Abstract
Background
Crohn's disease (CD) is characterised by periods of relapse and remission. Over time the disease leads almost inevitably to the complications of stricturing, penetration and fistulisation. Perianal CD involves areas of chronic abscess formation, ulceration, skin tags or fistula formation. This can be a particularly challenging and complex problem to manage, and a range of potential treatment modalities exist.
Methods
This review covers the management of perianal CD and provides recommendations for practice for the multidisciplinary team (MDT), including the use of wound management products and relevant clinical images.
Results
Current practice focuses predominantly on the use of antibiotic therapy, immunosuppression, immunomodulation and surgery. These therapies are used individually or in combination. The majority of evidence suggests that a combination of medical and surgical management produces the best disease outcomes. However, this treatment regime can be debilitating for the patient and compliance can be difficult. Published work on the use of topical therapy in the management of perianal CD focuses specifically on topical drug therapy; it does not, however, address the basic guiding principles of chronic wound management—in particular, optimal moisture control and the management of bacterial burden on the wound surface. Honey and silver-containing wound management products act as topical antimicrobial agents and therefore address these principles.
Conclusions
Perianal CD is the archetypal condition that exemplifies the need for an MDT approach in caring for patients with inflammatory bowel disease. A combination of treatment modalities that includes topical wound management is likely to produce the best patient outcomes.
Keywords: Crohn'S Disease
Introduction
Crohn's disease (CD) is an autoimmune inflammatory bowel disease (IBD) that has a relapsing and remitting course.1 2 The precise aetiology is unclear and is the focus of much contemporary research. Common symptoms include diarrhoea, bloody stools, abdominal pain, weight loss and fatigue.1 3 Fistulisation, stricturing and penetrating disease are common, with disease duration and severity being poor prognostic markers in the long term.4
Perianal CD affects approximately 21–23% of the population with CD5 and manifests in various ways, including skin tags, anal fissures, perianal ulceration, anal strictures, perianal abscesses and perianal fistulae.6 7 Each manifestation carries varying degrees of risk in terms of medical or surgical management.2 8 Conservative management is favoured for the less complex manifestations due to the high risks of incontinence following surgical intervention.8 9 In addition, the presence of active rectal inflammation is known to be a poor prognostic indicator for perianal CD.4
Classification of disease phenotype is vital in predicting an individual's prognosis and disease course. The unpredictable nature of CD makes the management of perianal lesions challenging and this is recognised in the literature.8–11 While the use of the perianal Crohn's disease activity index (PCDAI)48 (table 1) is widely published in the literature, it is noted that it is not widely used in the clinical setting. One reason for this has been postulated by Safar and Sands8 who suggest that the lack of specific guidance offered by the tools in terms of clinical management strategies makes them less useful in the clinical setting.
Table 1.
Perianal Crohn's disease activity index
| Score | |
|---|---|
| Discharge | |
| No discharge | 0 |
| Minimal mucous discharge | 1 |
| Moderate mucous or purulent discharge | 2 |
| Substantial discharge | 3 |
| Gross faecal soiling | 4 |
| Pain/restriction of activities | |
| No activity restriction | 0 |
| Mild discomfort, no restriction | 1 |
| Moderate discomfort, some limitation of activities | 2 |
| Marked discomfort, marked limitation | 3 |
| Severe pain, severe limitation | 4 |
| Restriction of sexual activity | |
| No restriction in sexual activity | 0 |
| Slight restriction in sexual activity | 1 |
| Moderate limitation in sexual activity | 2 |
| Marked limitation in sexual activity | 3 |
| Unable to engage in sexual activity | 4 |
| Type of perianal abscess | |
| No perianal disease/skin tags | 0 |
| Anal fissure or mucosal tear | 1 |
| <3 Perianal fistulae | 2 |
| ≥3 Perianal fistulae | 3 |
| Anal sphincter ulceration or fistulae with significant undermining of skin | 4 |
| Degree of induration | |
| No induration | 0 |
| Minimal induration | 1 |
| Moderate induration | 2 |
| Substantial induration | 3 |
| Gross fluctuance/abscess | 4 |
Perianal disease is debilitating and has a significant effect on the quality of life for the patient.1 10 12 Published data on the management of perianal CD favours a combined radiological, medical and surgical approach to produce the best outcomes.1 10 13–17
The principal author has noted, through clinical practice as a tissue viability nurse specialist and subsequently as an inflammatory bowel disease nurse specialist, that wound management is not often included in the treatment paradigm for perianal CD.
While there is a plethora of evidence available on this subject in general, none of the papers specifically relating to perianal CD mention it as a treatment option despite most surgical interventions leaving the patient with a wound. Furthermore, the management of anal ulceration, fissures and abscesses after drainage is commonly led by physicians despite the reality of these manifestations involving open wounds in the perianal area.
In particular, this review aims to analyse critically the evidence base for the management of perianal CD and chronic wounds and provide recommendations for practice for use by the multidisciplinary team (MDT). These recommendations will include the incorporation of wound management strategies in the current treatment paradigm. The review will concentrate on the more complex aspects of perianal CD, namely fistulae and abscesses, as the greatest volume of evidence exists in this area.
Radiology
Assessment of the nature and extent of perianal CD is carried out through a combination of diagnostic imaging and direct visualisation. If there is a clinical suspicion of fistulising disease after initial assessment, imaging followed by examination under anaesthetic (EUA) is recommended.17 18 Potentially useful imaging modalities include endo-anal ultrasound scanning and MRI.
