactivity among individuals with sIgE for the same allergens as we have done here. Consistent data from other studies include: (i) less SPT reactivity in Estonian compared to Swedish children despite similar frequencies of sIgE in both populations [2]; (ii) large dissociations between SPT reactivity and sIgE among children with helminth infections [4,5].
Possible explanations for the down-modulation of skin sensitivity to allergens are (i) induction of mechanisms regulating allergic effector responses [3,5,24], thus suppressing immediate skin hypersensitivity or (ii) differences in the affinity of IgE antibodies [25]. Chronic helminth infections have been shown to modulate the host immune system, and may have an important role in this immunomodulatory process [24]. Several studies have reported a reduced prevalence of SPT among children infected with systemic or intestinal helminths [26,27], but without reference to sIgE levels. We have reported previously in this population that T. trichiura infection early in life was associated with a reduced prevalence of SPT later in childhood [14]. Furthermore, we have shown that Toxocara spp. seropositivity acts as an effect modifier of the association between SPT and sIgE [15]. The data from the present study indicate that current A. lumbricoides or T. trichiura infections, measured by the presence of parasite eggs in stool, are associated with a reduced SPT positivity to any allergen among children with sIgE. These findings may reflect possible IgE cross-reactivity between helminths and arthropods (such as mites and cockroaches) antigens. A proportion of low-titer IgE antibodies detected by the IgE assay used in our study could be low-affinity IgE antibodies to helminth antigens that cross-react with arthropod allergens. Such low titer IgE may be less effective in interacting with allergens to activate mast cells [25,28,29]. In support, our data showed that children with negative SPTs had lower levels of sIgE to B. tropicalis than children SPT positives. It is possible that the sIgE threshold for SPT reactivity is higher than the assay cut-off. Although very low specific IgE levels have been associated with anaphylaxis to venom allergens [30] this may not be true for aeroallergens such as mite.
There is growing evidence for significant IgE cross-reactivity between helminths and a wide range of invertebrate allergens [31,32]. A previous observation from our research group showed that the absorption of sera with an extract of A. lumbricoides antigens decreased levels of sIgE to B. tropicalis[16]. An alternative hypothesis to explain the role played by helminths in the down-modulation of SPT reactivity is the enhancement of Treg cells and regulatory cytokines production, such as IL-10 and TGF-β, that may inhibit mast cell degranulation [24,29,31]. Although regulatory cytokines can theoretically also down-modulate IgE production [33,34], they likely do not do this as efficiently as inhibition of the effector phase of the type I hypersensitivity response in skin. In fact helminths are strong inducers of total and parasite-specific IgE despite the high levels of IL-10 produced during chronic helminth infections [13].
We investigated the effect of markers of viral infections (i.e. Hepatitis A, Herpes simplex, Herpes zoster and Epstein-Barr viruses) on SPT reactivity. The only association observed was a reduced SPT reactivity to D. pteronyssinus in sIgE positive children among those with IgG to H. simplex virus. The association of infections with reduced SPT reactivity could be explained by the induction of antigen-specific regulatory responses by pathogens that share sequence homology with allergens, but also with non-specific suppression of the immune response.
The finding of low biologic activity of IgE cross-reactive to carbohydrate epitopes of glycoproteins has been reported in grass pollen sensitized patients who had specific IgE to peanut but no skin sensitivity to peanut [7]. The authors conclude that ‘non-effector’ IgE contributed to the discrepancy between sIgE and SPT. For this reason, we investigated also if carbohydrate determinants of B. tropicalis allergens could play a role in the discrepancy between sIgE and SPT. We observed that carbohydrate depletion of B. tropicalis extracts led to a significant reduction in sIgE levels among children who were sIgE+/SPT- to B. tropicalis. Although the glycoproteins in this case were from B. tropicalis, the same is likely to be true for carbohydrate molecules present in helminth extracts for which cross-reactive IgE responses have been reported with dust mites [35].
Although helminths are important inducers of polyclonal IgE as we [27] and others [2, 3, 4] have shown, our data provided only limited evidence for high levels of policlonal IgE explaining the dissociation between sIgE and SPT. There was some evidence that the SPT-sIgE association was weaker among children with the highest total IgE levels compared with children with ‘moderate’ or ‘low’ levels, although a statistically significant interaction was seen only for the former comparison (S2 Table). Previous study showed that total IgE may be contribute to the dissociation between sIgE and SPT observed in some populations [1,36].
