Abstract
Background and aims
Low 25-hydroxyvitamin D [25(OH)D] concentrations have been associated with peripheral artery disease (PAD). Prevalence of low 25(OH)D and PAD differ between whites and blacks. However, these associations have not been studied prospectively or in a population based cohort. We tested the hypothesis that low 25(OH)D is associated with greater risk of incident PAD in white and black adults.
Methods
25(OH)D was measured in serum collected at ARIC visit 2 (1990 – 1992). We followed 11,789 ARIC participants free of PAD at visit 2 through 2011 for incident PAD events. 25(OH)D (ng/mL) was categorized as deficient (<20), insufficient (20 to <30) or sufficient (≥30). PAD was defined by an ankle brachial index (ABI) of <0.9 at ARIC visits 3 or 4 or a hospital diagnosis with an ICD-9 code indicating PAD during follow-up. Analysis used multivariable-adjusted Cox proportional hazards regressions.
Results
Over a mean follow-up of 17.1 years 1,250 incident PAD events were identified. 22% of whites and 61% of blacks were 25(OH)D deficient. After adjustment for demographic characteristics the hazard ratio (95% CI) of PAD in participants with deficient versus sufficient 25(OH)D was 1.49 (1.26, 1.76). Inclusion of BMI, physical activity, and smoking status attenuated the association [1.25 (1.06, 1.48)]. The association between 25(OH)D and PAD was qualitatively stronger in blacks (p for interaction = 0.20).
Conclusions
Deficient 25(OH)D was associated with increased risk of PAD in black and white participants. Whether treatment of low vitamin D through supplementation or modest sunlight exposure prevents PAD is unknown.
Introduction
Atherosclerotic lower extremity peripheral arterial disease (PAD) is usually characterized by at least one high grade (>75%) stenosis in the arteries that supply the legs, which present in the infrarenal aorta or more distal arteries. Prevalence of PAD among US adults aged 40 or older is approximately 7%, which equates to 8.5 million individuals.[1, 2] Black race is a strong PAD risk factor; the prevalence of PAD is approximately 1.7 times higher in blacks than in whites.[3] Individuals with PAD may suffer from leg pain while exercising, and in severe cases PAD patients experience constant leg pain, gangrene, or amputation. In addition to negatively impacting quality of life, PAD has been associated with increased risk of major coronary events, cardiovascular mortality, and mortality.[4] Although up to two-thirds of PAD cases are asymptomatic, these individuals are still at elevated risk of death and atherosclerotic cardiovascular disease (CVD) events.[5]
Vitamins D2 and D3 are acquired though exposure to ultraviolet B light or ingestion and are converted to serum 25-hydroxyvitamin D [25(OH)D]. Although 1,25(OH)2D is the active hormone, 25(OH)D has traditionally been viewed as the best biomarker for assessing vitamin D status. The Endocrine Society defines sufficient concentrations of 25(OH)D as 30–100 ng/mL, insufficiency as 21–29 ng/ml, and deficiency as below 20 ng/ml.[6] However, these cut-points are not based on recommendations for cardiovascular health. Deficient 25(OH)D levels are common, and prevalence varies by race/ethnicity; an analysis that used 2003–2006 National Health and Nutrition Examination Survey (NHANES) data found that 28% of whites and 81% of blacks had concentrations of ≤20 ng/mL.[7]
Research has associated low 25(OH)D with increased risk of CVD events[8, 9], and a 35% increased risk of CVD death.[10] There is, however, a growing body of evidence suggesting that the association between 25(OH)D and CVD outcomes is stronger in whites than blacks.[11–14] Adverse CVD outcomes observed in individuals with low levels of 25(OH)D are thought to be mediated through traditional CVD risk factors such as hypertension [15–20], diabetes [9, 21–24], and inflammation.[25–31]
To date, little research has evaluated whether low 25(OH)D is associated with the development of PAD. The studies that have looked at this relation are limited in that they were cross-sectional, in clinical populations, or limited to a white population.[32–34] Using data from the community-based and prospective Atherosclerosis Risk in Communities (ARIC) study we tested the hypothesis that deficient 25(OH)D compared to sufficient levels is associated with greater risk of incident PAD, and that the association is stronger among whites than blacks.
