Abstract
Background
ESO-2 video capsule endoscopy provides images of the oesophageal mucosa and continues to transmit gastric, and often small bowel images, for up to 30 min. This study compares ESO capsule endoscopy capsule oesophago-gastro-duodenoscopy (Cap-OGD) with conventional endoscopy (OGD).
Methods
50 outpatients with uncomplicated dyspepsia underwent Cap-OGD followed by OGD which was recorded on DVD. Cap-OGD and OGD were each reported independently by two gastroenterologists. A benchmark report was also produced by two gastroenterologists viewing both Cap-OGD and OGD on side-by-side monitors. Major findings included large hiatus hernia, Barrett's oesophagus, oesophagitis, erosive gastritis, tumour and ulceration. Minor findings included histologically-proven superficial gastritis, pouting gastric folds and fundic gland polyps. A questionnaire assessed the patient experience.
Results
49 patients completed the study. In 61%, Cap-OGD transmitted in the duodenum. In the benchmark study, all the major OGD findings were observed on Cap-OGD. Cap-OGD revealed fewer minor findings. When reported independently, Cap-OGD and OGD reports indicated differences in interpretation most marked between the capsule readers with or without previous ESO-2 experience. Patients expressed a clear preference for Cap-OGD.
Conclusions
When compared side-by-side, all the major findings on OGD are seen on Cap-OGD while there is under-reporting of minor findings. Previous experience of ESO-2 capsule reporting improves reading accuracy and indicates the need for training. This pilot study provides a backdrop to explore the possible role of Cap-OGD, especially where patients are reluctant to undergo conventional OGD or where there is risk of prion contamination of the endoscope.
Keywords: Endoscopy, Dyspepsia
Introduction
The fibre-optic endoscope made it possible to deliver light and capture images of the gastrointestinal tract. This allows precise tip control, air inflation and fluid suction to clear the visual field, in addition to both diagnostic and therapeutic intervention. Because intubation is distressing, patients require either topical anaesthesia or intravenous sedation.
Video capsule endoscopy represents an evolutionary shift in gastrointestinal imaging. The miniaturisation of a light emitting diode, a miniature battery, tiny optical lenses and wireless data transmission have freed bowel imaging from connections to the outside world.
The PillCam ESO capsule (Given Imaging, Yoqneam, Israel) has been designed to image the oesophagus. This has a fore and aft facing camera, providing a 312° field of vision at a frame rate of 14 frames per second.1 Studies have revealed that oesophageal capsule endoscopy is a highly accurate method for the diagnosis of oesophageal pathologies.2 Although the capsule continues to transmit images of the stomach and, often, the proximal small intestine, there has been little interest in the images transmitted during the gastric residence period. This is because of a perception that air insufflations and some form of guidance system are necessary to inspect the gastric mucosa.
Gastroscopy is performed in the fasted state and endoscopists are used to examining a near motionless stomach. This is unlike the gastric phase of capsule endoscopy when, following ingestion of the capsule with water, the stomach becomes motile and the capsule triturates moving up and down within the stomach, thus transmitting good quality images of the gastric mucosa.
The aim of this study was to compare images obtained at same day video capsule oesophago-gastro-duodenoscopy (Cap-OGD) and conventional OGD in outpatients presenting with uncomplicated heartburn or dyspepsia.
Patients and methods
A total of 50 outpatients, patients aged 18 and older, were invited to participate in the study which had been approved by the hospital ethics committee. Admission to the study was restricted to patients presenting with heartburn or dyspepsia defined by the Rome III criteria.3 Patients with alarm symptoms including dysphagia, vomiting, haematemesis or melena, weight loss and iron deficiency were excluded. In addition, patients with previous history of bowel surgery, Crohn's disease and symptoms of bowel obstruction (vomiting and severe cramps) were excluded to avoid the risk of capsule retention.