The use of MRI as a clinical evaluation tool is also recommended in the literature. In their review, Tozer et al17 highlight the benefits of sequential MRI during the medical and surgical management of perianal CD. It has been noted, particularly following the introduction of some medical therapies, that rapid healing can occur in fistulising disease.4 13 However, despite closure of the external wound, fistula tracks may remain and their identification is important when planning the patient's subsequent care. Tozer et al17 argue that MRI plays a role in assessing the progression or deterioration of fistulae that may not be visible during clinical examination. In terms of assessing the extent of disease, a combination of endo-anal ultrasound scanning or MRI with EUA is recognised as the gold standard for fistulising disease.7
Medical
The European Crohn's and Colitis Organisation guidance (2010) on perianal CD management makes several recommendations to aid with diagnosis and management. Clarity around the severity of the fistula should be sought through the use of MRI or EUA by an experienced surgeon. A proctosigmoidoscopy is also recommended due to the poor prognostic indication of rectosigmoid inflammation. Thereafter, medical management usually focuses on the reduction of the risk of infection. Most commonly in clinical practice, metronidazole and ciprofloxacin are the drugs used; however, despite widespread clinical experience with these antibiotics the supporting published evidence base is sparse.19
Despite this low quality published evidence, topical and systemic antibiotics are commonly used to treat perianal CD, and indeed, their use is recommended in the European Crohn's and Colitis Organisation guidelines. Consensus opinion is that the inclusion of these therapies improves the symptomatic management of perianal disease. It is, however, worth noting that systemic antibiotics also carry a recognised side-effect profile. This includes nausea, diarrhoea and a metallic taste in the mouth. The increase in faecal flow caused by diarrhoea may consequently lead to an increased bacterial burden on the wound. Paradoxically, this is likely to have a negative effect on wound healing. Furthermore, peripheral neuropathy may occur after the long-term use of metronidazole.8 The recommended time to continue taking oral antibiotics for perianal CD is 3–4 months.10
Anti-tumour necrosis factor (TNF) α (infliximab and adalimumab) therapy has now become the medical mainstay of complex perianal CD management and has the potential to have a significant effect on the previously described natural history of fistulising CD.2 Infliximab is known to be effective in the management of severe luminal and fistulising CD.5 20–23 In addition, the CHOICE trial demonstrated the efficacy of adalimumab in achieving fistula closure, improved quality of life and increased work productivity in patients with CD who had either failed or lost response to infliximab.22 Furthermore, Oussalah et al21 demonstrated the long-term efficacy of both anti-TNF agents in maintaining fistula closure after 1 year.
Clearly, the role of anti-TNF therapy in the management of perianal CD is well established; however, caution must be exercised, particularly if sepsis is suspected and should be actively excluded by EUA/MRI if needed before treatment is started.1
Surgical
The surgical options for managing perianal CD are varied and range from basic EUA to the formation of myocutaneous flaps in the most severe cases. For the purposes of this review, the most common types of surgical procedure discussed in the literature—incision and drainage of abscess, fistulotomy and placement of Seton suture—will be discussed. Most evidence on the surgical management of perianal CD focuses on the drainage of sepsis and is largely conservative due to the risk of incontinence.8 9 24 25 It is well established that colonic and specifically rectal inflammation are poor prognostic indicators, and healing after a surgical procedure in these cases is likely to be complicated.8 15 24 An understanding of the anatomy of the anus is identified as being fundamental to the understanding and management of perianal CD.7 10 13
A classification system for perianal CD was designed by Parks in 1976. This system identifies the most common types of perianal fistulae from an anatomical perspective and is used to help guide the surgical management of perianal CD (see supplementary figure S1, available online only).
However, the systematic review by Malik and Nelson26 found that despite this and other classifications, significant variation in management still exists. They call for a standardised approach to fistula definition and head to head studies comparing the outcomes of the various surgical techniques. This would help determine which is the most effective and in what circumstance each one is applicable. Although this current review does not discuss CD fistulae specifically, the message regarding the need for standardised tools for assessment and management of perianal fistulae is clear.
The placement of loose Seton sutures as a means of reducing the risk of abscess formation is well reported in the literature.9 18 26–28 This involves passing a silastic suture through the fistula tract and feeding it down the anal canal. The suture is then tied and remains in situ until the fistula is closed. In this way, any infection is allowed to drain and abscesses are less likely to form (see supplementary figure S2, available online only).
This management is usually used in high and trans-sphincteric fistulae where laying open (fistulotomy) would involve incision across the anal sphincters thus increasing the risk of incontinence.8 In both cases (fistulotomy and Seton suture placement) the patient is left with an open or draining wound. Clearly, this then has practical implications for the patients and their ability to function on a day-to-day basis.
The timing of surgical intervention in the treatment pathway is variable and widely dependent on the presence or absence of sepsis. Regardless of this, the patient is left with a draining wound that requires specific management in order for them to continue with their daily activities.
Topical antimicrobial management
The use of topical antimicrobial therapy is also addressed in the literature. However, within the context of perianal CD, it relates only to topical drug therapy. Stringer et al29 demonstrated a decrease in both PCDAI score and perianal pain with the topical application of 10% metronidazole three times daily. In contrast, Maeda et al30 demonstrated no effect on the PCDAI score using the same therapy applied at the same intervals. Both of those studies had significant methodological limitations in terms of short timelines (4 weeks), small patient numbers (14 and 74, respectively), heterogeneity in the study group and poorly controlled study drug administration. Interestingly, both studies reported a reduction in perianal pain with the application of topical antimicrobial therapy. In addition, Maeda et al30 comment on the absence of systemic adverse effects when using topical metronidazole compared with oral administration.