Materials and methods
Study design
The ARIC study is a prospective cohort designed to determine the causes of atherosclerosis and cardiovascular outcomes.[35] Using probability sampling, a total of 15,792 participants aged 45–64 years were initially enrolled in 1987–89 (visit 1), of whom 11,478 were white and 4,266 were black. Recruitment took place at 4 sites (suburban Minneapolis, MN; Forsyth Co., NC; Washington Co., MD; Jackson, MS). Participants have been followed over 27 years, with annual phone interviews (semi-annual since 2012) and 4 follow-up clinical study visits. Visits occurred at approximately year 3 (visit 2, 1990–1992), 6 (visit 3, 1993–95), 9 (visit 4, 1996–98), and 24 (visit 5, 2011–2013). 25(OH)D was measured in participant serum collected at visit 2; therefore, for the present analysis, visit 2 was used as baseline. Participants were followed for outcomes until the last date of active surveillance collection, death, or December 31, 2011.
ABI, the ratio of SBP in the ankle to the SBP in the upper arm, was not measured at visit 2. Since ABI was not measured concurrently with 25(OH)D, as a surrogate we defined prevalent PAD according to visit 1 ABI or hospital detection between visits 1 and 2. Participants were excluded from the analysis if they had prevalent PAD at visit 1 or hospital detected PAD between visits 1 and 2 (n=503), missing ABI information at ARIC visit 1 (n=457), an ABI of greater than 1.4 at visit 1 (indicative of arterial stiffness that interferes with ABI as a diagnostic tool for PAD) (n = 315), were neither African American nor white or if they were African Americans from the MN and MD centers (n = 78), did not attend visit 2 (n = 1,444), had missing data for 25(OH)D (n = 1,198), or missing information on other key covariates (n=8). The final analytic sample included 11,789 participants. The institutional review committees at each study center approved the study protocol, and all participants provided informed consent.
25(OH)D measurement
Serum 25(OH)D was measured in 2012–2013 using previously unthawed serum samples from visit 2 by liquid chromatography high-sensitivity mass spectrometry (Waters Alliance e2795; Waters, Milford, MA, USA) at the University of Minnesota Molecular Epidemiology and Biomarker Research Laboratory. Annual average serum 25(OH)D levels were estimated by accounting for seasonality using a residuals approach, as in previous ARIC papers.[36] Serum 25(OH)D levels were analyzed categorically, as deficient (<20 ng/mL), insufficient (20 to <30 ng/mL), and sufficient (≥30 ng/mL). Blind duplicate serum samples from ARIC visit 2 were used to calculate the coefficient of variation (CV) and correlation (r): 25(OH)D3 CV=6.9, r=0.97; 25(OH)D2 CV=20.8, r = 0.98.[36] Measurements of 25(OH)D3 and 25(OH)D2 were summed to calculate total serum 25(OH)D.
Incident PAD ascertainment
Incident PAD was defined as an ABI of less than 0.9 at ARIC visit 3 or 4, or a hospital discharge diagnosis of PAD, leg amputation, or leg revascularization procedure (leg endarterectomy, aorto-iliacfemoral bypass surgery, or leg bypass surgery) during follow-up.
ABI was measured in all participants at visit 1 and a random sample of participants at visit 3 (n=3,787) and 4 (n=5,143).[37] Trained staff used the Dinamap 1846 automated oscillometric device (Criticon, Tampa, FL) to measure ankle SBP at the posterior tibial artery with the participant prone, and brachial SBP in the right arm with the participant supine.[38] At visit 1 average ankle and brachial SBPs were calculated from two measurements in a randomly selected leg and (usually) the right arm. At visits 3 and 4 one ankle SBP and one brachial SBP were measured. ABI was defined as the ratio of the ankle SBP to the brachial SBP.
Participants were contacted by phone (annually through 2012) to identify intermittent claudication symptoms and all hospitalizations. Hospital discharge diagnosis International Classification of Disease, Ninth Revision codes of 443.9 (peripheral vascular disease, unspecified), 84.11 (toe amputation), 84.12 (foot amputation), 84.15 (below-knee amputation), 84.17 (above-knee amputation), 38.18 (leg endarterectomy), 39.25 (aorto-iliac-femoral bypass), and 39.29 (leg bypass surgery) were considered to be hospitalized PAD. Participants were assumed not to have had incident PAD if ABI was not low at visits 3 or 4 and no event was recorded through active surveillance. Date of incidence was defined as the first date of PAD based on either low ABI at visit 3 or 4, or hospital discharge with PAD.