After an overnight fast, each patient first underwent a Cap-OGD followed within 3 h by OGD. The first 25 patients swallowed the ESO capsule in accordance with the simplified ingestion protocol4 (SIP Group). The second group of 25 patients swallowed the capsule while standing (Standing Group) and remained upright and walking at their leisure for the full 30 min duration of the test.
Immediately following the Cap-OGD, the data were downloaded, read using Rapid 5 software and reported by a capsule reader (Cap-OGD1), who had reported over 200 small bowel capsule endoscopies, but had no prior experience of ESO capsule.
According to the patient's preference, the OGDs were performed with either topical anaesthesia or intravenous midazolam. The OGDs were performed by an experienced endoscopist (OGD1) who was unaware of the Cap-OGD1 findings. A DVD of each endoscopy was recorded for later review. On discharge, patients were asked to complete a questionnaire on tolerability and acceptability of Cap-OGD and OGD, including a Lickerd analogue scale.
On completion of the first Cap-OGD and OGD reporting, a second capsule reader (Cap-OGD2), who had experience of reporting 12 ESO capsule endoscopies, rereported the capsule studies without knowledge of the Cap-OGD1 report. Similarly, a second endoscopist (OGD2) used the DVD recording to rereport the OGDs.
At a third and final read, a benchmark report was agreed by two gastroenterologists viewing the Cap-OGD and OGD images in tandem: rapid 5 and a DVD player, connected to side-by-side monitors, were used to run each patient's capsule and OGD in close anatomical synchrony with the possibility of using slow motion or frame-by frame view.
For the purpose of this study, pathological findings were categorised as ‘major’ or ‘minor’. Major diagnoses included Barrett's oesophagus (figure 1), large hiatus hernia characterised by the presence of an obvious hernial sac, oesophagitis (at least one linear erosion) (figure 2), erosive gastritis (figure 3), peptic ulcer (figure 4) and tumours. Minor diagnoses included erythema with intact mucosa, small hiatus hernia defined as pouting gastric folds but no hernial sac, fundic gland polyps (figure 5) and Schatzki ring (figure 6).
Figure 1.
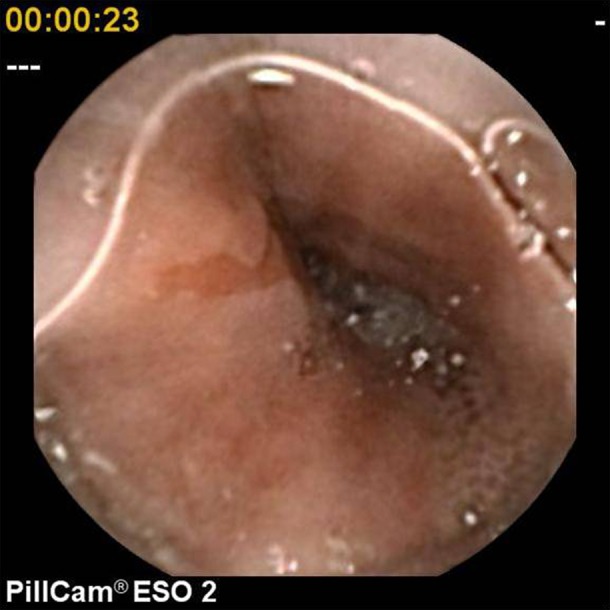
Barrett's oesophagus.
Figure 2.