Another possible explanation for absence of SPT reactivity in the presence of specific IgE for the same allergen could be host genetic factors. Previous studies linked polymorphisms on FCER1A with a reduction in sIgE levels and SPT reactivity [8]. However, none of these studies were done in a sIgE-positive population perhaps explaining why we did not observe relationships between SNPs on FCER1A and SPT reactivity here. Alternatively, one might think that SNPs on Immunoglobulin Heavy Constant Epsilon gene (IGHE; located at 14q32) could also play a role in SPT reactivity. In previous studies the genes most consistently shown to have effects included IL4, IL13, IL4RA and FCERB1 and more recently IL33 and ST2 and the IGH genes, both IGHG and IGHE [37,38]. In the present study, we have described for the first time a negative association between rs61737468 on IGHE and SPT reactivity. We could speculate that changes in the IgE heavy chain could decrease its affinity for FCɛRI and interfere with mast cell degranulation in the skin. Further molecular studies are required in other populations to elucidate how rs61737468 might affect SPT reactivity.
Conclusions
In conclusion, we have explored the role of poor hygiene exposures and genetic factors as an explanation for reduced SPT positivity observed in some LMIC settings in children with specific IgE for the same aeroallergens. Our observations do provide support for a role of poor hygiene exposures including childhood infections in mediating this effect, but other factors may have a role including host genetic factors, indicating that diverse mechanisms could be important. Potential mediating mechanisms identified here were increased glycosylation of aeroallergen-specific IgE and high levels of total IgE. A better understanding of how allergic effector responses are modulated could lead to the development of novel strategies for the prevention and control of allergic diseases.
Supporting information
*Adjusted for the a priori confounders (age, gender and parental asthma); **Ascaris lumbricoides and Trichuris trichiura
(DOCX)
p-valor (Breslow-Day). OR: 1st with 2nd tertile p = 0,531; 1st with 3rd tertile p = 0,174 and 2nd with 3rd tertile = 0,040.
(DOCX)
Abbreviations
- Bla g 2
recombinant allergen 2 from Blatella germanica
- Blo t 5
recombinant allergen 5 from Blomia tropicalis
- BMI
body mass index
- Can f 1
recombinant allergen 1 from Canis familiaris
- Der p 1
recombinant allergen 1 from Dermatophagoides pteronysinus
- EBV
Epstein-Baar virus
- FcɛRI
High affinity IgE receptor
- FCER1A
FcɛRI gene
- Fel d 1
recombinant allergen 1 from Felis domesticus
- GNI
gross national income
- HAV
Hepatitis A virus
- HSV
Herpes simplex virus
- HZV
Herpes zoster virus
- ISAAC
International Study of Asthma and Allergies in Childhood
- IgE
Immunoglobulin E antibody
- IGHE
IgE gene
- IgG
Immunoglobulin G antibody
- kU/L
kilounits per liter
- SCAALA
Social Change, Asthma and Allergy in Latin America
- sIgE
aeroallergen-specific immunoglobulin E antibody
- SNP
single nucleotide polymorphisms
- SPT
skin prick test
- Th1
T helper 1
- Th2
T helper 2
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: Wellcome Trust through the HCPC Latin America Excellence Centre Programme (072405/Z/03/Z) and grant 088862/Z/09/Z; and INCT/MCT/CNPq Programme (Contract no. 5737862008-9). We thank CAPES, CNPq and FAPESB for scholarships that supported some of the authors.