Covariates
Covariate information was from visit 2 (1990–1992), unless otherwise noted. Race, sex, age, education level (<high school degree, high school degree or some college, college graduate at visit 1), and smoking status (current, former, never) were self-reported. Physical activity was measured on a scale from 1 (low) to 5 (high) using the Baecke sports questionnaire at visit 1.[39] Medications for treatment of hypertension, diabetes, or high cholesterol were self-reported and transcribed from bottles participants brought to the visit. Height, weight, and SBP were measured. Fasting cholesterol and triglyceride levels were measured according to ARIC standard procedures.[40] Glucose was measured at visit 2 using a hexokinase assay. Diabetes mellitus was defined as fasting blood glucose level of ≥ 126 mg/dL, a non-fasting blood glucose level of ≥ 200 mg/dL, a self-reported physician diagnosis of diabetes, or the use of anti-diabetes medication in the previous 2 weeks. Serum creatinine was measured using the Jaffe method at visit 2. Cystatin C, serum calcium, and serum phosphorus were measured using a Roche Modular P Chemistry analyzer. Estimated glomerular filtration (eGFR) was calculated from serum creatinine and cystatin C levels using the CKD-EPI equation.[41] Serum parathyroid hormone (PTH) was measured using a Roche Elecsys 2010 Analyzer with a sandwich immunoassay method. High sensitivity C-reactive protein (hs-CRP) was measured using a Roche latex particle enhanced immunoturbidimetric assay kit and Modular P Chemistry analyzer. Serum fibroblast growth factor 23 was measured on a 2-site enzyme-linked immunosorbent assay (KainosLaboratories, Inc., Tokyo, Japan). Vitamin D binding protein SNPs rs7041 and rs4588 SNP genotypes were obtained using the ITMATBroad-CARe Chip.[12]
Statistical methods
Descriptive statistics of all potential covariates and effect modifiers, stratified by categorical 25(OH)D exposure groups, were calculated. The primary analysis used Cox proportional hazards regression to model the relationship between 25(OH)D categories and time to incident PAD. Person-time accrued from visit 2 until a PAD event occurred, end of follow-up (December 31st 2011), the participant was lost to follow-up, or died, whichever occurred earlier. The relation between 25(OH)D and incident PAD was adjusted for several sets of covariates in nested models. Model 1 adjusted for age, educational category, race, sex. Model 2 further adjusted for BMI, physical activity, and smoking status. We used Cox regression with restricted cubic splines to visually depict the association between 25(OH)D and incident PAD separately in black and white participants. 5 knots were placed at the 5th, 25th, 50th, 75th, and 95th percentiles of race specific 25(OH)D. The value at the 10th percentile was used as the referent, similar to previous publications.[33]
In addition to adjustment for baseline demographic and behavioral variables, secondary analysis looked at the association between 25(OH)D and incident PAD after adjustment for known PAD risk factors, some of which may be mediators. Model 3 added to model 2 prevalent diabetes, hypertension medication use, SBP, LDL cholesterol, HDL cholesterol, cholesterol medication use, and hs-CRP. Additionally, we separately added to model 2 eGFR, serum calcium, serum fibroblast growth factor 23, serum parathyroid hormone, and serum phosphorus. As a sensitivity analysis, we restricted the analysis to participants who were free of PAD and had ABI of ≥0.9 at visit 3 (n = 3,579). The outcome in this analysis was incident PAD after visit 3.
Effect modification by age, race, sex and key SNPs associated with vitamin D binding protein levels (i.e. rs7041, rs4588) was tested by including cross product terms in the models. Regardless of the presence of race-interactions, a priori we planned to present race-stratified models, given inherent interest.
Analyses were performed with SAS 9.3 (SAS Institute, Inc. Cary, North Carolina). Spline regression was performed with STATA 13.1 (StataCorp, College Station, Texas). Analyses were two sided with a type 1 error of 0.05 or less considered statistically significant.