This is a concept that merits further study. However, the poor quality of evidence in this area so far prevents one from making firm conclusions for patient management.
It appears clear, therefore, that a combined approach appears to be the most sensible way forward when dealing with perianal CD and this is borne out in the literature.17 31 What is not clear, however, is how the effects of these interventions are managed. The reality of draining abscesses, fistulotomy and Seton suture placement is that a wound is present. Wounds create exudate, which, when inappropriately managed, can subsequently have a detrimental effect on the integrity of the surrounding perianal skin.32 33 We would argue that the failure to recognise or address this could be a factor in prolonging the healing rates of these types of wounds.
Cleansing
No wound cleansing mechanisms are reported in the literature for perianal wounds. Genua and Vivas24 make passing reference to it in their review; however, their suggestion of a combination of hygiene, debridement of necrotic tissue and patience is not helpful in guiding practice. Some debate exists around the value of wound cleansing and the most effective solution for this.34 35 It seems reasonable, however, that in the case of perianal wound management where there is a faecal flow, the use of water as a cleansing agent would be both appropriate and practical. Furthermore, it seems counterintuitive not to cleanse a perianal wound that is producing significant amounts of exudate.
The use of regular, planned perianal hygiene in conjunction with topical antimicrobial therapies may help reduce the bacterial burden on the wound and is likely to be of benefit to the patient in terms of comfort and ability to function on a day-to-day basis.
Wound management
The use of topical wound management products in perianal CD cannot be considered without first addressing the basic principles of wound healing. Traditional wound management practice was challenged by Winter36 who proved the theory of moist wound healing. This seminal work demonstrated increased epithelialisation rates in superficial wounds when a moist environment was maintained. This mechanism of action is now well established, and controlling moisture levels at the wound surface remains a fundamental underlying principle of most wound management strategies. Classification of the wound type is also fundamental to the implementation of an effective wound management strategy. The exudate produced by acute and chronic wounds is not the same. In particular, exudate produced by chronic wounds can be detrimental to the healing process, prolonging inflammation and causing degradation of the wound bed.37 Chronicity is defined as any wound in which healing is delayed or the natural healing process is disrupted.38
Furthermore, a heavy bacterial burden is a defined cause of chronic wounds.33 Clearly, perianal CD falls into this category because the pathogenesis of CD is one of chronic inflammation, and the anatomical position of the wound makes a high bacterial burden very probable.
The review by Ousey and McIntosh39 identified key features of antimicrobial wound management products, which help maintain an optimal environment for healing. These are listed in supplementary box 1 (available online only).
There remains some clinical anxiety around the maintenance of a moist wound environment and the associated potential to harbour bacteria. This has been addressed in a systematic review by Slater,40 who concluded that there was no evidence of increased infection, although no conclusion could be drawn regarding any reduction in infection rates when using advanced wound management products.
The use of topical antimicrobial agents in wound management is well documented.33 41 42 Their use has gained momentum in recent years in light of increasing bacterial resistance to many systemic antibiotics. Silver and honey-containing wound management products have also been cited as effective antimicrobial agents.42–44 Bioavailable silver ions affect the bacterial cell wall causing interference with the cellular DNA and subsequent failure in bacterial cell reproduction.44 The high sugar content in honey exerts an osmotic effect on the wound surface and surrounding tissues encouraging lymph drainage and reduction in inflammation.43 45 Moreover, a by-product of the reaction between honey and the wound exudate produces a low concentration hydrogen peroxide, which is an established antiseptic product.43 The relative merit of both products in their role as antimicrobial agents is well established in practice. Advanced wound management products are designed to maintain the optimal wound environment. The clinical skill in the use of these products lies in choosing the most appropriate product for the individual wound.
This review has therefore explored clinical practice in perianal CD management. In line with published evidence, the principal author is recommending a multidisciplinary approach. However, this should also include members of the nursing team to improve the patient journey further. In particular, the tissue viability or IBD nurse specialist should be involved.
Practical application of evidence
The case studies below illustrate how the combined approach in the management of perianal CD can work in practice, as discussed in this article.
Case study 1
The patient was a 14-year-old boy diagnosed with panenteric CD in summer 2011 (figures 1–3). Significant perianal abscess and fistulisation was evident at diagnosis. Small bowel imaging was normal. He commenced 8 weeks of exclusive enteral nutrition and was given mercaptopurine at 1.5 mg/kg in conjunction with oral ciprofloxacin and metronidazole. Infliximab therapy was withheld at this time as perianal sepsis was a significant clinical concern.
Figure 1.
