Abstract
The widespread use of imaging techniques has led to an increased diagnosis of incidental liver tumours. The differential diagnosis is extremely broad since it may range from benign asymptomatic lesions to malignant neoplasms. The correct characterisation of a liver mass has become a diagnostic challenge for most clinicians. They can be divided in two major categories; cystic lesions, usually benign with excellent long-term outcome, and solid lesions, in which malignancy should be excluded. A particular population is those patients with cirrhosis, who have high risk for hepatocellular carcinoma development. Dynamic imaging techniques have a pivotal role in the diagnostic work-up of liver tumours, allowing a confident diagnosis in most cases. If imaging is not conclusive, a biopsy should be requested to obtain a definitive diagnosis.
Keywords: Hepatocellular Carcinoma, Computer Tomography, Magnetic Resonance Imaging, Ultrasonography
Introduction
A liver mass is defined as a focal solid or cystic lesion that can be differentiated from the surrounding liver parenchyma by imaging techniques. The detection of liver masses has dramatically increased in recent years due to the widespread use of imaging techniques for evaluation of the abdomen.1 The differential diagnosis is extremely broad since it may range from benign asymptomatic lesions to malignant neoplasms and, in most cases, the correct characterisation of a liver mass poses a diagnostic challenge for most clinicians. The diagnosis of a focal liver lesion is based on clinical background, imaging findings and, in some cases, on pathologic analysis.2 An incidental lesion detected by imaging in an asymptomatic patient with no history of chronic liver disease or known neoplasia is usually benign, being simple cysts, haemangiomas and focal nodular hyperplasia (FNH) the most frequent entities. However, in a patient with a known cancer of any origin, metastases will be the most probable diagnosis. Finally, a liver mass in a patient with cirrhosis is most likely a hepatocellular carcinoma (HCC).3–5 The clinical background may also suggest a diagnosis. Thus, a highly vascularised mass in a healthy young woman on oral contraceptives should raise the suspicion of hepatocellular adenoma (HCA), and a liver tumour in a patient with primary sclerosing cholangitis should suggest the presence of intrahepatic cholangiocarcinoma (ICC). Imaging techniques show whether the tumour has a liquid or solid content. The vascularisation profile after contrast administration may also suggest its possible diagnosis. However, both benign (FNH or HCA) and malignant (HCC, ICC, carcinoid, metastases) tumours may show arterial contrast uptake. Contrast-enhanced ultrasound,6 dynamic CT, and dynamic MRI define the vascular pattern and, together with analysis of the nodule characteristics, may strongly suggest the diagnosis. Nevertheless, in a relevant number of cases the final diagnosis will be established solely by pathological analysis obtained by biopsy.7
The following sections will separately review the epidemiology, clinical presentation, diagnosis and treatment of the most frequent cystic and solid hepatic lesions.
Cystic lesions
Cystic lesions comprise a wide group of entities with different aetiologies, clinical manifestations and outcomes. The majority of cysts arise in patients with no underlying liver disease, are incidentally found on liver imaging, and have a benign course. The distinction among different cystic lesions is particularly important as different lesions have a different clinical significance and management.
Simple cyst
The real prevalence of simple cysts is unknown, but recent series suggest that 18% of the general population is affected by a simple cyst.8 In most cases, they are solitary and small. If multiple, this should prompt the suspicion of hepatic and/or renal polycystic disease. Simple cysts are usually asymptomatic and incidentally found by CT or ultrasound. Only some large cysts may produce abdominal discomfort, and anecdotally, they may cause jaundice, haemorrhage or infection.9 Cysts contain a serous liquid, are covered by a single-layer, cuboidal epithelium resembling biliary epithelial cells, and do not communicate with the biliary ducts.9 10 Diagnosis is easily established by ultrasound demonstrating an anechoic lesion with well defined thin walls, associated with strong posterior wall echoes.11 Other imaging procedures have less utility compared with ultrasound, and generally are not required. Treatment, if any, should be symptomatic. Only complicated cysts may benefit from percutaneous sclerotherapy or surgical resection.12
Hydatid cyst
Hydatid disease, or echinococcosis, is a zoonosis caused by cestodes belonging to the genus Echinococcus (family Taeniidae). Hydatid disease is most frequently caused by Echinococcus granulosus. It can affect the liver, lung, central nervous system and other organs.13 The clinical manifestations depend upon the organ involved and viability of the cyst's contents. Usually, they are asymptomatic and incidentally found on routine imaging or at biopsy. Symptoms are related to expanding mass, pressure to adjacent structures, infection and rupture of cyst contents into surrounding body cavities. Diagnosis relies on ultrasound and serology. Thicker walls with potential calcification, septa and split walls with floating membranes differentiate hydatid from simple cysts.13 Differential diagnosis should be carried out with biliary cystadenoma or cystadenocarcinoma. Serology by ELISA is positive in 70% of cases and may remain positive years after surgical removal. Treatment should be based on the administration of mebendazole or albendazole, alone or associated with surgical resection.14 Cyst fluid spillage can occur during surgery resulting in anaphylaxis and/or secondary echinococcosis. Another therapeutic strategy is percutaneous treatment guided by ultrasound, known as PAIR (Puncture, Aspiration, Installation of scolicidal agent and Reaspiration), with promising results.15 16