Results
A total of 11,789 participants were included in the final analysis; 2839 (24.0%) were black, 6668 (56.6%) were female, and the mean ± SD age was 56.8 ± 5.7 years. Mean 25(OH)D level was 24.3 ± 8.5 ng/ml overall, and was higher in white (26.1 ± 8.3) than black (18.9 ± 6.7) participants. Among white participants, 29% had a 25(OH)D level ≥30 ng/mL (sufficient), 49% had a level 20 to <30 ng/mL (insufficient), and 22% had a level <20ng/mL (deficient). Only 6% of black participants had a 25(OH)D level ≥30 ng/mL, 32% had a level 20 to <30 ng/mL, and 61% had a level <20ng/mL.
Baseline characteristics by 25(OH)D categories are summarized in Table 1. Briefly, participants with deficient 25(OH)D tended to be black, female, less educated, current smokers, and have an overall worse CVD risk factor profile.
Table 1.
Baseline (1990–1992) participant characteristics by 25(OH)D levels in the Atherosclerosis Risk in Communities Study.
| 25(OH)D level, ng/mL | |||
|---|---|---|---|
| Deficient <20 | Insufficient 20 to <30 | Sufficient ≥30 | |
| N (%) | 3790 (32) | 5260 (45) | 2739 (23) |
| Demographic characteristics | |||
| Age (years), mean ± SD | 56.3 ± 5.7 | 57.0 ± 5.7 | 57.3 ± 5.7 |
| Female, N (%) | 2595 (68) | 2728 (52) | 1345 (49) |
| Race, N (%) | |||
| Black | 1742 (46) | 921 (18) | 176 (6) |
| White | 2048 (54) | 4339 (82) | 2563 (94) |
| Education, N (%) | |||
| ≤11 years | 911 (24) | 1046(20) | 500 (18) |
| 12–16 years | 1519 (40) | 2191 (42) | 1229 (45) |
| 17+ years | 1354 (36) | 2015(38) | 1005 (37) |
| Behavioral characteristics and BMI | |||
| BMI (kg/m2), mean ± SD | 29.4 ± 6.2 | 27.7 ± 4.9 | 26.3 ± 4.2 |
| Smoking, N (%) | |||
| Current | 976 (26) | 1033 (20) | 526 (19) |
| Never | 1200 (32) | 2034 (39) | 1204 (44) |
| Former | 1600 (42) | 2187 (42) | 1009 (37) |
| Physical activity (1–5), mean ± SD | 2.2 ± 0.7 | 2.5 ± 0.8 | 2.7 ± 0.9 |
| Physiologic characteristics | |||
| Calcium (mg/dl), mean ± SD | 9.4 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.4 |
| eGFR (ml/min per 1.73 m2), mean ± SD | 97.6 ± 18.1 | 95.3 ± 15.9 | 93.0 ± 15.8 |
| FGF-23 (pg/ml), mean ± SD | 43.5 ± 20.3 | 44.5 ± 16.0 | 45.3 ± 16.7 |
| Parathyroid hormone (pg/ml), mean ± SD | 48.2 ± 24.5 | 41.2 ± 17.6 | 36.8 ± 12.6 |
| Phosphorus (mg/dl), mean ± SD | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.5 |
| Physiologic characteristics | |||
| hs-CRP (mg/l), mean ± SD | 5.2 ± 8.2 | 3.9 ± 6.5 | 3.7 ± 6.6 |
| Diabetes mellitus, N (%) | 732 (19) | 694 (13) | 240 (9) |
| HDL (mg/dl), mean ± SD | 50.4 ± 17.0 | 48.7 ± 16.2 | 51.3 ± 17.8 |
| LDL (mg/dl), mean ± SD | 133.7 ± 37.9 | 134.0 ± 36.3 | 131.7 ± 35.7 |
| SBP (mm Hg), mean ± SD | 123.7 ± 18.0 | 120.6 ± 18.0 | 118.9 ± 17.6 |
| Medication | |||
| Hypertension medication, N (%) | 1413 (37) | 1642 (31) | 748 (27) |
| Cholesterol medication, N (%) | 349 (9) | 185 (4) | 185 (7) |
The mean follow-up was 17.1 (SD 5.7) years, with a maximum of 22.8 years. A total of 1,250 (10.6%) participants developed incident PAD, with 15% (n=186) of events detected at visit 3, 24% (n=305) detected at visit 4, and 61% (n=759) detected through hospital surveillance. The unadjusted incidence rate was 6.2 per 1000 person-years. Among white participants, there were 907 events for an incidence rate of 5.9 per 1000 person-years. Among black participants there were 343 events for an incidence rate of 7.3 per 1000 person-years.
