Abstract
Guidelines published by the British Society of Gastroenterology have standardised the care of patients attending for endoscopy procedures, taking aspirin, clopidogrel and warfarin.
Two new oral anticoagulant drugs, Rivaroxaban and Dabigatran, are licensed and National Institute of Clinical Excellence (NICE) approved for use in the UK. Unlike warfarin, these drugs do not require regular monitoring, and are at least as effective in preventing stroke in non-valvular atrial fibrillation. As such, they are likely to become popular among patients and clinicians alike. This paper summarises the practical management of patients taking these drugs attending for endoscopic procedures.
Keywords: Endoscopy, Bleeding
Introduction
Dabigatran works by directly inhibiting (both free and bound) thrombin.1 It is licensed in the UK for the prevention of stroke in patients with non-valvular atrial fibrillation (AF) with one or more risk factors (including previous stroke or Transient ischaemic attack (TIA), New York Heart Association (NYHA) class II or, worse, heart failure, left ventricular (LV) ejection fraction less than 40%, age over 75 years, and those aged over 65 years who have diabetes, hypertension or ischaemic heart disease).2 At the standard dosing regimen of 150 mg twice daily, the RE-LY study3 demonstrated lower risk of stroke compared with warfarin. It is recommended that patients over the age of 80 years, or who are at particular risk of haemorrhage, receive a lower dose of 110 mg twice daily which has equivalent efficacy in stroke prevention compared with warfarin, but lower bleeding risk. It is also licensed for the prevention of deep vein thrombosis (DVT) and pulmonary emboli (PE) in patients who have undergone hip or knee replacement.4
The prodrug, dabigatran etexilate, is mainly converted to the active substrate in the liver. Unlike warfarin, however, this conversion does not involve the cytochrome p450 system, thus reducing the potential for drug interactions. Its half-life is between 14 and 17 h. As the drug is eliminated by the kidneys, the dose must be reduced in renal impairment,5 and it should be avoided altogether if the creatinine clearance is <30ml/min. In most cases, the estimated glomerular filtration rate, eGFR is quoted by most laboratories, and can be used as a surrogate for creatinine clearance6 in the majority of patients, and has been used throughout this article.
Rivaroxaban, a direct factor Xa inhibitor is licensed for the treatment of DVT, PE and for preventing recurrent DVT and PE in patients with a previous DVT,7 for the prevention of venous thromboembolism (VTE) in patients undergoing elective hip and knee replacements,8 9 and for use in the prevention of stroke in patients with non-valvular AF10 with one or more risk factors for stroke as above. The Rocket AF study demonstrated non-inferiority compared with warfarin for the primary endpoint of stroke prevention.11
The half-life of rivaroxaban is short at 4–9 h, but this may be prolonged in the elderly. In addition, the drug is also excreted renally and, therefore, should be avoided if the eGFR<30 units. It is administered once daily at a dose of 20 mg for patients in AF (15 mg if creatinine clearance is less than 50 ml/min). In the treatment of VTE the standard dose is 15 mg twice daily for 3 weeks, followed by 20 mg once daily thereafter.8
Apixaban, like rivaroxaban is a direct factor X inhibitor and is licensed for thromboprophylaxis following hip and knee replacement. The Aristotle study12 demonstrated effectiveness in stroke prevention for patients with AF and although not a licence for this indication has recently been given. It has a half-life of around 10–14 h and is partially metabolised in the liver by CYP 3A4/5 and partially excreted by the kidneys. It should be avoided in patients with an eGFR<30 units and used with caution in those with hepatic impairment.
Unlike warfarin, the new anticoagulant drugs do not require routine monitoring. They also have fewer drug and dietary interactions than warfarin, and have a more predictable dose response. Because of their short half-lives, these drugs can usually be stopped 24 h prior to endoscopic procedures (if the renal function is normal), and in those considered low risk, can be restarted 6–8 h afterwards, reducing the time that patients spend off anticoagulants (and therefore potentially reducing the associated risks), while also saving on the need for patients to administer clexane therapy preprocedure and postprocedure for those perceived to be at highest risk of procedure-related thrombosis.