Hepatic abscess
Pyogenic hepatic abscesses are usually produced by bacteria from the gastrointestinal tract. Currently, the most frequent cause of liver abscesses is biliary infection, followed by portal pyaemia secondary to gastrointestinal infections such as diverticulitis or appendicitis. The clinical suspicion is based on the presence of fever, malaise, anorexia, right upper quadrant pain and signs of systemic sepsis. CT is considered the most important modality; it confirms the diagnosis by demonstrating one or more hypoattenuated lesions with ill-defined margins that can present rim enhancement, septa or gas, and may also detect other intra-abdominal disorders that could be the cause of the abscess. Blood cultures are positive in 50%–60% of cases. The basis of treatment relies on complete drainage of pus and infected debris by percutaneous or surgical procedures, initiation of adequate antibiotic therapy, and resolution of the underlying cause.17 A pyogenic abscess must be distinguished from amoebic hepatic abscess secondary to infection by Entamoeba histolytica. This is uncommon in developed countries, but may occur in travellers to endemic areas. Clinical manifestations and imaging techniques do not allow distinction from pyogenic abscess. Amoebic serology is highly sensitive and specific in the differentiation between pyogenic and amoebic hepatic abscess since this is positive in more than 95% of cases. The best treatment is metronidazole, frequently associated with percutaneous drainage.18
Cystic neoplasms
Some malignant lesions may present as complex cysts. Mural nodularity, thick septa, thick tumour rim and contrast enhancement are imaging findings suspicious for malignancy. Biliary cystadenoma and cystadenocarcinoma are tumours that originate from biliary epithelium and mainly affect women. They are detected as multiloculated, large cystic lesions. Cystoadenocarcinomas are usually well differentiated adenocarcinomas, often with an intracystic papillary component, and they are composed of malignant epithelial cells. These tumours tend to grow slowly, and the sole treatment option is surgical resection. Primary papillary tumour of the bile duct is a rare malignant tumour that distends the bile duct and appears as a cystic mass with intraductal tumour bulging into the lumen. Cystic liver metastases may occur frequently from sarcoma, gastrointestinal stromal tumour, melanoma, colon and ovarian cancer. Other metastases may also cavitate, frequently as a response to chemotherapy.
Solid lesions
Hepatic haemangioma
Haemangioma is the most frequent tumour of the liver, with a prevalence of 3%–20%.1 19 20 Haemangiomas are usually solitary and small, but can reach 20 cm in diameter.1 20 Even then, most patients are asymptomatic and diagnosis is incidental. They are most frequently detected in women between the ages of 30 and 50 (female:male rate, 3:1).20 The pathogenesis of haemangioma is not well understood; these lesions are considered congenital but there has been some suggestions that they might be hormonally mediated.21 It is composed of large vascular channels lined by mature, flattened, endothelial cells, enclosed in a fibroblastic stroma. Their course is benign, although they may grow slightly during pregnancy or oestrogen treatment.1 21 Bleeding is extremely infrequent and should suggest another diagnosis. Giant haemangiomas may become symptomatic in the event of infarction or thrombosis. Exceptionally, a haemangioma can lead to a rare complication known as Kasabach–Merritt syndrome, characterised by thrombocytopenia, consumptive coagulopathy and microangiopathic haemolytic anaemia. Ultrasound shows a well defined hyperechogenic lesion, that after contrast administration, displays an initial peripheral globular-nodular enhancement and is followed by a centripetal fill-in.6 MRI is the best technique to establish the diagnosis (100% sensitivity, 95% specificity). The classic appearance is that of hypointense lesion on T1-weighted sequences, strongly hyperintense on T2-weighted sequences, and a typical globular peripheral enhancement with progressive hyperintense filling after contrast administration (figure 1).22 23 Treatment should be symptomatic.1 20
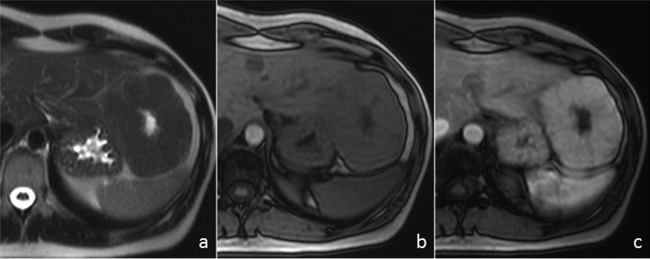
Figure 1.
MRI of focal nodular hyperplasia. (A) Axial T2-weighted image shows a large mass in the left hepatic lobe that is isointense to the liver parenchyma with a hyperintense central scar. (B) The mass is slightly hypointense on unenhanced axial T1-weighted image with the central scar being more hypointense. (C) On the gadolinium-enhanced T1-weighted image obtained during the arterial phase the mass shows intense and homogeneous enhancement, except for the central scar.