References
- 1.Weinmayr G, Genuneit J, Nagel G, Björkstén B, Van Hage M, Priftanji A, et al. International variations in associations of allergic markers and diseases in children: ISAAC Phase Two. Allergy. 2010;65: 766–775. 10.1111/j.1398-9995.2009.02283.x [DOI] [PubMed] [Google Scholar]
- 2.Julge K, Vasar M, Bjorksten B. Development of allergy and IgE antibodies during the first five years of life in Estonian children. Clin Exp Allergy. 2001;31: 1854–1861. [DOI] [PubMed] [Google Scholar]
- 3.Perzanowski MS, Ng’ang’a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140: 582–588. 10.1067/mpd.2002.122937 [DOI] [PubMed] [Google Scholar]
- 4.van den Biggelaar AHJ, Lopuhaa C, van Ree R, van der Zee JS, Jans J, Hoek A, et al. The Prevalence of Parasite Infestation and House Dust Mite Sensitization in Gabonese Schoolchildren. Int Arch Allergy Immunol. 2001;126: 231–238. [DOI] [PubMed] [Google Scholar]
- 5.Cooper PJ, Mitre E, Moncayo AL, Chico ME, Vaca MG, Nutman TB. Ascaris lumbricoides–Induced Interleukin-10 Is Not Associated with Atopy in Schoolchildren in a Rural Area of the Tropics. J Infect Dis. 2008;197: 1333–1340. 10.1086/586904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320: 412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100: 327–334. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V, Michel S, Gaertner V, Franke A, Vogelberg C, von Berg A, et al. A role of FCER1A and FCER2 polymorphisms in IgE regulation. Allergy. 2014;69: 231–236. 10.1111/all.12336 [DOI] [PubMed] [Google Scholar]
- 9.Barreto ML, Cunha SS, Alcântara-Neves N, Carvalho LP, Cruz AA, Stein RT, et al. Risk factors and immunological pathways for asthma and other allergic diseases in children: background and methodology of a longitudinal study in a large urban center in Northeastern Brazil (Salvador-SCAALA study). BMC Pulm Med. 2006;6: 15 10.1186/1471-2466-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto ML, Genser B, Strina A, Teixeira MG, Assis AMO, Rego RF, et al. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet (London, England). 2007;370: 1622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa GNO, Dudbridge F, Fiaccone RL, da Silva TM, Conceição JS, Strina A, et al. A genome-wide association study of asthma symptoms in Latin American children. BMC Genet. BioMed Central; 2015;16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14: 397–400. [PubMed] [Google Scholar]
- 13.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78: 3160–7. 10.1128/IAI.01228-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara‐Neves NM, Genser B, Cruz AA, et al. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008;38: 1769–1777. 10.1111/j.1365-2222.2008.03027.x [DOI] [PubMed] [Google Scholar]
- 15.Mendonça LR, Veiga RV, Dattoli VCC, Figueiredo CA, Fiaccone R, Santos J, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6: e1886 10.1371/journal.pntd.0001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponte JC, Junqueira SB, Veiga R V, Barreto ML, Pontes-de-Carvalho LC, Alcântara-Neves NM. A study on the immunological basis of the dissociation between type I-hypersensitivity skin reactions to Blomia tropicalis antigens and serum anti-B. tropicalis IgE antibodies. BMC Immunol. BioMed Central Ltd; 2011;12: 34 10.1186/1471-2172-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19: 1655–1664. 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baqueiro T, Pontes-de-Carvalho L, Carvalho FM, Santos NM, Alcântara-Neves NM. Asthma and rhinitis symptoms in individuals from different socioeconomic levels in a Brazilian city. Allergy and asthma proceedings. 2007. pp. 362–367. [DOI] [PubMed] [Google Scholar]
- 19.Chew FT, Yi FC, Chua KY, Fernandez-Caldas E, Arruda LK, Chapman MD, et al. Allergenic differences between the domestic mites Blomia tropicalis and Dermatophagoides pteronyssinus. Clin Exp Allergy. 1999;29: 982–988. [DOI] [PubMed] [Google Scholar]