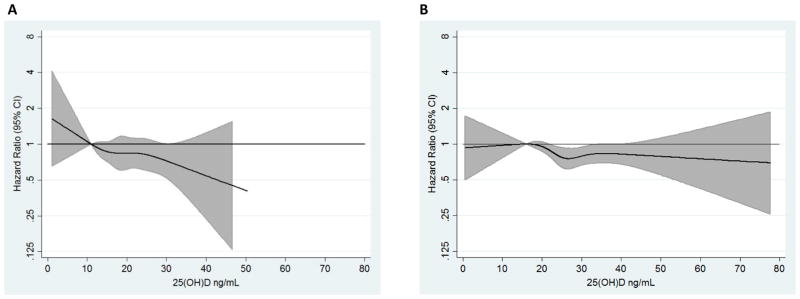
The association between 25(OH)D and incident PAD is visually depicted using race-stratified restricted cubic splines (Fig. 1) and presented according to 25(OH)D categories (Table 2). As shown in the race-stratified spline results, the hazard of PAD was inversely associated with level of 25(OH)D in both black and white participants, although the association appeared somewhat stronger in blacks. As shown in Table 2, after adjustment for baseline demographic characteristics (model 1) 25(OH)D level was inversely associated with incident PAD (2 df, Chi-square p<0.0001) and there was evidence of linear dose-response (1df, p<0.0001). The hazard of incident PAD in participants with deficient and insufficient 25(OH)D compared to participants with sufficient 25(OH)D was 1.49 (95% CI: 1.26, 1.76) and 1.14 (95% CI: 0.98, 1.32) respectively.
Fig. 1. Multivariable-adjusted hazard ratio of PAD at different levels of 25(OH)D based on restricted cubic spline.
(A) Black and (B) white participants. Restricted cubic splines adjusted for age, education level, sex, BMI, physical activity, and smoking status. Knots at the 5th, 25th, 50th,75th, and 95th percentiles of the race specific 25(OH)D. The 10th percentile was the referent.
Table 2.
Hazard ratios of peripheral artery disease by categorical 25(OH)D levels.
| Hazard ratios (95% confidence intervals) for 25(OH)D level (ng/mL) | ||||
|---|---|---|---|---|
| Sufficient ≥30 | Insufficient 20 to <30 | Deficient <20 | pa | |
| All participants | ||||
| Events, N (%) | 256 (9) | 533 (10) | 461 (12) | |
| Model 1 | 1 | 1.14 (0.98, 1.32) | 1.49 (1.26, 1.76) | <.0001 |
| Model 2 | 1 | 1.08 (0.92, 1.25) | 1.25 (1.06, 1.48) | 0.02 |
| Model 3 | 1 | 1.05 (0.90, 1.22) | 1.15 (0.97, 1.37) | 0.22 |
| Whites | ||||
| Events, N (%) | 242 (9) | 423 (10) | 242 (12) | |
| Model 1 | 1 | 1.10 (0.94, 1.29) | 1.48 (1.23, 1.77) | <.0001 |
| Model 2 | 1 | 1.03 (0.88, 1.21) | 1.23 (1.02, 1.48) | 0.06 |
| Model 3 | 1 | 1.02 (0.87, 1.20) | 1.13 (0.93, 1.37) | 0.41 |
| Blacks | ||||
| Events, N (%) | 14 (8) | 110 (12) | 219 (13) | |
| Model 1 | 1 | 1.58 (0.91, 2.77) | 1.94 (1.12, 3.34) | 0.03 |
| Model 2 | 1 | 1.70 (0.97, 2.97) | 1.84 (1.07, 3.19) | 0.09 |
| Model 3 | 1 | 1.50 (0.85, 2.63) | 1.64 (0.95, 2.85) | 0.19 |
Model 1 is adjusted for age, education level, sex, and race (overall results only).
Model 2 is adjusted for age, education level, sex, race (overall results only), BMI, physical activity, and smoking status.
Model 3 is adjusted for age, education level, sex, race (overall results only), BMI, physical activity, smoking status, SBP, HDL, LDL, CRP, diabetes mellitus, cholesterol medication use, and hypertension medication use.