There is little published evidence available to guide clinicians on the best management for patients taking these drugs undergoing interventional procedures such as those performed by gastroenterologists. The guidance given in this article has, therefore, had to adopt a pragmatic approach reflecting the opinion of the authors based on very limited data.
Side effects/interactions of the new oral anticoagulants
Dabigatran interacts with drugs that act on the transporter P-glycoprotein as this transporter is responsible for uptake of the drug across the gut wall. Quinidine inhibits the p-glycoprotein and should be used with caution in patients taking dabigatran. Other p-glycoprotein inhibitors include ketoconazole, amiodarone and verapamil. St John's Wort and rifampicin induce the P-glycoprotein and should also be avoided.8 Drugs which may interact with rivaroxaban include the azole antimycotics and HIV protease inhibitors, St Johns Wort, rifampicin and dronaderone. This list is not exhaustive, and prescribers are referred to the specialty product certificate (SPC) for full listings of the interactions.
Common side effects include dyspepsia, vomiting, change in bowel habit, deranged liver function tests, anaemia and bleeding. Non-bleeding upper gastrointestinal events in patients taking dabigatran in the RE-LY study13 were almost twice as common as those taking warfarin, but most were described as mild-moderate with only 4% of patients having to discontinue the medication. The RE-LY study3 also highlighted the risk of gastrointestinal haemorrhage in patients taking dabigatran in a dose of 150 mg was higher than in those patients taking warfarin, but there was no increased risk for patients taking 110 mg. Those at greatest risk were more than 75 years old who were also taking aspirin, clopidogrel or both. Rivaroxaban therapy is also associated with a higher incidence of gastrointestinal (GI) haemorrhage as compared with warfarin, but a lower risk of intracranial haemorrhage.11 As a result, patients taking these medications may more frequently be referred for endoscopy.
Because of the potential for increased risk of bleeding, these drugs should be used with caution in combination with antiplatelet drugs, such as aspirin and clopidogrel.8 14
Although these new drugs appear at first much more convenient than warfarin given the lack of need for monitoring, unlike warfarin they cannot be easily reversed with vitamin K or prothrombin factor concentrate if the patient bleeds during or after their procedure. Measurement of the INR cannot be used to assess the risk of bleeding, nor is it useful in the monitoring of the anticoagulant effects of rivaroxaban or dabigatran and should not be used to monitor or measure their effects. For dabigatran, the APTT (activated partial thromboplastin time), diluted TT (thrombin time) and ecarin clotting time 15 can be used to measure the anticoagulant effect of the drug. However, as the ecarin test is not widely available, the most useful tests are likely to be the APTT and the TT. For rivaroxaban the prothrombin time (PT) may provide a useful measure of the drug effect, as will a modified anti-Xa assay. Like the ecarin clotting time, this is unlikely to be routinely available in most laboratories. Routine measurement of clotting studies is not required in patients taking rivaroxaban and dabigatran if their renal function is normal. However, their effects can be measured to assess likelihood of bleeding at the time of surgery, or assess whether the anticoagulant effect is ongoing in patients with gastrointestinal haemorrhage referred for emergency endoscopy.
Pre-endoscopy management
Endoscopic procedures with a low risk of bleeding1 are summarised in table 1. While it is safe for patients on warfarin to have biopsy samples taken without the need to discontinue the drug (assuming the INR is within the therapeutic range), with no reports of uncontrolled bleeding following biopsy over more than a decade,1 there is currently no evidence to suggest that this policy can safely be adopted for patients taking rivaroxaban and dabigatran. Although pinch biopsies at endoscopy would be classed as being associated with a low risk of bleeding, until further evidence is forthcoming we recommend that patients should take the last dose 24 h pre-endoscopy, and restart treatment 6–8 h postprocedure. This policy means that patients are without anticoagulation for only a short period of time, and because of their rapid onset of action, patients will be fully anticoagulated within a few hours of restarting treatment.
Table 1.
