Abstract
Objective
Recognition of Non alcoholic fatty liver disease (NAFLD) and metabolic syndrome in patients with gallstones undergoing laparoscopic or open cholecystectomy, along with it we will also study the life style of patients with gall stones.
Background
Patients with gallstones have associated NAFLD, with concurrent metabolic syndrome and these ailments share similar factors for example obesity, hypertriglyceridemia and diabetes mellitus. Factors like body mass index, gender, raised lipid levels, use of contraceptives and alcohol and having diabetes, physical inactiveness, multiparous women, water with excessive iron content, metabolic syndrome, and NAFLD are accountable factors for gallstones formation.
Methodology
This was a case series done at Surgical Unit 1 of Civil Hospital Karachi. Selective samples of 88 patients were included. Duration was 3 months. We included both sexes with ultrasound proof of gall stone irrespective of cholecystitis. Excluded patients with history of seropositive viral hepatitis, autoimmune and wilson's disease. As these conditions can act as a confounder to our variables.
Results
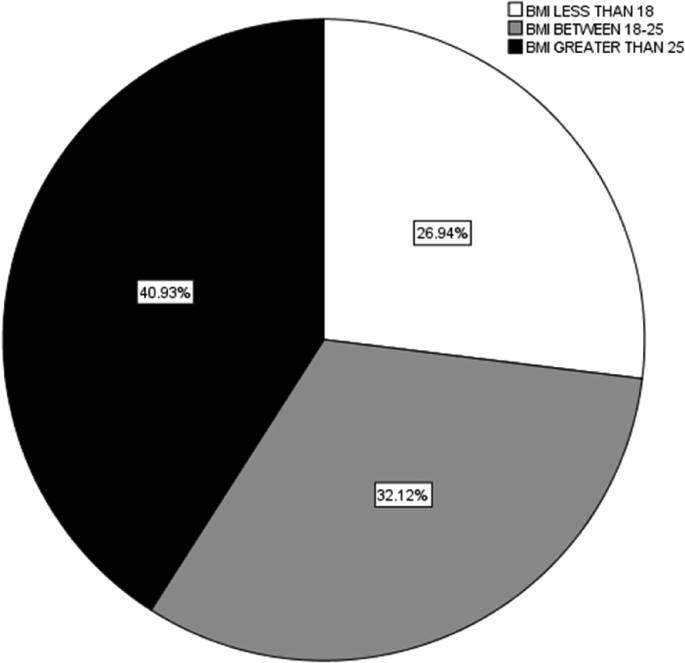
Nafld was present in 62.5%(n = 55) while 28.4% (n = 25) had metabolic syndrome. 26.94% had BMI less than 18, 32.12 had BMI between 18 and 25 and majority had BMI greater than 25 i.e in 40.93%. Of all 46.6% had a family history of cholelithiasis. Gallstone patients with NAFLD reported about their first degree relative being suffering from cholelithiasis at a significant p-value of 0.034 while this was not significant in cases of metabolic syndrome and the p -value was 0.190.
Conclusion
We found association of metabolic syndrome with gallstones and NAFLD. Non alcoholic fatty liver was highly prevalent in our study subjects. Huge percentage of first degree relatives of gall stone patients had gallstones and this relation was more pronounced patients who had associated NAFLD.
Keywords: Non alcoholic fatty liver, Metabolic syndrome, Gallstones, Cholycystectomy, Gallbladder
Highlights
-
•
Most patients with gallstones have associated NAFLD.
-
•
Metabolic syndrome, NAFLD, gallstones share common factors.
-
•
We recommend health education and lifestyle modification in gall stone patients.
-
•
Majority first degree relatives of gall stone patients had gallstones.
-
•
First degree relatives of patients had gallstones and this relation was more pronounced patients who had associated NAFLD.