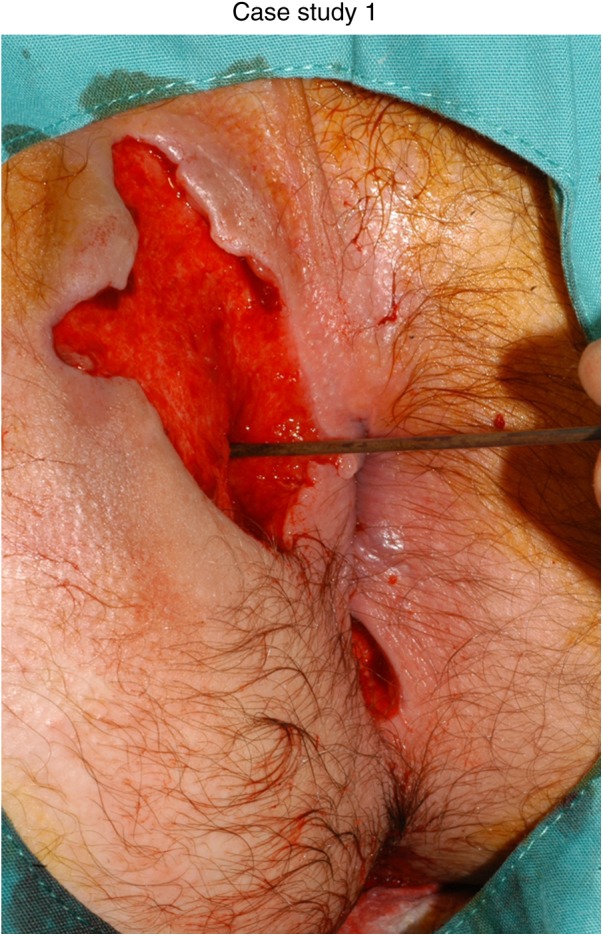
At diagnosis.
Figure 2.
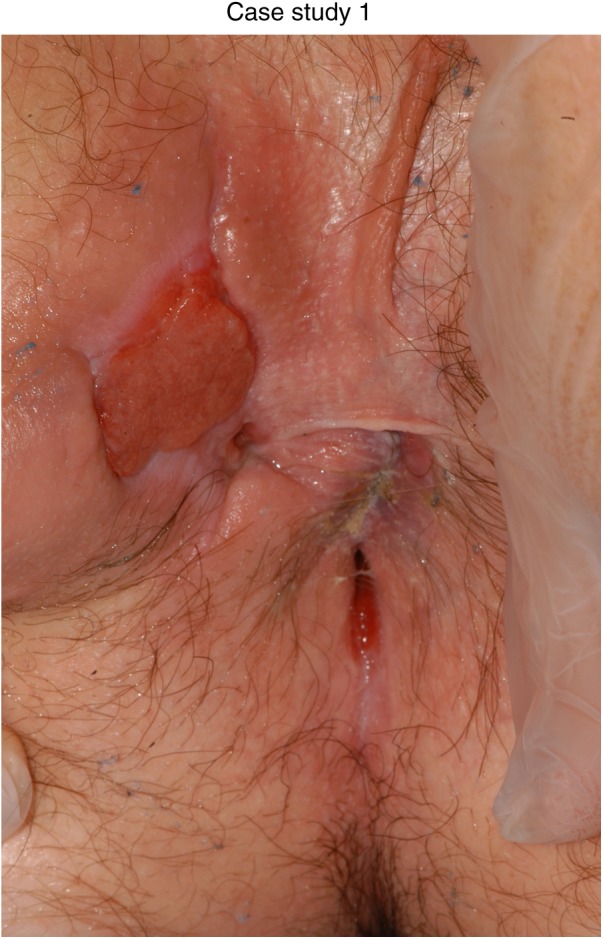
Three months after defunctioning: exclusive enteral nutrition; mercaptopurine at 1.5 mg/kg and topical wound management.
Figure 3.
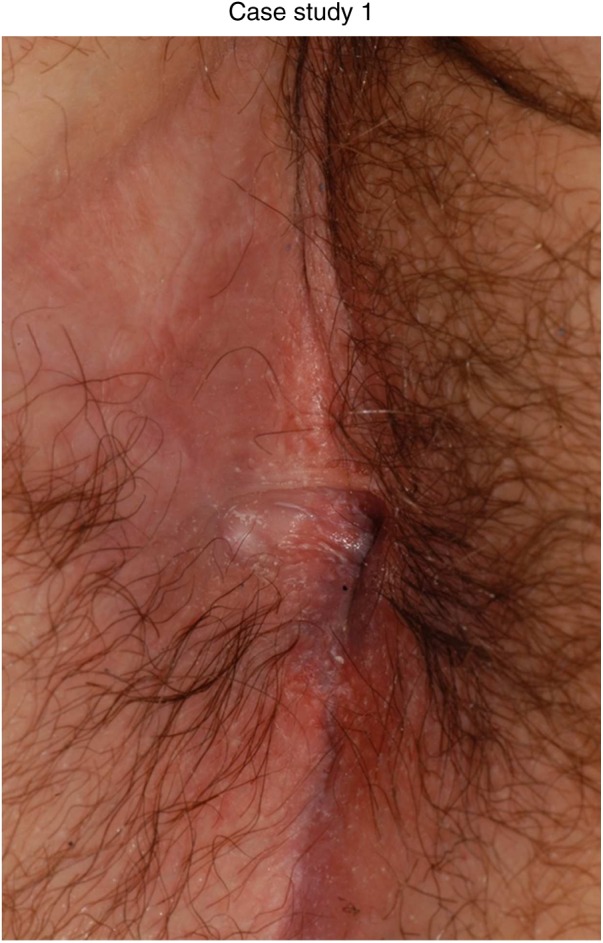
Twelve months later after addition of infliximab at 5 mg/kg and continued topical wound management.
MRI at presentation demonstrated a complex right-sided perianal fistula with a trans-sphincteric component, which crossed the external sphincter at the 9 o'clock position. There was a small right ischio-anal abscess and extension of the fistula cavity inferiorly ending in the right perineum. The fistula also branched medially in an intersphincteric location at the 6 o'clock position.