2 degree of freedom type 3 Wald Chi square test of significance.
Inclusion of BMI, physical activity, and cigarette smoking status (model 2) attenuated the association between 25(OH)D and PAD, though it remained statistically significant (p=0.02); HR for deficient vs. sufficient 25(OHD) was 1.25 (95% CI 1.06, 1.48). Adjustment for cardiovascular risk factors (model 3) further attenuated the association (HR: 1.15 95% CI: 0.97, 1.37, p=0.22). There were no multiplicative interactions of the association between 25(OH)D and incident PAD by age, race, sex or SNPs rs7041 or rs4588. Despite the lack of significant race interaction (model 2 interaction p=0.20), as specified a priori, race-stratified results are presented. The magnitude of the association between deficient vs. sufficient 25(OH)D and incident PAD was qualitatively stronger in blacks (HR: 1.84 95% CI: 1.07, 3.19) than whites (HR: 1.23 95% CI: 1.02, 1.48), after accounting for demographics and behavioral factors.
We also explored the impact of further adjustment for variables that are known to be biochemically related to 25(OH)D level (i.e. serum calcium, FGF-23, PTH phosphorus, eGFR). Adding these biomarkers to model 2 did not substantially change the association between 25(OH)D and incident PAD. Results of these analyses are shown in Table 3.
Table 3.
Hazard of peripheral artery disease by categorical 25(OH)D exposure levels after adjustment for baseline variables and biomarkers biochemically associated with 25(OH)D.a
| Hazard ratios (95% confidence intervals) for 25(OH)D level (ng/mL) | ||||
|---|---|---|---|---|
| Additional adjustment variable | Sufficient ≥30 | Insufficient 20 to <30 | Deficient <20 | pb |
| eGFR | 1 | 1.09 (0.93, 1.29) | 1.25 (1.04, 1.51) | 0.05 |
| Calcium | 1 | 1.08 (0.92, 1.25) | 1.26 (1.06, 1.49) | 0.02 |
| FGF-23 | 1 | 1.08 (0.93, 1.26) | 1.27 (1.07, 1.50) | 0.01 |
| PTH | 1 | 1.06 (0.91, 1.23) | 1.22 (1.03, 1.44) | 0.05 |
| Phosphorus | 1 | 1.07 (0.92, 1.25) | 1.26 (1.06, 1.49) | 0.02 |
Adjusted for age, education level, sex, race, BMI, physical activity, smoking status, and the listed variable.
2 degree of freedom type 3 Wald Chi square test of significance.
In sensitivity analyses results were similar when the analyses were restricted to non-smokers (data not shown). Results were qualitatively stronger compared to the main analyses when we restricted to the 3,579 participants who at baseline were free of PAD and at visit 3 had a measured ABI of ≥0.9 (to exclude the potential for incident ABI <0.9 between visit 1 and 2). With this restriction, the HR of incident PAD after visit 3 in participants with deficient 25(OH)D compared to participants with sufficient 25(OH)D was 1.66 (95% CI: 1.14, 2.40) after adjustment for demographic characteristics (model 1), and 1.46 (95% CI: 1.00, 2.15) after further adjustment for BMI, physical activity, and smoking status (model 2).
Discussion
In this analysis of nearly 12,000 participants from the community-based ARIC study cohort, those with deficient levels of 25(OH)D were at approximately 30% greater risk of developing incident PAD, relative to those with sufficient 25(OH)D levels, after accounting for demographics and lifestyle factors. Although there was not a significant interaction by race, the association between low 25(OH)D level and incident PAD was qualitatively stronger in blacks than it was in whites. As predicted, adjustment for known PAD risk factors attenuated the association of 25(OH)D and PAD. These variables may be mediators of the relation or share common cause with low 25(OH)D.