The risk of bleeding based on type of endoscopic procedure
| Low risk procedures | High risk procedures |
|---|---|
| Diagnostic OGD ± biopsy | Polypectomy |
| Diagnostic colonoscopy ± biopsy | ERCP with sphincterotomy |
| Biliary stenting | Endoscopic mucosal resection (EMR) |
| Pancreatic stenting | EUS with FNA |
| Diagnostic EUS | PEG insertion |
| Variceal banding | |
| Stricture dilatation |
ERCP, Endoscopic retrograde cholangiopancreatography; EUS, Endoscopic ultrasound; FNA, Fine needle aspiration; OGD, Oesophagogastroduodenoscopy; PEG, Percutaneous endoscopic gastrostomy.
In the RE-LY3 study, a total of 4591 patients underwent interventional procedures including 8.6% undergoing colonoscopy. Healey16 and Garcia et al17 have summarised the outcome of these patients (although they did not specifically report on the cohort undergoing endoscopy procedures). In those patients attending for elective procedures, the last dose of dabigatran had been given a mean of 49 h (range 35–85) prior to the procedure, compared with 114 h (range 87–144) for those taking warfarin. No significant difference in procedural bleeding rates were seen with either dose of dabigatran when compared with warfarin. Ischaemic stroke or VTE occurred in 0.5%, but the incidence of periprocedural bleeding was up to 5.1%, that is an eightfold increased risk of major bleeding episodes than stroke for both dabigatran and warfarin,17 even though only 28.5% of patients on warfarin and 16% taking dabigatran received bridging anticoagulation with low molecular weight heparin.
For patients requiring urgent surgery, major bleeding requiring transfusion (± other supportive measures) occurred in 17.8% and 17.7% for patients taking dabigatran 110 mg and 150 mg compared with 21.6% for those taking warfarin.16
The British Society of Gastroenterology guidelines1 recommend bridging anticoagulation in patients deemed to be high risk for recurrent VTE or stroke although there are no randomised trials that provide direct evidence for this approach. The results from the PERIOP-218 and Bridge19 studies when available, may provide helpful advice to clarify management further, although these studies did not examine the role of the newer oral anticoagulants for bridging.
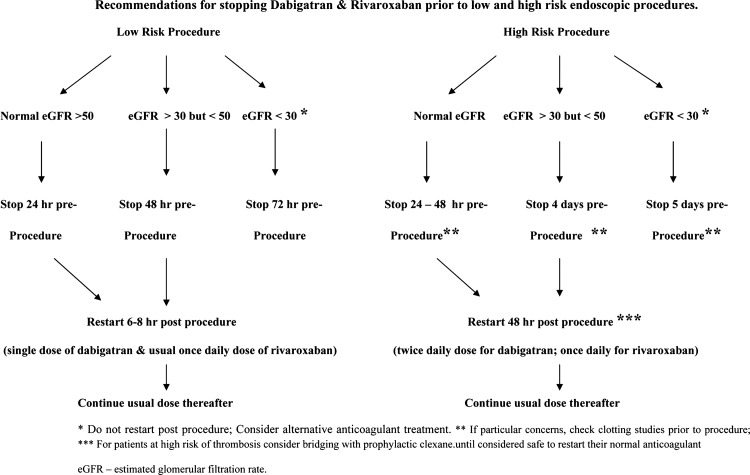
Figure 1 summarises the advice on management of these drugs prior to low-risk and high-risk endoscopic procedures with respect to risk of bleeding. Patients should have had an assessment of renal function prior to starting them, but if there is any clinical reason to believe this might have changed, a repeat should be arranged preprocedure.
Figure 1.
Recommendations for stopping Dabigatran and Rivaroxaban prior to low-risk and high-risk endoscopic procedures.
For patients requiring emergency endoscopy taking either of these drugs, the PT APTT and TT should be measured. If all are normal, there is unlikely to be a significant anticoagulant effect of the drugs, and the procedure can be performed safely. However, if the clotting is even mildly abnormal, there may be significant drug effect, and the procedure should be delayed if clinically acceptable to do so.