Focal nodular hyperplasia
FNH accounts for the second most frequent benign tumour of the liver with an estimated prevalence of 2.5% in the general population. It is predominantly diagnosed in women with a peak incidence between 30 and 50 years of age. In most cases it is solitary and smaller than 5 cm, but it may be larger and multiple in 20% of cases. Usually, this represents an incidental finding in asymptomatic subjects. It is thought to represent a hyperplasic cell response to an aberrant dystrophic artery.1 24 It is composed by normal hepatocytes arranged in nodules, and in the margins of the nodule it presents prominent bile ductular reaction.24 The presence of a central fibrotic scar containing the feeding artery is a characteristic finding,24 and is used to establish the CT or MRI diagnosis in the absence of biopsy. At molecular level, FNH is characterised as a polyclonal lesion with activation of the β-catenin pathway without β-catenin mutation. Accordingly, staining for glutamine synthetase, a target gene of β-catenin, is frequently positive in typical FNH.24 On MRI, FNH is usually hypointense or isointense on T1-weighted images, and slightly hyperintense or isointense on T2-weighted images, with a central scar hypointense on T1-weighted images, and hyperintense on T2-weighted images. Following contrast administration, FNH displays intense, homogeneous enhancement in the arterial phase sparing the central scar, whereas, it becomes isointense to liver parenchyma in the portal venous and delayed phases with enhancement of the central scar25 (figure 1). The clinical evolution is uneventful with no potentially severe complications. Thus, no treatment is recommended.1 24
Hepatocellular adenoma
HCA is a very uncommon tumour (prevalence 0.001%) that is found more frequently in young women. It is associated with oral contraceptives or anabolic treatment, and with glycogen storage disease types I and III. In most instances it is a solitary lesion, but in up to 10%–20% of cases more than one adenoma can be detected. In these patients, hepatic adenomatosis due to a genetic abnormality has to be considered.26 HCA is composed of normal hepatocytes without atypia, arranged in plates separated by dilated sinusoids, in absence of portal spaces or biliary ducts.1 Around 25% of patients with HCA report mild abdominal pain. The most frequent complication is necrosis and bleeding, leading to a severe hemoperitoneum. The risk of bleeding is increased with pregnancy, history of prolonged oral contraceptive use, larger lesions and subcapsular location.
Molecular approaches have demonstrated that HCA is a heterogeneous entity. Genotype classification of HCA has allowed the identification of three subtypes: HNF1A-mutated HCA (H-HCA) in 35% of cases, β-catenin-mutated HCA (b-HCA) in 10%, and inflammatory HCA in 55%. The main molecular, clinical and radiological characteristics are summarised in table 1. Malignant transformation has been demonstrated in a minority of patients (approximately 1%–5% of cases), and recent studies have suggested that those HCAs with β-catenin activation have higher risk for malignant degeneration.24 Regrettably, no clinical or radiologic signs can predict preoperatively the diagnosis of degenerated HCA.
Table 1.
Molecular, clinical and radiological characteristics of HCA
| Subtype | Mutated gene | Immuno-histochemistry | Clinical features | Radiological features |
|---|---|---|---|---|
| H-HCA (HNF1α inactivated) | TCF1 | LFABP − |
|
|
| b-HCA (β-catenin activated) | CTNNB1 | β-catenin + | Risk of malignant transformation |
|
| GS + | ||||
| IHCA* (inflammatory) | IL6ST | SAA + |
|
|
| CRP + |
|
|
||
| Unclassified HCA | – | – | – | – |
*Around 10% of IHCA present β-catenin activation (b-IHCA).
CRP, C-reactive protein; CTNNB1, catenin β-1; GS, glutamine synthetase; HCA, hepatocellular adenoma; HNF1α, hepatocyte nuclear factor 1α; IHCA, inflammatory HCA; IL6ST, interleukin 6 signal transducer; LFABP, liver fatty acid binding protein; SAA, serum amyloid A; TCF1, transcription factor 1.
Distinction between HCA and FNH may be difficult, even with the most sensitive imaging techniques and pathologic examination. HCA can show a variable signal intensity related to tissue components. On T1-weighted images lesions are frequently heterogeneous and may appear homogeneously or heterogeneously hyperintense on T2-weighted images. A peripheral rim corresponding to a fibrous capsule may be seen. There is rapid contrast uptake during the arterial phase, with the lesion remaining isointense with respect to the liver tissue in delayed sequences.27 MRI with liver-specific contrast agents, such as gadoxetic acid, may help in the differential diagnosis since FNH appears iso-hyperintense in the hepatobiliary phase while HCA is usually hypointense.28 In some cases a biopsy is needed for its correct characterisation. However, despite the pathological analysis, the differential diagnosis with FNH may be impossible in some cases.
The decision regarding management of hepatic adenomas depends on symptoms, size, number of lesions and risk of bleeding, rupture and malignant transformation. Some authors have suggested that small lesions (<5 cm) can be managed conservatively with repeated periodic imaging; oral contraceptives and steroids should be discontinued. In adenomas that enlarge despite discontinuation of oestrogens, as well as those that are symptomatic, larger than 5 cm or those diagnosed in men, resection should be considered. In those patients in whom surgery is contraindicated, percutaneous ablation with radiofrequency may be an effective option.29
Nodular regenerative hyperplasia
Nodular regenerative hyperplasia (NRH) of the liver is a benign proliferative process in which normal hepatic parenchyma is replaced by diffuse regeneration of hepatocytes with minimal associated fibrosis.30 Many aetiological factors have been associated with the development of NRH, mostly through vascular damage. The pathogenesis of NRH is unknown; it has been hypothesised that the main event leading to NRH is sinusoidal portal venous hypertension caused by thrombosis because of endothelial injury, hypercoagulability or autoimmune injury. The resulting centrolobular hepatocyte atrophy produces a compensatory proliferation of portal hepatocytes, which form regenerative nodules.31 Most patients with NRH are asymptomatic; however, patients may present with signs of portal hypertension. Imaging findings can be subtle in the diffuse form with widespread nodularity at ultrasound. In focal NRH, CT or MRI shows multiple hypervascular masses that may suggest metastases or HCC, although in a patient with long-standing Budd–Chiari syndrome stability on follow-up, studies should help in making the correct diagnosis.32 Biopsy is required to make a definitive diagnosis of NRH. Microscopically, the nodules are clustered around portal triads, with the centre of the regenerative nodules containing hypertrophied hepatocytes arranged in plates. The peripheral cells are thin and atrophic, associated with sinusoidal dilatation. Prognosis reflects the development and progression of portal hypertension. Treatment for NRH is directed at treating the underlying medical condition and preventing complications of portal hypertension.