Our finding that low 25(OH)D was associated with an approximately 30% increased risk of PAD is consistent with previous research. The idea that, among individuals with PAD, the presence and development of atherosclerosis may be influenced by disorders of calcium metabolism is not new. It has been previously reported that, relative to healthy controls, patients with PAD have lower bone density and abnormal bone turnover.[42] Fetuin-A, which is a member of the cystatin superfamily of cysteine protease inhibitors involved in vascular pathology and bone metabolism, has also been linked to PAD.[43]
Looking specifically at studies of 25(OH)D and PAD, a study that used the electronic medical records of 41,504 patients in the Intermountain Healthcare system who had 25(OH)D measured for clinical indications (e.g. osteoporosis risk), found approximately 40% increased risk of PAD in patients with low 25(OH)D (≤15 ng/ml) compared to those with sufficient concentrations (>30 ng/mL).[34] Cross-sectional analyses that used NHANES data found an 80% higher prevalence of PAD in participants in the lowest quartile of 25(OH)D compared to the highest quartile after adjustments.[32] However cross-sectional analysis may be complicated by reverse causation if patients with PAD are unable to participate in outdoor physical activity due to limitations from their PAD, and thus have less sunlight exposure and lower 25(OH)D levels. Therefore, our prospective study of incident PAD enhances existing knowledge about the association between 25(OH)D and PAD, as results from studies of patients may be biased and lack generalizability, and results from cross-sectional research cannot establish temporality.
Although interaction by race was not statistically significant, our finding that the magnitude of association was qualitatively higher in black participants was opposite of the hypothesized effect. It is unclear why, in the ARIC population, the association was qualitatively stronger in blacks than whites. Importantly, there were few events among blacks with sufficient 25(OH)D levels, and confidence intervals for hazard estimates were wide.
Prior research on 25(OH)D and PAD is limited in that it has been cross-sectional, or prospective using a clinical sample of white patients.[32–34] This analysis used the ARIC study, a community based prospective cohort of blacks and whites with more than 20 years of follow-up and standardized procedures for collection of numerous variables related to cardiovascular health. This analysis also has limitations. First, 25(OH)D was measured only once, and there was a long time-span between that measurement and when some of the PAD events accrued, therefore exposure misclassification may have occurred. However, exposure misclassification would likely weaken the observed association. Second, ABI (measured at visits 1, 3 and 4) was not measured concurrently with 25(OH)D (measured at visit 2). For the primary analyses we used low ABI at visit 1 or diagnosis of PAD between visits 1 and 2 as a surrogate for PAD at visit 2. To address the limitation of lack of concurrent measurements, we conducted a sensitivity analysis that was restricted participants who did not have PAD at visit 3. Results in this sensitivity analysis were qualitatively stronger than those in the full cohort, with a HR of 1.46 (vs. 1.25) for deficient compared to sufficient participants after adjustments (model 2). Third, it is possible that 25(OH)D is not the optimal biomarker for assessing vitamin D status.[44, 45] Fourth, the ARIC study did not collect information on sunlight exposure or about how much time people spent outdoors. However, concentrations of 25(OH)D are downstream from sun exposure and nutritional intake, and are influenced by individual-level factors (e.g. amount of melanin in the skin which blocks the conversion of 7-dehydrocholestrol to vitamin D in the skin). As such, concentrations of 25(OH)D are important when thinking about the potential role of vitamin D in the development of PAD. Fifth, ABI was measured in one leg at visits 1, 3, and 4. Measurement of ABI in one leg could cause misclassification of participants with uni-lateral PAD as non-PAD cases. At visits 3 and 4 ABI was only performed on a randomly sample of participants. These would both cause the measured ABI incidence to be lower than the true rate, however this may be alleviated by the fact that >60% of cases were detected through hospital surveillance. Finally, although we controlled for a number of baseline demographic, behavioral, and health characteristics, this is an observational study and residual confounding may remain.
In this large prospective analysis, deficient levels of 25(OH)D were associated with an increased hazard of PAD in whites and blacks, and the association remained after adjustment for demographic and lifestyle variables. Estimates of the 25(OH)D and PAD association were stronger in black than white participants, however confidence intervals for the estimates in blacks were wide, and tests of multiplicative interaction by race were non-significant. It is possible that increasing levels of vitamin D in blacks and whites through supplementation or modest sunlight exposure could result in lower incidence of PAD, however additional research is needed to support these findings.
Highlights.
The first population based bi-racial prospective cohort study on 25(OH)D and PAD.
Deficient 25(OH)D was associated with increased PAD in whites and blacks.
The relation of 25(OH)D and PAD was qualitatively stronger in blacks.