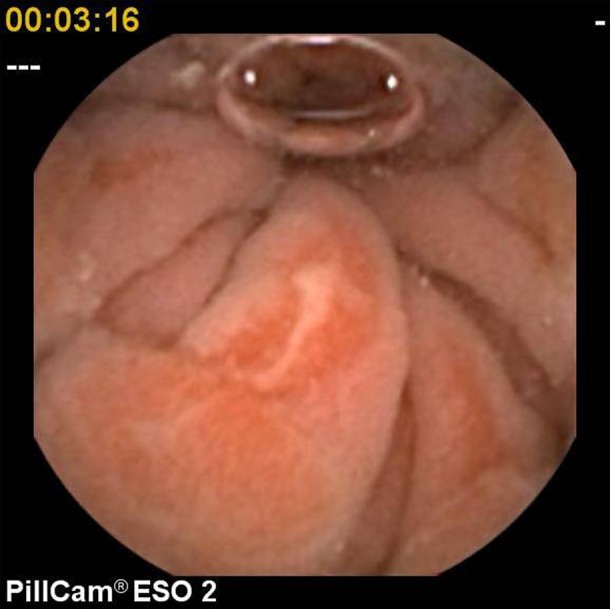
Oesophagitis.
Figure 3.
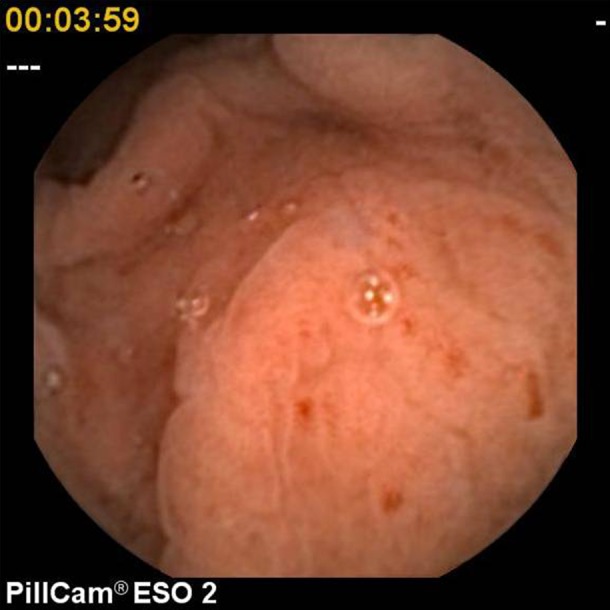
Erosive gastritis.
Figure 4.
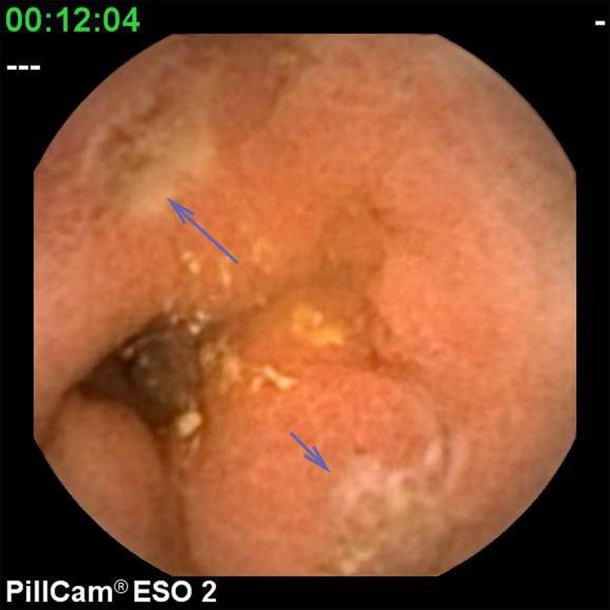
Duodenal ulcers.
Figure 5.

Fundic gland polyp.
Figure 6.
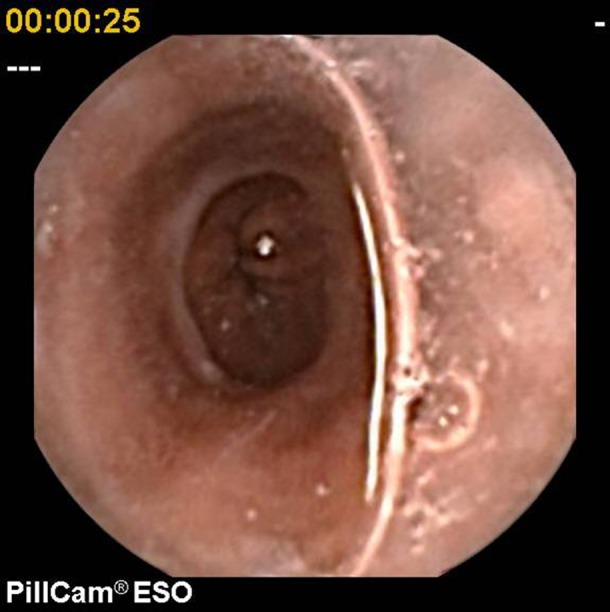
Schatzki ring.