He attended theatre three times weekly for 4 weeks for wound toileting. This allowed for effective wound cleansing as the patient was not able to tolerate dressing changes on the ward. In addition, persistent faecal flow was compounding much of his wound management. After discussion with the surgical team and in conjunction with the family, the decision was made to carry out a defunctioning ileostomy in July 2011. Sepsis was ruled out following defunctioning and the patient was commenced on standard infliximab induction therapy.
Throughout this time, his perianal wound persisted, requiring regular dressing changes. The reduction in faecal flow led to a marked improvement in both the perianal tissue and the patient's ability to cope with dressing changes. He attended the hospital as an outpatient twice weekly for dressing changes using nitrous oxide (entonox) as analgesia.
As his wound was highly exudative, we used Acticoat Absorbant (Smith and Nephew). This is a silver-containing product that comes as a ribbon to facilitate easy packing into the fistula cavity. We occluded the wound (where possible) using Allevyn Thin (Smith and Nephew) as a secondary product. This is a malleable foam dressing that has the capacity to absorb exudate while being flexible enough to conform to the perianal area. Surrounding skin integrity was maintained using Cavilon (3M), a non-sting barrier film product applied on alternate days.
As his wound progressed and exudate levels reduced, we discontinued the Acticoat Absorbant and used Allevyn Thin as a protective agent while his skin integrity resolved. He had no issues with infection during this time.
Throughout the course of his wound management, he was encouraged to irrigate his wound twice daily using the showerhead at home. The frequency of this was largely based around the likelihood that he would comply with the instruction given that he was an adolescent and poor compliance in this age group is well documented.46 In addition, it encouraged him to take an active part in his recovery—another important facet in managing the patient with a chronic condition.47 The perianal hygiene programme continued until his wound was completely healed.
He attended theatre on a further two occasions following faecal diversion as the external opening to his fistula became very overgranulated and required debridement under anaesthetic. Full healing was achieved on completion of his induction course of iInfliximab.
Follow-up MRI showed significantly less contrast enhancement and adjacent inflammation at the site of fistula and resolution of the abscess cavity in the right ischio-anal fossa.
Case study 2
The patient was a 13-year-old boy diagnosed with pancolonic CD including severe activity in the caecum (figures 4–6). Small bowel imaging showed no abnormalities. No perianal symptoms were evident at diagnosis. He responded well clinically to 8 weeks of exclusive enteral nutrition and commenced azathioprine at 2.5 mg/kg from diagnosis. Two months after diagnosis, he began complaining of pain and discomfort at his anal margins. He attended the IBD clinic and was noted to have a visible abscess on his left buttock superior to the perianal margin. This was excised and drained and a deep fistula was identified. He was commenced on an induction course of infliximab at 5 mg/kg in July 2011 after sepsis had been completely ruled out.
Figure 4.
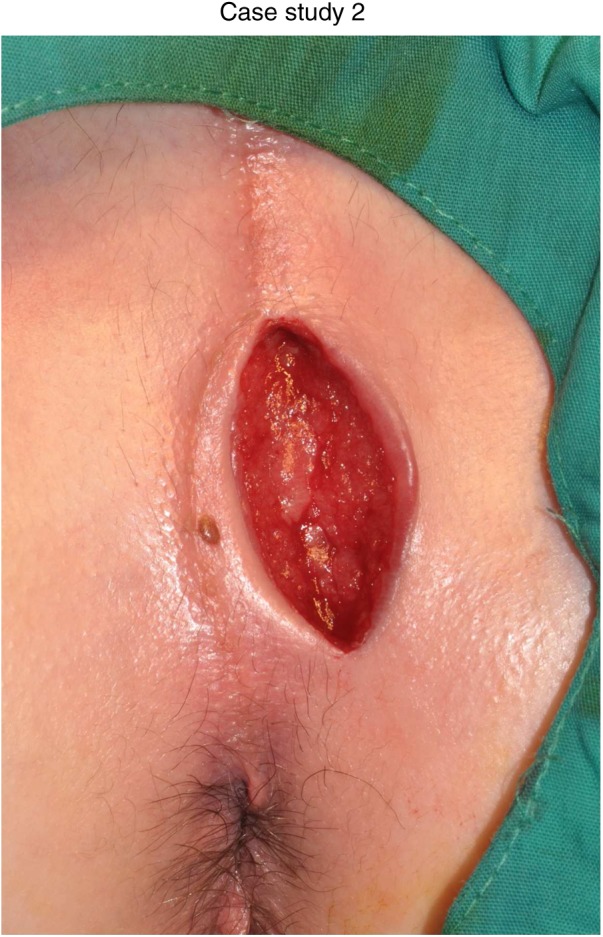
Six months after diagnosis.
Figure 5.