It is unknown if treatment of low vitamin D prevents PAD.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This research was further supported by grants from the NIH National Heart, Lung, and Blood Institute (R01 HL103706 to Dr. Lutsey) and the NIH Office of Dietary Supplements (R01 HL103706-S1 to Dr. Lutsey). Reagents for the C-reactive protein assays were donated by the manufacturers. Genotyping was supported through the NHLBI CARe (Candidate Gene Resource) grant (N01-HC-65226).
Footnotes
Author contributions
IRR and PLL planned and designed the analysis. IRR analyzed the data under the supervision of PLL. IRR wrote the first draft of the paper. All authors reviewed manuscript and provided critical scientific input. IRR and PLL had responsibility for final content. All authors approved of final manuscript draft.
Conflict of interest
I. R. Rapson reports grants from National Institutes of Health, during the conduct of the study; E.D. Michos reports honorarium from Seimens Diagnostics outside the submitted work; A. Alonso reports grants from National Institutes of Health, during the conduct of the study; A. T. Hirsch reports grants from AstraZeneca, grants from Pluristem, grants and other support from Merck, and other support from Bayer, outside the submitted work; J. P. Reis has nothing to disclose; P.L. Lutsey reports grants from National Institutes of Health, during the conduct of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison MA, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Ostchega Y, et al. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2007;55(4):583–9. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Ankle Brachial Index, C., et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criqui MH, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez OM, et al. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–53. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford JA, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–55. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury R, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michos ED, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241(1):12–7. doi: 10.1016/j.atherosclerosis.2015.04.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutsey PL, et al. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3(5):347–56. doi: 10.1016/j.jchf.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson-Cohen C, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–88. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michos ED, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28(4):367–71. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28(3):205–21. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 16.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27(10):1948–54. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 17.Vimaleswaran KS, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–29. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327–31. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 19.Xiang W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya A, Forman JP. Vitamin D and hypertension: current evidence and future directions. Hypertension. 2010;56(5):774–9. doi: 10.1161/HYPERTENSIONAHA.109.140160. [DOI] [PubMed] [Google Scholar]
- 21.Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’? British Journal of Nutrition. 2007;79(04):315. doi: 10.1079/bjn19980055. [DOI] [PubMed] [Google Scholar]
- 22.Palomer X, et al. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obesity and Metabolism. 2008;10(3):185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 23.Borissova AM, et al. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57(4):258–61. [PubMed] [Google Scholar]
- 24.Pittas AG, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikle DD. Vitamin D regulation of immune function. Vitam Horm. 2011;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 26.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Martín-Ventura JL, et al. Possible Role of Parathyroid Hormone–Related Protein as a Proinflammatory Cytokine in Atherosclerosis. Stroke. 2003;34(7):1783–1789. doi: 10.1161/01.STR.0000078371.00577.76. [DOI] [PubMed] [Google Scholar]
- 28.Somjen D, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111(13):1666–71. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 29.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79(6):1659–64. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michos ED, Blumenthal RS. Vitamin D supplementation and cardiovascular disease risk. Circulation. 2007;115(7):827–8. doi: 10.1161/CIRCULATIONAHA.106.686238. [DOI] [PubMed] [Google Scholar]
- 31.Schleithoff SS, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 32.Melamed ML, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–85. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis JP, et al. Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88(6):1469–77. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JL, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 35.INVESTIGATORS TA. THE ATHEROSCLEROSIS RISK IN COMMUNITY (ARIC) STUDY: DESIGN AND OBJECTIVES. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 36.Lutsey PL, et al. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC) Clin Chim Acta. 2015;442:75–81. doi: 10.1016/j.cca.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wattanakit K, et al. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18(2):629–36. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 38.Weatherley BD, et al. The reliability of the ankle-brachial index in the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI Family Heart Study (FHS) BMC Cardiovasc Disord. 2006;6:7. doi: 10.1186/1471-2261-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 40.National Heart, L., and Blood Institute. The ARIC Manuals of Operation Version 2.0. University of North Carolina; Chapel Hill: 1991. [Google Scholar]
- 41.Inker LA, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pennisi P, et al. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int. 2004;15(5):389–95. doi: 10.1007/s00198-003-1550-9. [DOI] [PubMed] [Google Scholar]
- 43.Fiore CE, et al. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195(1):110–5. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 44.LeFevre ML U.S.P.S.T. Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133–40. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 45.Chun RF, et al. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–7. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]