Hepatic metastasis
Most malignant liver tumours are metastases from cancers that originate in other organs, the most frequent being lung, colon, stomach, pancreas, gallbladder, breast and ovaries.33 Metastatic involvement of the liver usually implies a poor prognosis. Searching for the primary tumour and biopsy confirmation is justified if the patient may benefit from therapy, such as surgery or systemic chemotherapy.33 Fine-needle aspiration biopsy has an 85% diagnostic sensitivity with more than 95% specificity. Serum biomarkers may be useful in the follow-up of some tumours after treatment. In the context of a focal liver lesion without a known primary neoplasia, serum biomarkers lack proper specificity, although in some cases they can help in orienting the primary origin. On CT or MRI, liver metastases are usually hypovascular lesions and frequently show peripheral rim enhancement, but some neoplasms may have a different pattern. Contrast uptake in the arterial phase on CT or MRI suggests neuroendocrine tumour, melanoma, sarcoma, hypernephroma, or thyroid neoplasia.9 Isotopic studies using labelled somatostatin analogues can identify neuroendocrine tumours. Surgical resection of liver metastases may prolong survival in patients with colorectal cancer,34 neuroendocrine tumours,35 and some renal carcinomas, but for other neoplasms the indication of surgery is still controversial.
Hepatocellular carcinoma
HCC constitutes the sixth most common neoplasm in the world, the third most frequent cause of cancer-related death,36 and is currently considered the main cause of death in cirrhotic patients.5 HCC usually develops in the setting of chronic liver disease, and cirrhosis represents the strongest predisposing factor. There are significant geographic differences in HCC incidence, reflecting the heterogeneous distribution of its main etiologic factors. In Asia and sub-Saharan Africa, where the incidence exceeds 20–30 cases/105 inhabitants, hepatitis B virus infection is the predominant risk factor, and the risk is increased by ingestion of aflatoxin B1-contaminated food. By contrast, in developed countries with low HCC incidence, hepatitis C virus (HCV) or alcohol-related cirrhosis are the most frequent predisposing factors.37 Surveillance is aimed for decreasing the HCC-related mortality, and the majority of scientific societies agree that it is indicated in those patients at high risk of HCC development, and should rely on abdominal ultrasound every 6 months.3 4 38 Once a nodule is detected, a recall strategy should be immediately implemented since the likelihood of this being a HCC is very high. In cirrhotic patients, the HCC confirmation can be established by imaging in nodules larger than 1 cm (figure 2).3 These non-invasive criteria are based on the finding by dynamic imaging techniques of a specific vascular profile characterised by intense and homogeneous contrast uptake in arterial phase followed by contrast washout in venous phases (figure 3). When this specific vascular pattern is not found, or the nodule arises in a healthy liver, a biopsy should be requested. Several groups have prospectively validated the near-absolute specificity of these non-invasive criteria, but the sensitivity for nodules <2 cm is around 50%, so nearly half of these small nodules need pathological confirmation.39–41 However, the biopsy of these nodules is also associated to a non-negligible false negative rate. Consequently, a negative result does not confidently discard the HCC diagnosis, and a new biopsy, or close follow-up, is recommended.3 Nodules smaller than 1 cm are usually benign, and their small size hampers its correct characterisation, so the best strategy is to closely monitor them for discarding growth that could suggest a malignant nature.
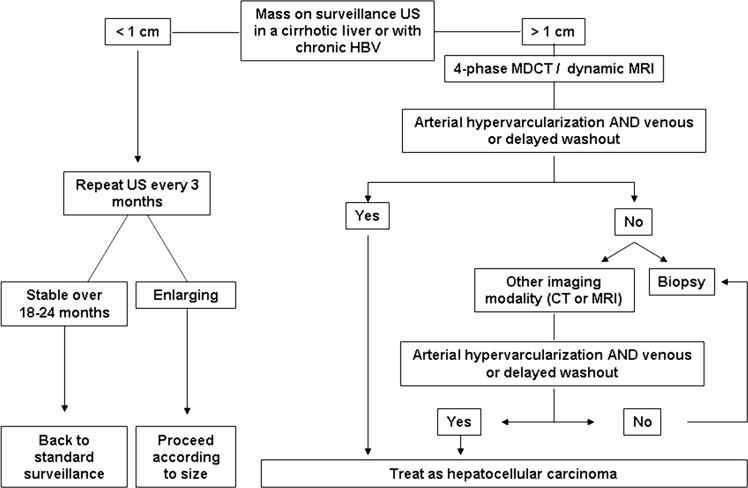
Figure 2.