Restarting treatment after endoscopy
Restarting both dabigatran and rivaroxaban depends upon the risk of bleeding postprocedure (see figure 1). For low-risk procedures, both drugs can be restarted approximately 6–8 h after the procedure, if there has been no significant bleeding. Dabigatran should be given as a single dose of either 110 mg or 150 mg (whichever is the normal dose for that patient) and the following day, and in the absence of significant bleeding, patients should continue with their normal twice daily dosing schedule. Rivoroxaban can be continued at the standard once daily dosage19–21 restarted 6–8 h postprocedure.
Following high-risk procedures, both drugs should be omitted for 48 h and then restarted in the standard twice daily dosing for dabigatran and once daily for rivoroxaban if there has been no excessive bleeding. For patients considered to be at high risk of thrombotic complications, and who have undergone a high-risk procedure, consideration should be given to using a prophylactic dose of low molecular weight heparin until the oral anticoagulant can be safely restarted. Although not currently supported by published evidence, Schulman et al15 have also suggested the use of smaller doses of the new oral anticoagulants—75 mg of dabigatran or 10 mg of rivaroxaban in place of low molecular weight heparin. The risks of bleeding should be weighed against the risk of thrombosis in these patients on an individual basis.
Patients undergoing colonoscopic polypectomy may develop bleeding immediately or up to 4 weeks postprocedure.22 These studies were performed in an era prior to the new endoscopic modalities of haemostasis. The use of injection therapy, endoloops,23 or clips,24 significantly reduced the risk of postpolypectomy bleeding, and such procedures should be used in this patient cohort at colonoscopy. They may also be used in the event of a delayed bleed requiring intervention. Should the endoscopist believe at the time of the index procedure that the risk of (delayed) bleeding is very high, consideration should be given for the use of low molecular weight heparin over a more extended period prior to the reintroduction of these drugs. Perhaps reassuringly, Majeed et al25 reviewed all the reports of the 1121 major bleeds identified in 5 phase III clinical trials of dabigatran in 27 419 patients found that by stopping the drug and providing supportive measures most cases of bleeding were manageable, and the bleeding stopped (but note that they did not include patients with impaired renal function, patients aged over 75 years or with other comorbidities that might have affected the bleeding risk).
Management of acute gastrointestinal haemorrhage for patients on dabigatran or rivaroxaban of bleeding
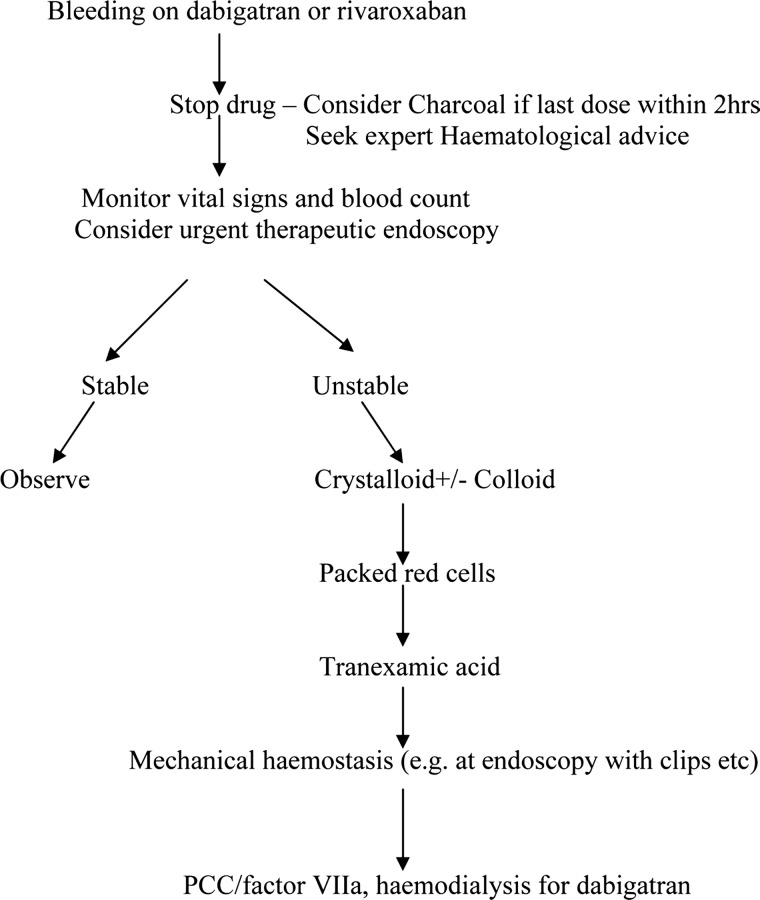
This is summarised in figure 2. Initial management is supportive and includes stopping the anticoagulant drug (as both drugs have relatively short half-lives, this is the first step in reversing their anticoagulant effects). Activated charcoal has been suggested if ingestion was within 2 h, for example in overdose.2 26 The patient's heart rate, blood pressure and haemoglobin should be carefully monitored, and they should receive appropriate fluid resuscitation as well as packed red cells, if indicated.