Results
A total of 50 patients (17 male and 33 female subjects) aged 18–80 (mean age 56) agreed to participate in the prospective study. A single patient was unable to swallow the capsule. In the OGD arm of the study, 35 patients chose topical anaesthesia and 15 intravenous sedation.
Cap-OGD transit times
The SIP Group demonstrated a significantly longer oesophageal transit time (median 14 s, 95% CI 4 to 86) than the Standing Group (median 3 s, 95% CI 3 to 8; p<0.0001). Cap-OGD gastric transit time was similar in the SIP and Standing Groups with a median gastric transit of 10 min (95% CI 8 to 18) for the SIP Group and 12 min (95% CI 9 to 17) for the Standing Group. In 30 of 49 Cap-OGDs (61%), the capsule was still transmitting when entering the duodenum. This was similar for the SIP and Standing Groups.
Benchmark results
On joint reporting of the 49 capsule and conventional OGDs viewed in parallel, 17 patients had one or more major pathologies readily identifiable on both Cap-OGD and OGD (tables 1 and 2). In 16 patients, one or more minor pathologies were identified on Cap-OGD and/or OGD (tables 1 and 2). When comparing Cap-OGD and OGD side-by side, only two of the 12 small hiatus hernia seen on OGD were evident on Cap-OGD. Of 13 patients with mucosal erythema viewed on OGD, seven were judged to have erythema on Cap-OGD. Fundic gland polyps were seen on OGD in four patients, two of whom had fundic polyps seen on the Cap-OGD. In a further patient, fundic polyps were seen on Cap-OGD, but not on OGD.
Table 1.
Benchmark findings versus OGD1 and OGD2 reports
| Pathology | Benchmark | OGD1 | OGD2 |
|---|---|---|---|
| Barrett's oesophagus | 6 | 4 | 6 |
| Peptic ulcer | 1 | 0 | 1 |
| Oesophagitis | 5 | 5 | 5 |
| Erosive gastritis | 4 | 4 | 4 |
| Large HH | 4 | 4 | 4 |
| Submucosal lesion | 1 | 1 | 1 |
| Superficial erythema | 13 | 26 | 20 |
| Schatzki ring | 1 | 0 | 0 |
| Small HH | 12 | 13 | 14 |
| Fundic polyps | 5 | 4 | 4 |
HH, hiatus hernia; OGD, oesophago-gastro-duodenoscopy.
Table 2.
Benchmark findings versus Cap-OGD1 and Cap-OGD2 reports
| Pathology | Benchmark | Cap-OGD1 | Cap-OGD2 |
|---|---|---|---|
| Barrett's oesophagus | 6 | 4 | 6 |
| Peptic ulcer | 1 | 1 | 1 |
| Oesophagitis | 5 | 4 | 4 |
| Erosive gastritis | 4 | 3 | 4 |
| Large HH | 4 | 1 | 4 |
| Submucosal lesion | 1 | 1 | 1 |
| Superficial erythema | 13 | 9 | 13 |
| Schatzki ring | 1 | 1 | 1 |
| Small HH | 12 | 2 | 2 |
| Fundic polyps | 5 | 2 | 2 |
HH, hiatus hernia; Cap-OGD, capsule oesophago-gastro-duodenoscopy.
Benchmark findings versus OGD1 and OGD2 reports
The major and minor pathologies reported by OGD1 and OGD2 are summarised in table 1.