Diagnostic algorithm for suspected hepatocellular carcinoma upon the detection of a liver nodule by ultrasound. HBV, Hepatitis B virus; MDCT, multidetector CT; US, ultrasound. (Adapted with permission from Bruix and Sherman (3)).
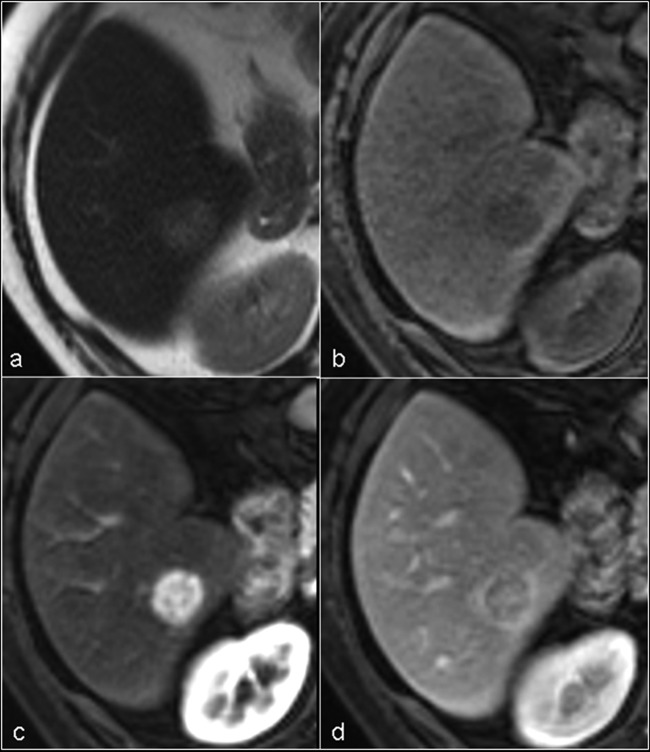
Figure 3.
MRI appearance of typical hepatocellular carcinoma. (A) Axial T2-weighted image shows a well defined, slightly hyperintense nodule in segment VI of the right hepatic lobe. (B) The nodule is slightly hypointense on unenhanced axial T1-weighted image. (C) The nodule shows intense and homogeneous contrast uptake on the gadolinium-enhanced T1-weighted image obtained during the arterial phase (wash-in). (D) On the portal phase the lesion is hypointense (wash-out).
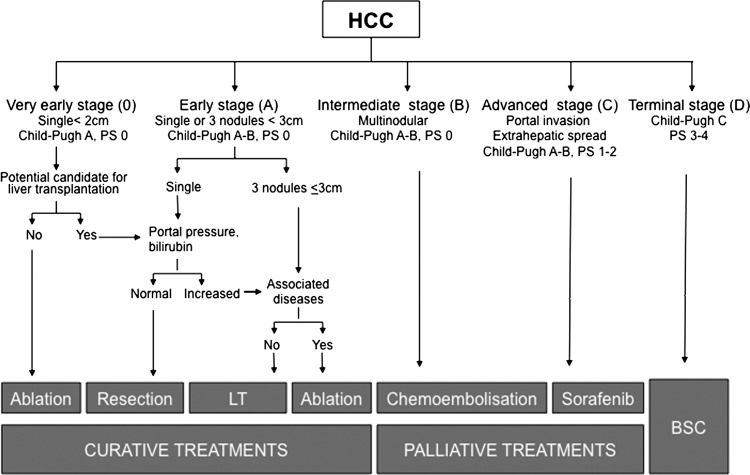
Prognosis of HCC depends not only on tumour stage, but also on the degree of liver function impairment and the general health status.42 Several staging systems have been proposed during the last decade. Among them, the Barcelona-Clinic-Liver-Cancer (BCLC) system has been externally validated in the USA, Europe and Asia as the only one that links prognosis assessment and treatment indication, and it is the recommended staging system in the USA3 and Europe.4 The BCLC staging system considers the tumour stage (size, number of nodules, presence of vascular invasion and extrahepatic spread), liver function (Child–Pugh score and presence of portal hypertension) and general health (ECOG Performance Status), and stratifies patients into five major categories, each with a different treatment (figure 4).5 43 The very early stage (Stage 0) corresponds to patients with preserved liver function without clinically relevant portal hypertension, and a solitary tumour ≤2 cm, well differentiated, and theoretically with minor ability to disseminate. Ablation may be considered the first option, and resection could be reserved for those patients who are potential candidates for liver transplantation, or those in whom ablation fails. With these potential curative therapies, the 5-year survival rate may be greater than 80%.43 Early stage (Stage A) HCC corresponds to patients with preserved liver function, no cancer-related symptoms, and single tumours or with up to three nodules ≤3 cm. These patients are suitable for potential curative treatment (resection, liver transplantation or percutaneous ablation), and the expected 5-year survival after treatment is 50%–70%. The optimal candidates for resection are those with solitary tumours in whom significant portal hypertension or hyperbilirubinemia have been discarded.44 45 Liver transplantation is the preferred approach for patients with impaired liver function in whom resection does not offer an optimal long-term outcome. The best results are obtained with the Milan criteria (single tumour <5 cm or up to three nodules smaller that 3 cm).46 Finally, percutaneous ablation by ethanol injection or, preferably if technically feasible, by radiofrequency47 48 should be considered in patients at stage 0 or A who are not eligible for surgery, or as a bridge during the waiting period.3 4 Intermediate stage (Stage B) patients are still asymptomatic with large or multifocal HCC exceeding the criteria for applying curative therapies, with neither vascular invasion nor extrahepatic spread. The only option that up to now has shown survival benefit is transarterial chemoembolisation.49 This requires selective catheterisation of the hepatic artery feeding the tumour, with injection of a chemotherapeutic agent associated with an embolising agent. The procedure is well tolerated, achieves tumour necrosis in more than 50% of the patients, and in well-selected candidates using a state-of-the-art technique, the median survival surpasses 4 years.50 A novel therapeutic approach is the use of internal radiotherapy through the endovascular injection of microspheres charged with Yttrium-90. Cohort studies coming from the USA51 and Europe52 have shown promising results, but further prospective clinical trials are needed to confirm the potential benefit of this approach. The advanced stage (Stage C) denotes patients with large/multifocal tumours and vascular involvement, extrahepatic spread or physical impairment. The expected median survival without treatment is 4–8 months, and until recently, therapies were not available. The improvements in the knowledge of the molecular pathogenesis of cancer have allowed the development of agents that act by blocking the altered pathways. Active research is currently ongoing and until now, the only agent that has demonstrated survival benefit in randomised, placebo-controlled trials is sorafenib.53 54 Finally, patients with terminal stage (Stage D) have a very poor physical status and/or major tumour burden, and should receive symptomatic treatment. HCC patients with Child–Pugh grade C who are not candidates for liver transplantation belong to this group, and do not benefit from antitumoral treatment, as outcome is poor due to liver function impairment.5
Figure 4.
The Barcelona Clinic Liver Cancer staging and treatment strategy. LT, liver transplantation; PS, performance status. (Adapted with permission from Forner et al5 BSC, best supportive care).