Figure 2.
Management of acute gastrointestinal haemorrhage in patients on Rivaroxaban and Dabigatran.
There is no evidence for the use of tranexamic acid specifically in patients taking these drugs who sustain a major bleed, but it is clearly a useful agent in management of massive haemorrhage, as demonstrated in the CRASH 2 trial,27 and is unlikely to be harmful. A review of seven randomised trials of the use of tranexamic acid against placebo in patients with gastrointestinal bleeding suggested that the drug may reduce all-cause mortality, although the trials quoted all predated modern endoscopic intervention. No increase in thromboembolic events was noted.28
Therapeutic endoscopy performed by an experienced operator may provide haemostasis (eg, clips, injection therapy, heater probe etc).29
Dabigatran inhibits the last stage of the coagulation cascade by blocking thrombin. In life-threatening bleeding, where supportive measures have been unsuccessful, dabigatran can be removed with haemodialysis; however, due to its high protein binding (approximately 90%) this is not the case with rivaroxaban.5
There is no place for the use of vitamin K or fresh frozen plasma (FFP) in the reversal of these agents. Prothrombin factor concentrate (PCC)30 contains factors II, VII, IX and X or factor viii inhibitor bypass activity (FEIBA)31 containing activated clotting factors II, VII, IX and X may be partially effective in overcoming the effect of dabigatran and also rivaroxaban. Recombinant factor VIIa has also been suggested for use in life-threatening bleeding. Both PCC and Factor VIIa should only be used if the clotting is abnormal. However, most of these studies have involved animal models or healthy, non-bleeding volunteers, and have not been used in patients taking the drug who have experienced serious bleeding.32 33
Conclusions
Since its introduction in the 1940s, warfarin has been the oral anticoagulant of choice, despite its many drawbacks. Dabigatran and Rivaroxaban seem to offer simpler and more straightforward dosing and monitoring options for patients.
Awareness of the indications and complications of these drugs for patients attending for endoscopy procedures is clearly essential. Knowing when to stop and restart the medications in low-risk or higher-risk procedures (with knowledge of renal function) will guide safe management. The main drawback of these new drugs is the lack of a direct ‘antidote’ should a patient bleed during or after their procedure.
This review provides guidelines on how to safely manage patients attending our endoscopy units who are taking these medications.
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributors: See previous submission information.
References
- 1.Veitch AM, Baglin Tp, Gershlick AH, et al. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergiong endoscopic procedures. Gut 2008;1322–9. [DOI] [PubMed] [Google Scholar]
- 2.6. NICE Guidance TA249 Atrial fibrillation- Dabigatran etexliate (TA249). Dabigatran etexilate for the prevention of stroke and systemic embolisation in atrial fibrillation. NICE technology appraisal issued March 2012.
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 4.NICE Guidance TA157 venous thromboembolism- Dabigatran: a quick reference guide 24th September 2008.