1. Introduction
Non alcoholic fatty liver disease (NAFLD) has the prevalence of 15%–20%. It is the amassing of fats in the hepatic tissue without significant alcohol intake that result in hepatic steatosis [1]. NAFLD includes a broad range of liver conditions including plain fattiness in hepatic tissue which is static to progressive steatohepatitis that may progress to cirrhotic changes and hepatic carcinoma [2], [3], [4]. NAFLD contributes as the commonest cause that leads to cirrhosis and approximately 22% of NAFLD progresses to cirrhosis as reported by studies. Currently there is no medical remedy to stop this progression or to reverse the cirrhotic condition. How ever weight reduction is the mainstay of preventing cirrhosis in NAFLD patients [5]. Non alcoholic fatty liver disease happens in two phases. First triglycerides get accumulates in liver without hepatic damage and later on hepatic damage takes place. Lipid peroxidation and oxidative stress are the understood noxious pathologies [6], [7].
Patients with gallstones have associated NAFLD, with concurrent metabolic syndrome and these ailments share similar factors for example obesity, hypertriglyceridemia and diabetes mellitus [8].
Gallstone forms by the precipitation of calcium, bilirubin, cholesterol mucous and proteins. It is one of the commonest ailments presenting to surgical units. A study reported an incidence of 9.03% [9]. Nearly 75% of the patients with gall stones are symptomless [10].
Factors like body mass index, gender, raised lipid levels, use of contraceptives and alcohol and having diabetes [11], [12], physical inactiveness [13], multiparous women [14], water with excessive iron content [15], metabolic syndrome [16], and NAFLD [1] are accountable factors for gallstones formation.
The presence of three or more of the following five factors is defined as the Metabolic Syndrome by Adult Treatment Panel III (ATP III) criteria. [17], [18]: (1) hypertriglyceridemia: (higher TG) greater than 150 mg/dL; (2)abdominal obesities e waist girth in men greater than 90 cm and in women greater than 80 cm (3) lower HDL-C: serum HDL-C less than 40 mg/dL in men and <50 mg/dL in women; (4)hyperglycemia: fasting plasma glucose greater than 100 mg/dL or DM history (including medical record or self-reported). (5) elevated blood pressure (systolic blood pressure greater than 130 mmHg or diastolic blood pressure greater than 85 mmHg or hypertension history (including medical record or self-reported).
The study will be a paradigm to identify the presence of Non alcoholic fatty liver disease (NAFLD) and metabolic syndrome in patients with gallstones undergoing laparoscopic or open cholecystectomy along with it we will also study the life style of patients with gall stones. Also role of metabolic syndrome in pathophysiology of Non alcoholic fatty liver disease and gallstone will be identified as well as their interrelation.
2. Objective
Recognition of Non alcoholic fatty liver disease (NAFLD) and metabolic syndrome in patients with gallstones undergoing laparoscopic or open cholecystectomy, along with it we will also study the life style of patients with gall stones.
3. Methodology
This was a case series done at Surgical Unit 1 of Civil Hospital Karachi. Selective samples of 88 patients were included in this which was calculated by www.OpenEpi.com taking the population size of 95 at 95% CI and 3% margin of error [19]. Duration was 3 months. Institutional review board approval for research proposal was also taken from Dow University Of Health Sciences with reference id of IRB-748/DUHS/Approval/2016/240. Random sampling was done. We included both sexes with ultrasound proof of gall stone irrespective of cholecystitis. Excluded patients with history of seropositive viral hepatitis, autoimmune and wilson's disease. As these conditions can act as a confounder to our variables. After signing a consent all the relevant data was collected on a proforma. To make the liver ultrasound report more authentic and to pick up NAFLD more critically a well experience sonographer was given the duty to see the participants of our research. All data collected data was entered and analyzed via SPSS-20.
4. Results
Analysis of the data showed 37.5% (n = 33) were males and 62.5%(n = 55) were females. The mean age of the participants was 36.40 ± 12.97. Majority were married (78.4%). 37.5% belonged to the rural areas while 62.5% were from urban areas. A huge percentage (45.5%) had history of addiction Beatle nut chewing was reported by 40.5% and smoking in 5%. 39% drink un-boiled water. 38.77% reported taking oral contraceptive pills. About 38.6% had abdominal surgery in the past.