When compared with the benchmark study, OGD1 failed to report two patients with Barrett's oesophagus and one pyloric channel ulcer. OGD2 reported all the major pathologies seen on the benchmark report. The diagnosis of Barrett's oesophagus was confirmed on histology in the four cases identified in OGD1 since the endoscopist took biopsy; the other two cases reported by OGD2 were diagnosed based only on the benchmark report since histology was not available.
OGD1 reported 26 superficial mucosal erythema. Histology was available in 21, and inflammation was present in 11 (52%). OGD2 reported mucosal erythema in 20 patients. Histology was available in 17 and inflammation confirmed in 11 (65%) patients. Two patients with normal gastric mucosa reported by both OGD1 and OGD2 had histological gastritis. OGD1 and OGD2 reported pouting gastric folds in 13 and 14 patients, respectively, compared with 12 diagnosed on benchmark imaging. Both OGD1 and OGD2 reported four patients with fundic polyps compared with five in the benchmark study. A Schatzki ring observed in the benchmark study was not reported by either OGD1 or OGD2.
Benchmark findings versus Cap-OGD1 and Cap-OGD2
The reported major and minor pathologies seen by Cap-OGD1 and Cap-OGD2 are summarised in table 2. Compared with the benchmark, Cap-OGD1 failed to recognise three large hiatus hernias, two patients with Barrett's oesophagus, one oesophagitis and one erosive gastritis. Cap-OGD2 failed to report only a single finding of oesophagitis.
Cap-OGD1 reported nine patients with superficial mucosal erythema. Histology was available in eight and inflammation was confirmed in 5 (63%) patients. Cap-OGD2 reported 13 superficial erythema. Histology was available in 11 and inflammation confirmed in 7 (64%) patients. Cap-OGD1 and Cap-OGD2 both reported pouting gastric folds in two patients, compared with 12 reported on benchmark OGD. Both Cap-OGD1 and Cap-OGD2 recognised a Schatzki ring that was not reported by OGD1 or OGD2. Both Cap-OGD1 and Cap-OGD2 reported only two of five cases with fundic gland polyps.
Patient acceptability of Cap-OGD versus OGD
The self-reported questionnaire indicated that Cap-OGD was well-tolerated by 94% of the patients. OGD was well-tolerated by only 28% of patients, 51% tolerated it rather badly and 21% badly. Cap-OGD was considered acceptable in 96% and 4% rated it acceptable but uncomfortable. OGD was considered acceptable in only 34%, 62% rated it acceptable but uncomfortable and 4% indicating that they would not undergo the procedure again. Median Lickerd scores for patient discomfort was 0 for Cap-OGD and 4.5 for OGD (0=no discomfort; 10=unbearable).
Discussion
This prospective study indicates that when compared with conventional OGD, ESO 2 capsule transmits useful images of the oesophageal, gastric and, in 61% of examinations, duodenal mucosa. By viewing the Cap-OGD and OGD images on side-by-side monitors, two gastroenterologists reporting in tandem could carefully correlate and aggregate pathologies seen on one or both imaging modalities.
In the benchmark study, all the major pathology seen on OGD was visible on Cap-OGD. There were, however, differences in the minor pathologies. When viewed side-by-side, OGD revealed 12 patients with small hiatus hernia, while in only two of the 12 (16%) was this visualised on Cap-OGD. Difference in pressure and posture might explain the difference. During OGD, the discomfort and involuntary retching that often occur during intubation and examination cause raised intra-abdominal pressure and this, in turn, might encourage a short segment of proximal stomach to herniate through the oesophageal hiatus. These forces do not occur with Cap-OGD. In addition, patients are positioned differently for Cap-OGD and OGD.