- 5.Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood 2010;115:15–20. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis 2009;54:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NICE Guidance—TA261- Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism July 2012.
- 8.Xarelto® Guidance for healthcare professionals (product leaflet, Bayer Healthcare).
- 9.NICE Guidance TA170 venous thromboembolism- Rivaroxaban: a quick ref guide 22nd April 2009.
- 10.NICE Guidance TA256 Atrial fibrillation (stroke prevention) –rivaroxaban: guidance 21st May 2012.
- 11.Patel MR, Mahaffey KW, Garj J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 12.Granger CB, Alexander JH, McMurray JJV, et al. Apixiban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 13.Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013;11: 246–52. [DOI] [PubMed] [Google Scholar]
- 14. Pradaxa® product information leaflet- undesirable effects http://www.medicines.org.uk (accessed Nov 2012).
- 15.Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012;119:3016–23. [DOI] [PubMed] [Google Scholar]
- 16.Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thrombotic events with dabigatran compated with warfarin. Circulation 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 17.Garcia DA, Granger CB. Anitcoagulation, novel agents and procedures: can we pardon the interuption? Circulation 2012;126:255–7. [DOI] [PubMed] [Google Scholar]
- 18.PERIOP-2: A safety and effectiveness study of LMWH bridging therapy versus placebo bridging therapy for patients on long term warfarin and require temporary interuption of their warfarin. http://clinicaltrials.gov/ct2/show/NCT00432796
- 19.The Bridge Study: Bridging anticoagulation in patients who require temporary interuption of warfarin therapy for an elective invasive procedure or surgery. https://bridge.dcri.duke.edu/
- 20.Considerations for surgery for patients treated with pradaxa® (dabigatran etexilate) (Boehringer Ingleheim product leaflet).
- 21.UpToDate. Anticoagulants other than heparin and warfarin (September 2012).
- 22.Rosen L, Bub DS, Reed JF, et al. Hemorrhage following colonoscopic polypectomy. Dis Col Rectum 1993;36: 1126–31. [DOI] [PubMed] [Google Scholar]
- 23.Kouklakis G, Mpoumponaris A, Gatopoulou A, et al. Endoscopic resection of large pedunculated colonic polyps and risk of postpolypectomy bleeding with adrenaline injection versus endoloop and hemoclip : a prospective, randomised study. Surg Endosc 2009;12:2732–7. [DOI] [PubMed] [Google Scholar]
- 24.Parra-Blanco A, Kaminaga N, Kojima T, et al. Hemoclipping for postpolypectomy and post biopsy colonic bleeding. Gastrointest Endosc 2000;51:37–41. [DOI] [PubMed] [Google Scholar]
- 25.Majeed A, Huang H-G, Brueckman M, et al. Management and outcome of major bleeding on dabigatran or warfarin. Blood 2012;120:A19. [Google Scholar]
- 26.Makris M, Van Veen JJ, Tait CR, et al. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol 2012;160:35–46. [DOI] [PubMed] [Google Scholar]
- 27.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376: 23–32. [DOI] [PubMed] [Google Scholar]
- 28.Gluud LL, Klingenberg SL, Langholz SE, et al. Systematic review: Tranexamic acid for upper gastrointestinal bleeding. Aliment Pharmacol Ther 2008;27:752–8. [DOI] [PubMed] [Google Scholar]
- 29.NICE guidance CG141 : Acute upper gastrointestinal bleeding : Management. June 2012. [Google Scholar]
- 30.Eerenberg ES, Kamphuisen PW, SIjpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomised, placebo controlled crossover study in healthy subjects. Circulation 2011;124:1573–9. [DOI] [PubMed] [Google Scholar]
- 31.Khoo TL, Weatherburn C, Kershaw G, et al. The use of FEIBA in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol 2013;35: 222–4. [DOI] [PubMed] [Google Scholar]
- 32.Pragst I, Zeitler SH, DOerr B, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost 2012:10: 1841–8. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda T, Honda Y, Kamisato C, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost 2012;107: 253–9. [DOI] [PubMed] [Google Scholar]