- 20.Weinmayr G, Weiland SK, Björkstén B, Brunekreef B, Büchele G, Cookson WOC, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007;176: 565–574. 10.1164/rccm.200607-994OC [DOI] [PubMed] [Google Scholar]
- 21.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38: 872–897. 10.1111/j.1365-2222.2008.02971.x [DOI] [PubMed] [Google Scholar]
- 22.Handoyo S, Rosenwasser LJ. Asthma phenotypes. Curr Allergy Asthma Rep. 2009;9: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelosi U, Porcedda G, Tiddia F, Tripodi S, Tozzi AE, Panetta V, et al. The inverse association of salmonellosis in infancy with allergic rhinoconjunctivitis and asthma at school-age: a longitudinal study. Allergy. 2005;60: 626–630. 10.1111/j.1398-9995.2005.00747.x [DOI] [PubMed] [Google Scholar]
- 24.Smits HH, Yazdanbakhsh M. Chronic helminth infections modulate allergen-specific immune responses: Protection against development of allergic disorders? Ann Med. 2007;39: 428–439. 10.1080/07853890701436765 [DOI] [PubMed] [Google Scholar]
- 25.Pierson-Mullany LK, Jackola DR, Blumenthal MN, Rosenberg A. Evidence of an affinity threshold for IgE-allergen binding in the percutaneous skin test reaction. Clin Exp Allergy. 2002;32: 107–116. [DOI] [PubMed] [Google Scholar]
- 26.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111: 995–1000. [DOI] [PubMed] [Google Scholar]
- 27.Flohr C, Quinnell RJ, Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy. 2009;39: 20–32. 10.1111/j.1365-2222.2008.03134.x [DOI] [PubMed] [Google Scholar]
- 28.Obeng BB, Hartgers F, Boakye D, Yazdanbakhsh M. Out of Africa: what can be learned from the studies of allergic disorders in Africa and Africans? Curr Opin Allergy Clin Immunol. 2008;8: 391–397. 10.1097/ACI.0b013e32830ebb70 [DOI] [PubMed] [Google Scholar]
- 29.Yazdanbakhsh M, Kremsner PG, Van Ree R. Allergy, parasites, and the hygiene hypothesis. Science (80-). 2002;296: 490–494. 10.1126/science.296.5567.490 [DOI] [PubMed] [Google Scholar]
- 30.Golden DBK, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107: 897–901. 10.1067/mai.2001.114706 [DOI] [PubMed] [Google Scholar]
- 31.Santos ABR, Rocha GM, Oliver C, Ferriani VPL, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121: 1040–1046. 10.1016/j.jaci.2007.12.1147 [DOI] [PubMed] [Google Scholar]
- 32.Acevedo N, Sanchez J, Erler A, Mercado D, Briza P, Kennedy M, et al. IgE cross-reactivity between Ascaris and domestic mite allergens: the role of tropomyosin and the nematode polyprotein ABA‐1. Allergy. 2009;64: 1635–1643. 10.1111/j.1398-9995.2009.02084.x [DOI] [PubMed] [Google Scholar]
- 33.Punnonen J, de Waal Malefyt R, van Vlasselaer P, Gauchat JF, de Vries JE. IL-10 and viral IL-10 prevent IL-4-induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol. 1993;151: 1280–1289. [PubMed] [Google Scholar]
- 34.Shin HD, Park BL, Kim LH, Kim J, Kim J. Interleukin-10 haplotype associated with total serum IgE in atopic dermatitis patients. Allergy. 2005;60: 1146–1151. 10.1111/j.1398-9995.2005.00839.x [DOI] [PubMed] [Google Scholar]
- 35.Arkestål K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α1, 3-galactose. J Allergy Clin Immunol. 2011;127: 1024–1028. 10.1016/j.jaci.2011.01.033 [DOI] [PubMed] [Google Scholar]
- 36.De Vos G, Nazari R, Ferastraoaru D, Parikh P, Geliebter R, Pichardo Y, et al. Discordance between aeroallergen specific serum IgE and skin testing in children younger than 4 years. Ann Allergy, Asthma Immunol. Elsevier; 2013;110: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ober C. Perspectives on the past decade of asthma genetics. J Allergy Clin Immunol. 2005;116: 274–278. 10.1016/j.jaci.2005.04.039 [DOI] [PubMed] [Google Scholar]
- 38.Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2013;46: 51–55. 10.1038/ng.2830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*Adjusted for the a priori confounders (age, gender and parental asthma); **Ascaris lumbricoides and Trichuris trichiura
(DOCX)
p-valor (Breslow-Day). OR: 1st with 2nd tertile p = 0,531; 1st with 3rd tertile p = 0,174 and 2nd with 3rd tertile = 0,040.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.