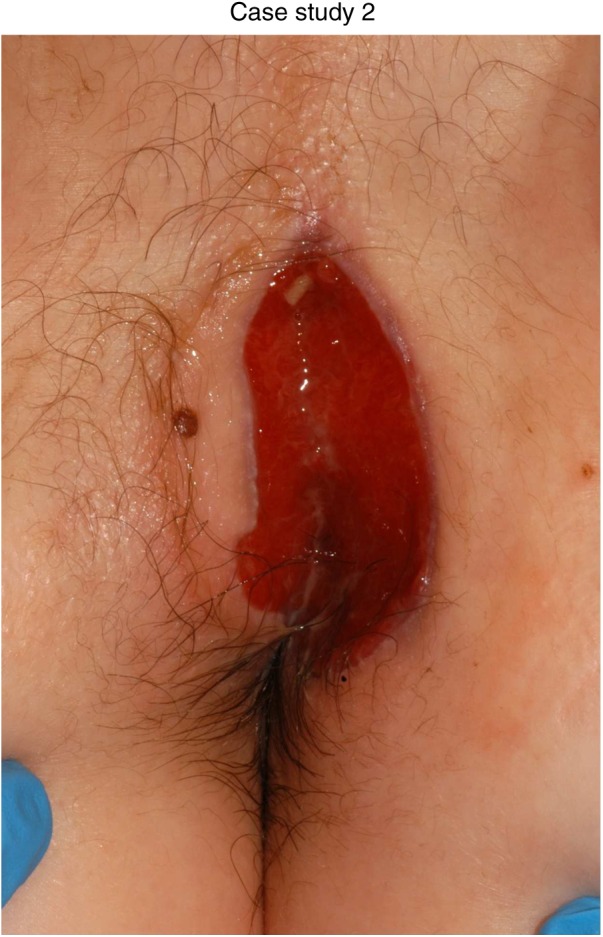
Seven months after diagnosis.Note the reduced distance from the mole to the tip of the wound in figures 5 and 6 when compared to figure 4. Note also, the reduction in oedema in figure 2.
Figure 6.
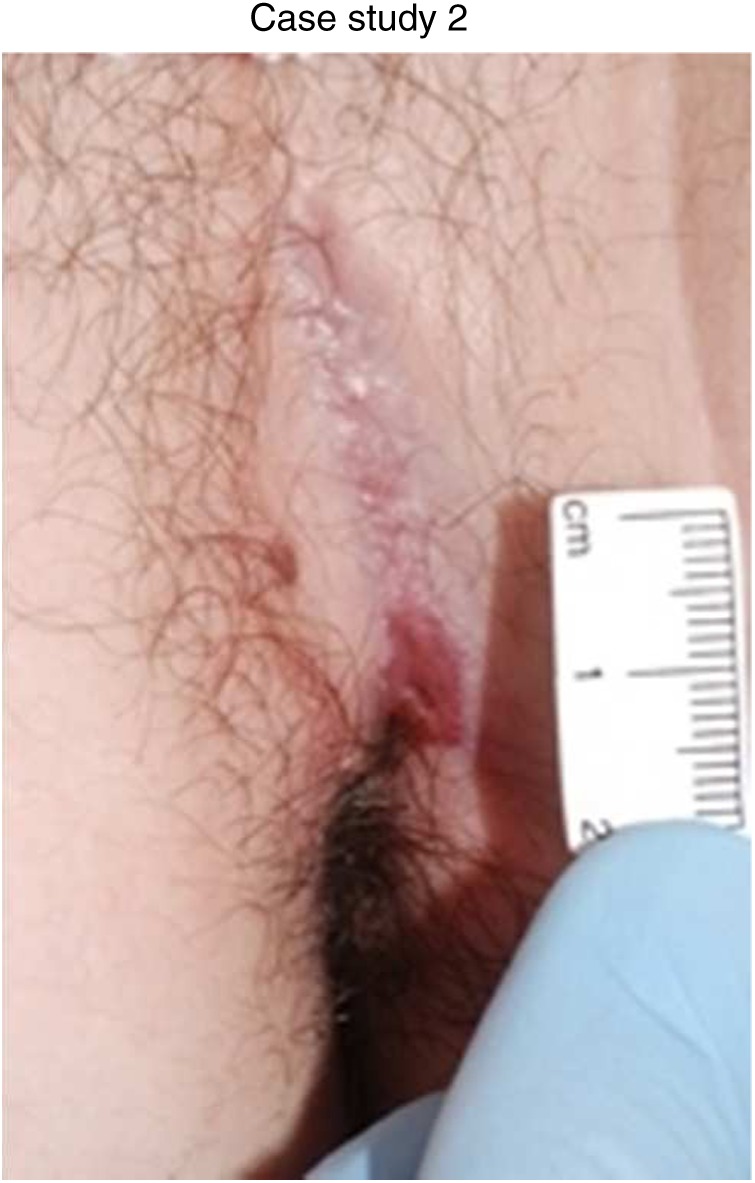
Eleven months after diagnosis: azathioprine at 2.5 mg/kg, infliximab at 5 mg/kg and continued topical wound management.
MRI at presentation demonstrated a complex left perianal fistula with a trans-sphincteric component, which crossed the external sphincter at the 2 o'clock position. There was extension of the fistula medially in an intersphincteric location, laterally into the left ischio-anal fossa and inferiorly into a large abscess cavity containing gas, which measured 4.2×2.2×3 cm in the left perineum.
Following incision and drainage, he attended the hospital three times per week and had his dressings changed using entonox as analgesia. In the initial stages as his wound was highly exudative, we used Acticoat Absorbant (Smith and Nephew) and Allevyn Thin (Smith and Nephew) as a secondary product to occlude the area.
As his wound progressed we used Allevyn Thin alone to help restore skin integrity. However, this wound was complicated by a high bacterial burden, which was demonstrated by repeated episodes of inflammation and overgranulation. In this case, we used Activon honey (Advancis Medical Ltd) to help redress the bacterial balance and treat the overgranulation. Melolite (Smith and Nephew) dressings held in place with Hypafix (3M) ensured the honey maintained contact with the wound surface in addition to keeping it contained in the dressing. Importantly, this allowed the patient to function and attend school despite having a significant perianal wound.
Again, he was encouraged to irrigate his wound with the showerhead twice daily and after every bowel motion when practical. Clinical experience in this area has demonstrated that irrigation is a most effective way of maintaining healthy tissue at the wound and on the surrounding skin. As previously stated, it also has the added benefit of involving the patient in their care and encouraging them to become more active in their recovery.