Fibrolamellar carcinoma
Fibrolamellar carcinoma is an uncommon variant of HCC (1%–9%), frequent in Western countries, and most prevalent in young patients with no association with underlying chronic liver disease. Typically, it appears as a large, single, well defined but non-encapsulated intrahepatic mass with prominent fibrous septa connected to a fibrotic, frequently calcified, central scar. The diagnosis is usually done late, when the patients refer symptoms related to mass-effect and constitutional syndrome. The α-fetoprotein level is normal in more than 90% of patients. Diagnosis and staging are based on CT and/or MRI,55 but percutaneous core biopsy may be needed in cases with atypical imaging features. Fibrolamellar HCC tends to be slow-growing and frequently resectable with better prognosis than those with classic HCC.56 Aggressive surgical resection or liver transplantation is feasible in around 80%, with a 5-year survival rate of 75%.57
Intrahepatic cholangiocarcinoma
ICC is an adenocarcinoma that originates from intrahepatic biliary epithelial cells. ICC is less common than extrahepatic ductal cholangiocarcinoma, and usually appears as a focal mass-forming lesion. Several risk factors for ICC development have been suggested. The most well known are primary sclerosing cholangitis, hepatobiliary flukes, hepatolithiasis and biliary malformations. In addition, cirrhosis, mainly secondary to chronic infection with HCV, associated with alcohol use and metabolic syndrome, have been recognised as important risk factors for ICC development, and this association has been speculated as the cause of the increasing incidence of ICC in recent years.58 59 Diagnosis could be suggested by dynamic imaging techniques. The typical radiological appearance is a lesion with progressive contrast uptake along the different phases, associated with a central scar, vascular encasement and capsule retraction. Dilatation of peripheral bile ducts is often seen.60 61 ICC can display a vascular profile indistinguishable from HCC by contrast-enhanced ultrasound (CEUS),62 63 and for that reason, CEUS is not further recommended for non-invasive HCC diagnosis in patients with cirrhosis.3 4 The tumour becomes symptomatic upon reaching a large size, and the best therapeutic option is surgical resection, which is an option in only a minority of patients who may then have a 3-year survival rate of 40%–60%.64 65 Liver transplantation has poor results and is not recommended.59 In those locally advanced or metastatic cholangiocarcinoma, systemic chemotherapy with cisplatin plus gemcitabine is associated with survival benefit.66
Angiosarcoma
This is the most common primary sarcoma of the liver.67 The peak incidence is during the sixth and seventh decades of life and appears more frequently in men (male:female ratio 3:1). It originates from the endothelial cells of the sinusoidal lining. The tumour cells infiltrate the sinusoids, hepatic and portal veins, and finally substitute the hepatic parenchyma. Angiosarcoma has been associated with Thorotrast, vinyl chloride and arsenic exposure.67 Symptoms may mimic those of chronic liver disease, but in 15% of patients, angiosarcoma is diagnosed because of acute hemoperitoneum due to tumour rupture. Dynamic CT or MRI shows gradual contrast enhancement and homogeneity in the late phase. MRI shows that the tumour may be hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging, features that may resemble a haemangioma.9 Liver biopsy establishes the diagnosis. Diagnosis is usually made at an advanced stage when surgery is not feasible, and prognosis is dismal with an expected survival of less than 6 months.67
Hepatic epithelioid hemangioendothelioma
Epithelioid hemangioendothelioma (EHE) is a rare, low-grade malignant tumour of vascular origin that may arise from liver, lung, soft tissue or bone in adults. EHE is more common in women. It originates from endothelial cells and, histologically, the tumour comprises small groups of tumour cells surrounded by a distinctive and abundant sclerotic stroma. In contrast with haemangiosarcoma, the hepatic acinar landmarks are preserved.68 Its pathogenesis is unknown; symptoms are non-specific, and the evolution is unpredictable. It can remain stable for years and then progress in a very aggressive manner. On imaging studies, EHE appears as multiple, peripheral, nodular lesions and large masses, mimicking metastatic disease.68 The diagnosis can only be done by pathological assessment demonstrating positive immunostaining for factor VIII-related antigen, CD31 and CD34. The treatment algorithm of EHE is far from standardised. The role of liver transplantation is questioned in view of the documented spontaneous, long-term survivals, the high incidence of extrahepatic disease (up to 45%), the lack of predictive clinical or histological criteria and, finally, the high incidence (up to 33%) of recurrent allograft disease.69 Transplantation is usually delayed until there is evidence of unequivocal tumour progression.70
Summary