Table 1 is representing the education statuses our participant which is showing a very less literacy rate. Most of our participants had no exercise habit as shown by Table 2.
Table 1.
Education status.
| Frequency | Percent | |
|---|---|---|
| Illiterate | 27 | 30.7 |
| School | 39 | 44.3 |
| Undergraduate | 20 | 22.7 |
| Graduate | 2 | 2.3 |
| Total | 88 | 100.0 |
Table 2.
Exercise habit of participants.
| Frequency | Percent | Valid Percent | Cumulative Percent | |
|---|---|---|---|---|
| No | 71 | 80.7 | 80.7 | 80.7 |
| Rarely | 2 | 2.3 | 2.3 | 83.0 |
| Once A Week | 2 | 2.3 | 2.3 | 85.2 |
| Twice A Week | 2 | 2.3 | 2.3 | 87.5 |
| Yes, Often | 11 | 12.5 | 12.5 | 100.0 |
| Total | 88 | 100.0 | 100.0 |
26.94% had BMI less than 18, 32.12 had BMI between 18 and 25 and majority had BMI greater than 25 i.e in 40.93%. The mean weight was 65.7 and standard deviation of ± 15.6 the mean height was 1.6 m and standard deviation of 0.109 m. Abdominal circumference came out to be 87.5 ± 5.8.
Of all 46.6% had a family history of cholelithiasis. Gallstone patients with NAFLD reported about their first degree relative being suffering from cholelithiasis at a significant p-value of 0.034 while this was not significant in cases of metabolic syndrome and the p -value was 0.190.
As far as the parity is concerned 90.73% were multipara. Systolic BP was greater than 130 in 55.30% and diasystolic BP was greater than 85 in 56.69%.
Nafld was present in 62.5% (n = 55) while 28.4% (n = 25) had metabolic syndrome.
The patients with metabolic syndrome had a co morbid of hypertension and diabetes at a p-values of 0.001. and 0.00, respectively which were highly significant.
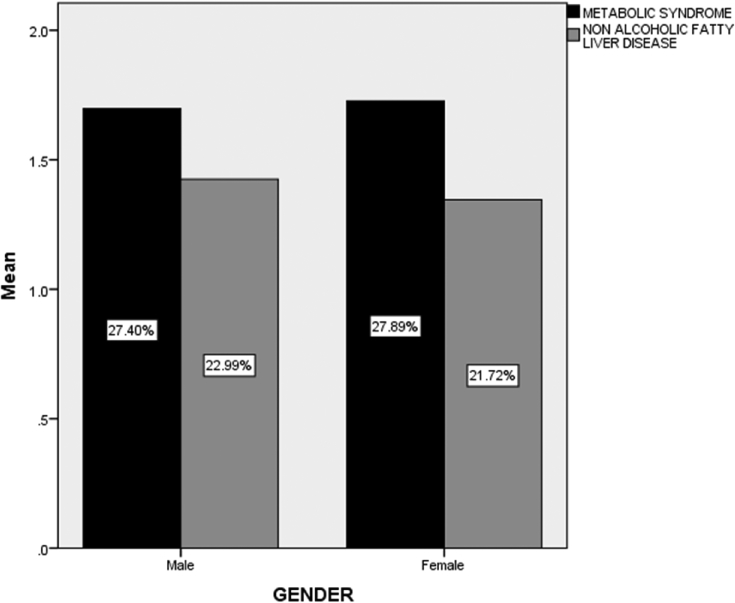
The patient with NAFLD had hypertension and diabetes as a co morbid at a p-values of 0.89 and 0.336, respectively. There is no gender related association of metabolic syndrome nor NAFLD as shown by Fig. 1, Fig. 2.
Fig. 1.
BMIs of the study participants.
Fig. 2.