Surrounding skin integrity was maintained using Cavilon (3M), a non-sting barrier film product applied on alternate days.
Follow-up MRI demonstrated resolution of the abscess cavity and a simple left trans-sphincteric fistula with no ramification and significantly less contrast enhancement and adjacent inflammation.
For both cases, regular meetings with the surgical team facilitated visits to theatre as necessary for debridement of overgranulated tissue or further detailed wound re-assessment. These were coordinated by the IBD nurse specialist who maintained regular communications between the surgical and GI teams. This communication was further facilitated through formal weekly meetings at which the patients were discussed in a multidisciplinary forum, which included the nurse specialist. This MDT model of care was also enhanced through joint meetings with radiology, medical, surgical and nursing teams when both patients were reviewed in a virtual manner.
It is worth noting that despite regular hospital attendance and significant perianal wounds, both young men attended school and had time off only for dressing changes. Had this cohesive approach not been taken, it could be argued that they would both have found it very difficult to manage this while dealing with the symptoms of active perianal CD. Key principles in the practical management of perianal wounds are shown in supplementary box 2 (available online only).
Conclusion
Perianal CD is complex in its aetiology and challenging in its management. The relapsing and remitting nature of CD and the anatomical position of perianal CD makes management difficult both in terms of reducing bacterial burden and maintaining an optimal environment for healing. Despite several assessment and classification tools being available, none of them help guide clinical practice in terms of leading the clinician towards the most appropriate management strategy for individual patients.
Radiological, medical and surgical management has been shown to provide the best outcomes; however, the practical management of the perianal wound has not been addressed in the literature pertaining to IBD. The inclusion of wound management products in the treatment paradigm is likely to improve the ability of the patient in terms of daily functioning and, we believe, should therefore become an integral part of the overall treatment plan.
Furthermore, a cohesive approach involving medical, surgical, radiological and nursing staff is fundamental to achieving the best outcome for the patient with perianal CD. The use of objective, validated and standardised markers to define the most appropriate treatment option and when to use it would greatly benefit clinicians in the management of this complex condition.
Supplementary Material
Footnotes
Contributors: VG wrote the manuscript and all authors critically reviewed and amended the manuscript. All authors agreed on the final version.
Funding: The IBD team at Yorkhill Hospital Glasgow are supported by the Catherine McEwan Foundation and the Yorkhill IBD fund. RKR is supported by an NHS Research Scotland career fellowship award.
Competing interests: VG has received honorarium or travel expenses from MSD, Warner Chilcott, Ferring and Dr Falk. RKR has received consultation fees, research grants, or honorarium, from MSD, Abbott, Dr Falk, Ferring and Nestle. ES and GH have no conflicts of interest.
Ethics approval:
Patient consent: Obtained.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 2.Ingle SB, Loftus EV., Jr The natural history of perianal Crohn's disease. Dig Liver Dis 2007;39:963–9. [DOI] [PubMed] [Google Scholar]
- 3.Panes J, Gomollon F, Taxonera C, et al. Crohn's disease: a review of current treatment with a focus on biologics. Drugs 2007;67:2511–37. [DOI] [PubMed] [Google Scholar]
- 4.Ingle SB, Loftus J. The natural history of perianal Crohn's disease. Dig Liver Dis 2007;39:963–9. [DOI] [PubMed] [Google Scholar]
- 5.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohn’s and Colitis 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 6.Singh B, McC Mortensen NJ, Jewell DP, et al. Perianal Crohn's disease. Br J Surg 2004;91:801–14. [DOI] [PubMed] [Google Scholar]
- 7.Vermeire S, Van AG, Rutgeerts P. Perianal Crohn's disease: classification and clinical evaluation. Dig Liver Dis 2007;39:959–62. [DOI] [PubMed] [Google Scholar]
- 8.Safar MD, Sands D. Perianal Crohn's Disease. Clin Colon Rectal Surg 2007;20:282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, George BD, Mortensen NJ. Surgical therapy of perianal Crohn's disease. Dig Liver Dis 2007;39:988–92. [DOI] [PubMed] [Google Scholar]
- 10.Griggs L, Schwartz DA. Medical options for treating perianal Crohn's disease. Dig Liver Dis 2007;39:979–87. [DOI] [PubMed] [Google Scholar]
- 11.Tozer PJ, Whelan K, Phillips RK, et al. Etiology of perianal Crohn's disease: role of genetic, microbiological, and immunological factors. Inflamm Bowel Dis 2009;15:1591–8. [DOI] [PubMed] [Google Scholar]
- 12.Ng SC, Plamondon S, Gupta A, et al. Prospective assessment of the effect on quality of life of anti-tumour necrosis factor therapy for perineal Crohn's fistulas. Aliment Pharmacol Ther 2009;30:757–66. [DOI] [PubMed] [Google Scholar]
- 13.Siemanowski B, Regueiro M. Management of perianal fistula in Crohn's disease. Inflamm Bowel Dis 2008;14(Suppl. 2):S266–8. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P. Review article: treatment of perianal fistulizing Crohn's disease. Aliment Pharmacol Ther 2004;20(4 (Suppl.)):106–10. [DOI] [PubMed] [Google Scholar]