The correct characterisation of a liver mass has become a diagnostic challenge for most clinicians. The diagnosis of a focal liver lesion is based on clinical background and imaging findings, but in some cases, a biopsy should be requested. In the case of cystic lesions, ultrasound is sufficient in most cases for diagnosing a simple cyst. If the lesion is not typical, clinical characteristics, hydatid and amoebic serology, and CT or MRI will allow the differential diagnosis between a simple cyst, hepatic and/or renal polycystic disease, hydatid cyst, pyogenic abscess and amoebic abscess. The differentiation between cystoadenoma and cystoadenocarcinoma is difficult and, if suspected, usually requires pathological assessment. Contrarily, in the case of a solid lesion, the differential diagnosis is very broad since it may range from benign asymptomatic lesions to malignant neoplasms. The clinical background will be extremely helpful. In healthy patients, haemangioma is the most prevalent lesion, which can be easily diagnosed by ultrasound and MRI. If the patient is a young woman with the antecedent of oral contraceptive use, it will be necessary to rule out FNH and HCA, since the prognosis and treatment differ significantly. If the nature of the lesion is still equivocal despite imaging and percutaneous biopsy, surgical resection should be recommended. In patients affected with a chronic liver disease, the most frequent diagnosis is, by far, HCC. There is a well established and prospectively validated diagnostic algorithm summarised in figure 2. The diagnosis of HCC in cirrhotics relies, in most cases, on the finding of a specific vascular profile on dynamic imaging techniques. Remarkably, there are other malignancies, such as ICC, that should be discarded, since the treatment and prognosis differ from HCC. Finally, a solid lesion in a patient with a known neoplasia is usually a liver metastasis. In this scenario, imaging and tumour markers are usually diagnostic, but in some doubtful cases, a biopsy should be requested.
References
- 1.Bahirwani R, Reddy KR. Review article: the evaluation of solitary liver masses. Aliment Pharmacol Ther 2008;28:953–65. [DOI] [PubMed] [Google Scholar]
- 2.Ishak KG, Goodman ZD, Stocker JT. Tumors of the liver and intrahepatic bile ducts. In: Rosai J SLH. ed. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology, 2001. [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 6.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall Med 2008;29:28–44. [DOI] [PubMed] [Google Scholar]
- 7.Verslype C, Libbrecht L. The multidisciplinary management of gastrointestinal cancer. The diagnostic and therapeutic approach for primary solid liver tumours in adults. Best Pract Res Clin Gastroenterol 2007;21:983–96. [DOI] [PubMed] [Google Scholar]
- 8.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol 2003;58:626–9. [DOI] [PubMed] [Google Scholar]
- 9.Fulcher AS, Sterling RK. Hepatic neoplasms: computed tomography and magnetic resonance features. J Clin Gastroenterol 2002;34:463–71. [DOI] [PubMed] [Google Scholar]
- 10.Horton KM, Bluemke DA, Hruban RH, et al. CT and MR imaging of benign hepatic and biliary tumors. Radiographics 1999;19:431–51. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Hann LE. A practical approach to analyzing focal lesions in the liver. Ultrasound Q 2005;21:187–200. [DOI] [PubMed] [Google Scholar]
- 12.Erdogan D, van Delden OM, Rauws EA, et al. Results of percutaneous sclerotherapy and surgical treatment in patients with symptomatic simple liver cysts and polycystic liver disease. World J Gastroenterol 2007;13:3095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus DP, Zhang W, Li J, et al. Echinococcosis. Lancet 2003;362:1295–304. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ 1996;74:231–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997;337:881–7. [DOI] [PubMed] [Google Scholar]
- 16.Smego RA, Jr, Bhatti S, Khaliq AA, et al. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis 2003;37:1073–83. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Tapias J. Bacterial, rickettsial and spirochaetal infections. In: Rodes J, Benhamou J, Blei A, Reichen J, Rizzetto M. eds. Textbook of hepatology: from basic science to clinical practice. 3rd edn Oxford: Blackwell, 2007:1001–11. [Google Scholar]
- 18.Stanley SL., Jr Amoebiasis. Lancet 2003;361:1025–34. [DOI] [PubMed] [Google Scholar]
- 19.Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis 1993;13:423–35. [DOI] [PubMed] [Google Scholar]
- 20.Gandolfi L, Leo P, Solmi L, et al. Natural history of hepatic haemangiomas: clinical and ultrasound study. Gut 1991;32:677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glinkova V, Shevah O, Boaz M, et al. Hepatic haemangiomas: possible association with female sex hormones. Gut 2004;53:1352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitney WS, Herfkens RJ, Jeffrey RB, et al. Dynamic breath-hold multiplanar spoiled gradient-recalled MR imaging with gadolinium enhancement for differentiating hepatic hemangiomas from malignancies at 1.5T. Radiology 1993;189:863–70. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell DG, Saini S, Weinreb J, et al. Hepatic metastases and cavernous hemangiomas: distinction with standard- and triple-dose gadoteridol-enhanced MR imaging. Radiology 1994;193:49–57. [DOI] [PubMed] [Google Scholar]