The benchmarking study revealed mucosal erythema in 13 OGDs but only in seven was this apparent on Cap-OGD. OGD provides higher contrast image and projects a deeper red colour compared with Cap-OGD. This difference in colour setting may account for discrepancy in interpretation of mucosal erythema. In addition, the histology from patients reported to have superficial mucosal erythema confirmed the well recognised overdiagnosis of gastritis and duodenitis when judged by colour.5 6
Fundic gland polyps are readily recognisable small benign sessile lesions, identical in colour to the surrounding gastric mucosa. Cap-OGD identified only two of five fundic gland polyps reported in the benchmark study, while OGD missed only one. This is probably because inflation of the stomach, which occurs only during OGD, allows separation of the gastric folds and visualisation of these small lesions.
Using the benchmark study for comparison, individual reporting of Cap-OGD and OGD revealed some inconsistency in recognising both major and minor pathologies. Cap-OGD1, who had no previous ESO capsule experience, failed to recognise three large hiatus hernias, one patient with oesophagitis, two with short segment Barrett's oesophagus and one with erosive gastritis. All the major pathologies except for a single case of oesophagitis were, however, recognised by Cap-OGD2 who, unlike Cap-OGD1, had previous experience reporting ESO capsule endoscopy. This probably reflects a learning curve for ESO capsule image recognition. In the side-by-side study it was clear that recognising a hiatus hernia on capsule endoscopy does not have the advantage of using diaphragmatic movement, instrument control and retroflexion to determine the anatomical landmarks. On Cap-OGD, the neck of the prolapsing hernial sac is not readily identified; however, a large hernia is characterised by a temporary holdup after passing the z-line, a distinctive downstream narrowing reflecting the neck of the hernial sac, and gastric folds visible proximal and distal to this point.
When comparing OGD with benchmark findings, OGD1 failed to recognise a pyloric channel ulcer that was seen by OGD2 who had the benefit of viewing the image on DVD, where suspicious areas can be reviewed in slow motion or frame-by-frame. The lesion miss rate on conventional OGD is well recognised.7–11
This study confirms the feasibility of adapting ESO capsule endoscopy for examination of the oesophagus and stomach, and in 61% of patients, of the proximal duodenum. Oesophageal transit time was longer in the SIP compared with the Standing Group, but there was no difference in the gastric transit time or the frequency with which the capsule was seen to pass through into the duodenum. Gastric transit time is readily measured on capsule endoscopy and it is possible that this procedure could offer some physiological as well as imaging information. The visualisation of the gastric mucosa appears to depend more on peristalsis than patient position change. These findings suggest that for ESO Cap-OGD, the initial ingestion should follow the SIP procedure, but after a few minutes in the right lateral decubitus position, patients should be free to stand up and walk about.
As expected, patients indicated a clear preference for Cap-OGD. However, the random movement of the capsule in the stomach raises the possibility of missed regions, and biopsy and endotherapy cannot be applied. Currently, the battery life of the ESO capsule is insufficient to guarantee images of the duodenum, although this could be achieved by extending battery life or administering a prokinetic.
The potential for probing the foregut with capsule gastroscopy was also indicated in the retrospective small bowel capsule endoscopy (SBCE) study of patients with previously normal OGD and colonoscopy. In these patients, a likely source of bleeding was detected proximal to the second part of the duodenum in 12.9% of patients. These findings strengthen the case that capsule gastroscopy can illuminate a wide range of potentially important gastric mucosal findings.
Conclusions
The benchmark study demonstrates that in patients presenting with dyspepsia and no alarm symptoms, ESO capsule endoscopy reliably recognises a normal gastric mucosa as well as the major, and most of the minor, pathologies seen on conventional OGD. For both capsule and conventional OGD, reports vary when different individuals observe the same images and endoscopy training should focus on image recognition as well as technical skills. By extending transmission time, ESO capsule endoscopy could offer an alternative choice for investigating dyspepsia uncomplicated by alarm features, where the need to biopsy is less likely than complicated dyspepsia. ESO capsule gastroscopy might also be considered when patients are intolerant of conventional OGD or where there is a risk of prion contamination of the endoscope. A small number of patients will require follow on conventional endoscopy to obtain targeted histology or clarify uncertainty. This ESO capsule study provides a backdrop for further evaluation of capsule endoscopy for imaging the mucosa of the oesophagus, stomach and duodenum.