- 15.Hyder SA, Travis SP, Jewell DP, et al. Fistulating anal Crohn's disease: results of combined surgical and infliximab treatment. Dis Colon Rectum 2006;49:1837–41. [DOI] [PubMed] [Google Scholar]
- 16.Guidi L, Ratto C, Semeraro S, et al. Combined therapy with infliximab and seton drainage for perianal fistulizing Crohn's disease with anal endosonographic monitoring: a single-centre experience. Tech Coloproctol 2008;12:111–17. [DOI] [PubMed] [Google Scholar]
- 17.Tozer PJ, Burling D, Gupta A, et al. Review article: medical, surgical and radiological management of perianal Crohn's fistulas. Aliment Pharmacol Ther 2011;33:5–22. [DOI] [PubMed] [Google Scholar]
- 18.Ardizzone S, Maconi G, Cassinotti A, et al. Imaging of perianal Crohn's disease. Dig Liver Dis 2007;39:970–8. [DOI] [PubMed] [Google Scholar]
- 19.Thia KT, Mahadevan U, Feagan BG, et al. Ciprofloxacin or metronidazole for the yreatment of perianal fistulas in patients with Crohn's disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2009;15:17–24. [DOI] [PubMed] [Google Scholar]
- 20.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 21.Oussalah A, Danese S, Peyrin-Biroulet L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: a systematic review. Curr Drug Targets 2010;11:156–75. [DOI] [PubMed] [Google Scholar]
- 22.Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther 2010;32:1228–39. [DOI] [PubMed] [Google Scholar]
- 23.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 24.Genua JCM, Vivas DAM. Management of nonhealing perineal wounds. Clin Colon Rectal Surg 2007;20:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sciaudone G, Di Stazio C, Limongelli P, et al. Treatment of complex perianal fistulas in Crohn disease: infliximab, surgery or combined approach. Can J Surg 2010;53:299–304. [PMC free article] [PubMed] [Google Scholar]
- 26.Malik A, Nelson R. Surgical management of anal fistulae: a systematic review. Colorectal Dis 2008;10:420–30. [DOI] [PubMed] [Google Scholar]
- 27.Gaertner WB, Decanini A, Mellgren A, et al. Does infliximab infusion impact results of operative treatment for Crohn's perianal fistulas? Dis Colon Rectum 2007;50:1754–60. [DOI] [PubMed] [Google Scholar]
- 28.Talbot C, Sagar PM, Johnston MJ, et al. Infliximab in the surgical management of complex fistulating anal Crohn's disease. Colorectal Dis 2005;7:164–8. [DOI] [PubMed] [Google Scholar]
- 29.Stringer EE, Nicholson TJ, Armstrong D. Efficacy of topical metronidazole (10 percent) in the treatment of anorectal Crohn's disease. Dis Colon Rectum 2005;48:970–4. [DOI] [PubMed] [Google Scholar]
- 30. doi: 10.1002/bjs.7121. Maeda Y, NG SC, Durdey P, et al. Randomised clinical trail of metronidazole ointment versus placebo in perianal Crohn's disease. BR J Surg 2010;97:1340–7. [DOI] [PubMed] [Google Scholar]
- 31.Ardizzone S, Porro GB. Perianal Crohn's disease: overview. Dig Liver Dis 2007;39:957–8. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher J. Wound assessment and the TIME framework. Br J Nurs 2007;16:462–6. [DOI] [PubMed] [Google Scholar]
- 33.Okan DBMc, Woo KMRAG, Ayello EAP, et al. The role of moisture balance in wound healing. Adv Skin Wound Care 2007;20:39–53. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez R, Griffiths R. Water for wound cleansing. Update of Cochrane Database Syst Rev 2002;(4):CD003861; Cochrane Database Syst Rev 2008;(1):CD003861. [DOI] [PubMed] [Google Scholar]
- 35.Moore Z, Cowman S. A systematic review of wound cleansing for pressure ulcers. J Clin Nurs 2008;17:1963–72. [DOI] [PubMed] [Google Scholar]
- 36.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 37.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 38.Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2008;(4):CD005083. [DOI] [PubMed] [Google Scholar]
- 39.Ousey K, McIntosh C. Topical antimicrobial agents for the treatment of chronic wounds. Br J Community Nurs 2009;14:S6–8. [DOI] [PubMed] [Google Scholar]
- 40. doi: 10.12968/bjon.2008.17.Sup9.31659. Slater M. Does moist wound healing influence the rate of infection? British Journal of Nursing 2008;17(20):S4–S15. [DOI] [PubMed] [Google Scholar]
- 41.Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49:1541–9. [DOI] [PubMed] [Google Scholar]
- 42.Cooper R, Jenkins L, Rowlands R. Inhibition of biofilms through the use of manuka honey. Wounds UK 2011;7: 24–32. [Google Scholar]
- 43.Sharp A. Beneficial effects of honey dressings in wound management. Nurs Stand 2009;24:66–8; 70; 72; 74. [DOI] [PubMed] [Google Scholar]
- 44.Lo SF, Chang CJ, Hu WY, et al. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs 2009;18:716–28. [DOI] [PubMed] [Google Scholar]
- 45.Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds 2006;5:40–54. [DOI] [PubMed] [Google Scholar]
- 46.McCartney S. Inflammatory bowel disease in transition: challenges and solutions in adolescent care. Frontline Gastroenterol 2011;2:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodhand JM, Dawson RM, Hefferon MM, et al. Inflammatory bowel disease in young people: the case for transitional clinics. Inflamm Bowel Dis 2010;16:947–52. [DOI] [PubMed] [Google Scholar]
- 48.Irvine EJ. Usual therapy improves perianal Crohn's disiease as measured by a new disease activity index. McMaster IBD Study Group. Journal of Clinical Gastroenterology 1995;1(20); 27–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