- 24.Bioulac-Sage P, Cubel G, Balabaud C, et al. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis 2011;31:91–103. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics 2004;24:3–17; discussion 18–19. [DOI] [PubMed] [Google Scholar]
- 26.Cherqui D, Rahmouni A, Charlotte F, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology 1995;22:1674–81. [PubMed] [Google Scholar]
- 27.Grazioli L, Federle MP, Brancatelli G, et al. Hepatic adenomas: imaging and pathologic findings. Radiographics 2001;21:877–92; discussion 892–874. [DOI] [PubMed] [Google Scholar]
- 28.Purysko AS, Remer EM, Veniero JC. Focal liver lesion detection and characterization with GD-EOB-DTPA. Clinical Radiology 2011;66:673–84. [DOI] [PubMed] [Google Scholar]
- 29.Atwell T, Brandhagen D, Charboneau J, et al. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol 2005;184:828–31. [DOI] [PubMed] [Google Scholar]
- 30.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 1990;11:787–97. [DOI] [PubMed] [Google Scholar]
- 31.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 2006;44:7–14. [DOI] [PubMed] [Google Scholar]
- 32.Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010;194:993–9. [DOI] [PubMed] [Google Scholar]
- 33.Pavlidis N, Briasoulis E, Hainsworth J, et al. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990–2005. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham D, Atkin W, Lenz H-J, et al. Colorectal cancer. Lancet 2010;375:1030–47. [DOI] [PubMed] [Google Scholar]
- 35.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 36.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 37.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5–16. [DOI] [PubMed] [Google Scholar]
- 38.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104. [DOI] [PubMed] [Google Scholar]
- 40.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging technique in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638–44. [DOI] [PubMed] [Google Scholar]
- 41.Khalili KT, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol 2011;54:723–8. [DOI] [PubMed] [Google Scholar]
- 42.Forner A, Reig ME, Rodriguez de Lope C, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61–74. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez de Lope C, Tremosini S, Forner A, et al. Management of HCC. J Hepatol 2012;56(Suppl):S75–87. [DOI] [PubMed] [Google Scholar]
- 44.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–40. [DOI] [PubMed] [Google Scholar]
- 45.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908–16. [DOI] [PubMed] [Google Scholar]
- 46.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- 47.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49:453–9. [DOI] [PubMed] [Google Scholar]
- 48.Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380–8. [DOI] [PubMed] [Google Scholar]
- 49.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429–42. [DOI] [PubMed] [Google Scholar]
- 50.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using DC Beads. Implications for clinical practice and trial design. J Hepatol 2012;56:1330–5. [DOI] [PubMed] [Google Scholar]
- 51.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497–507 e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868–78. [DOI] [PubMed] [Google Scholar]
- 53.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- 54.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 55.Ichikawa T, Federle MP, Grazioli L, et al. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology 1999;213:352–61. [DOI] [PubMed] [Google Scholar]
- 56.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004;39:798–803. [DOI] [PubMed] [Google Scholar]
- 57.Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006;106:1331–8. [DOI] [PubMed] [Google Scholar]
- 58.Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016–21. [DOI] [PubMed] [Google Scholar]
- 59.Patel T. Cholangiocarcinoma-controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683–700. [DOI] [PubMed] [Google Scholar]
- 61.Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology 2009;50:791–8. [DOI] [PubMed] [Google Scholar]
- 62.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010;51:2020–9. [DOI] [PubMed] [Google Scholar]
- 63.Chen LD, Xu HX, Xie XY, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 2010;20:743–53. [DOI] [PubMed] [Google Scholar]
- 64.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51(Suppl 6):VI1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol 2010;17:1823–30. [DOI] [PubMed] [Google Scholar]
- 66.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 67.Kim HR, Rha SY, Cheon SH, et al. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol 2009;20:780–7. [DOI] [PubMed] [Google Scholar]
- 68.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108–21. [DOI] [PubMed] [Google Scholar]
- 69.Lerut JP, Weber M, Orlando G, et al. Vascular and rare liver tumors: a good indication for liver transplantation? J Hepatol 2007;47:466–75. [DOI] [PubMed] [Google Scholar]
- 70.Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg 2007;246:949–57; discussion 957. [DOI] [PubMed] [Google Scholar]