What is already known on this topic.
ESO capsule endoscopy is a highly accurate method for the diagnosis of oesophageal pathologies
What this study adds.
ESO capsule endoscopy reliably recognises normal gastric mucosa as well as the major, and most of the minor pathologies seen on conventional upper endoscopy (OGD) in patients presenting with dyspepsia
How might it impact on clinical practice in the foreseeable future.
ESO capsule endoscopy could offer an alternative choice to conventional OGD for investigating dyspepsia, especially in patients with cardiac or respiratory co-morbidity, those reluctant to undergo OGD or where there is risk of prion contamination of the endoscope
Footnotes
Contributors: LM: study concept and design, acquisition, analysis and interpretation of data, drafting the article, final approval of the version published. FMJ, LJ and HP: acquisition of data. GE and MH: critical revision of the manuscript for important intellectual content. OE: study concept and design, critical revision of the manuscript for important intellectual content, final approval of the version published.
Financial support: Registration fee for the British Society of Gastroenterology (BSG) Annual Meeting 2011 paid by DiagMed for the first author and one coauthor (Linda Jackson). No other financial support given.
Funding: None.
Competing interests: None.
Ethics approval: Chair Moorfields & amp; Whiittington Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.http://www.givenimaging.com/en-int/HealthCareProfessionals/Products/Pages/PillCamESO.aspx (accessed 30 November 2012).
- 2.Sharma VK, Eliakim R, Sharma P, et al. ICCE consensus for esophageal capsule endoscopy. Endoscopy 2005;37:1060–4. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006;131;336. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Rabinovitz R, Afik D, et al. A simplified ingestion procedure for esophageal capsule endoscopy: initial evaluation in healthy volunteers. Endoscopy 2006;38:913–8. [DOI] [PubMed] [Google Scholar]
- 5.Sauerbruch T, Schreiber MA, Schussler P, et al. Endoscopy in the diagnosis of gastritis. Diagnostic value of endoscopic criteria in relation to histological diagnosis. Endoscopy 1984;16:101–4. [DOI] [PubMed] [Google Scholar]
- 6.Laine L, Cohen H, Sloane R, et al. Interobserver agreement and predictive value of endoscopic findings for H pylori and gastritis in normal volunteers. GIE 1995;42:420–3. [DOI] [PubMed] [Google Scholar]
- 7.Zaman A, Katon RM. Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointest Endosc 1998;47:372–6. [DOI] [PubMed] [Google Scholar]
- 8.Descamps C, Schmit A, Van Gossum A. “Missed” upper gastrointestinal tract lesions may explain “occult” bleeding. Endoscopy 1999;31:452–5. [DOI] [PubMed] [Google Scholar]
- 9.Fry LC, Bellutti M, Neumann P, et al. Incidence of bleeding lesions within reach of conventional upper and lower endoscopes in patients undergoing double-balloon enteroscopy for obscure gastrointestinal bleeding. Aliment Pharmacol Ther 2009;29:342–9. (24.3% upper and lower GI) [DOI] [PubMed] [Google Scholar]
- 10.Kitivara T, Selby W. Non-small-bowel lesions detected by capsule endoscopy in patients with obscure GI bleeding. GIE 2005;62:234–8. [DOI] [PubMed] [Google Scholar]
- 11.Vlachogiannakos J, Papaxoinis K, Viazis N, et al. Bleeding lesions within reach of conventional endoscopy in capsule endoscopy examinations for obscure gastrointestinal bleeding: is repeating endoscopy economically feasible? Dig Dis Sci 2011;56:1763–8. [DOI] [PubMed] [Google Scholar]