Gender wise ratios of Metabolic Syndrome and Non Alcoholic Fatty Liver Disease Occurrences among the participants.
Patients with the presence of metabolic syndrome had higher mean SGPT level i.e 86.96 as compared to patients with absent metabolic syndrome (48.09) at a p-value of 0.005. The triglyceride levels in patients with presence metabolic syndrome was higher(mean = 162.5200) and the p value was 0.00 and HDL was significantly low(mean = 98.3600) at a p value of 0.001(Table 3, Table 4).
Table 3.
Analysis of presence of metabolic syndrome relation status with SGPT, SGOT, BILIRUBIN, FBS, TRIGLYCERIDE and HDL.
| N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean |
Minimum | Minimum | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||
| SGPT | Yes | 25 | 86.9600 | 83.20931 | 16.64186 | 52.6129 | 121.3071 | 7.00 | 333.00 |
| No | 63 | 48.0952 | 43.48627 | 5.47875 | 37.1434 | 59.0471 | 7.00 | 300.00 | |
| Total | 88 | 59.1364 | 59.73604 | 6.36788 | 46.4795 | 71.7932 | 7.00 | 333.00 | |
| SGOT | Yes | 25 | 75.0400 | 88.94637 | 17.78927 | 38.3247 | 111.7553 | 8.00 | 333.00 |
| No | 63 | 55.5238 | 52.64484 | 6.63263 | 42.2654 | 68.7822 | 8.00 | 300.00 | |
| Total | 88 | 61.0682 | 65.08382 | 6.93796 | 47.2782 | 74.8581 | 8.00 | 333.00 | |
| Total BILIRUBIN | Yes | 25 | 1.9088 | 3.45250 | 0.69050 | 0.4837 | 3.3339 | 0.25 | 15.70 |
| No | 63 | 1.3695 | 3.29433 | 0.41505 | 0.5399 | 2.1992 | 0.10 | 23.80 | |
| Total | 88 | 1.5227 | 3.32898 | 0.35487 | 0.8174 | 2.2281 | 0.10 | 23.80 | |
| Fasting plasma glucose level(FBS) | Yes | 25 | 108.5600 | 37.12937 | 7.42587 | 93.2337 | 123.8863 | 75.00 | 200.00 |
| No | 63 | 102.4286 | 17.19688 | 2.16660 | 98.0976 | 106.7595 | 75.00 | 180.00 | |
| Total | 88 | 104.1705 | 24.47012 | 2.60852 | 98.9857 | 109.3552 | 75.00 | 200.00 | |
| TRIGLYCERIDE level | Yes | 25 | 162.5200 | 37.79453 | 7.55891 | 146.9192 | 178.1208 | 81.00 | 215.00 |
| No | 63 | 115.6190 | 45.58028 | 5.74258 | 104.1398 | 127.0983 | 51.00 | 210.00 | |
| Total | 88 | 128.9432 | 48.24038 | 5.14244 | 118.7220 | 139.1643 | 51.00 | 215.00 | |
| SERUM HDL | Yes | 25 | 98.3600 | 26.59085 | 5.31817 | 87.3838 | 109.3362 | 32.00 | 150.00 |
| No | 63 | 121.5556 | 27.50354 | 3.46512 | 114.6289 | 128.4822 | 50.00 | 175.00 | |
| Total | 88 | 114.9659 | 29.06570 | 3.09841 | 108.8075 | 121.1243 | 32.00 | 175.00 | |
Table 4.
Anova for metabolic syndrome status with SGPT,SGOT,BILIRUBIN,FBS,TRIGLYCERIDE and HDL.
| ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | Sig. | ||||
| SGPT | Between Groups | (Combined) | 27033.975 | 1 | 27033.975 | 8.203 | 0.005 | |
| Linear Term | Unweighted | 27033.975 | 1 | 27033.975 | 8.203 | 0.005 | ||
| Weighted | 27033.975 | 1 | 27033.975 | 8.203 | 0.005 | |||
| Within Groups | 283416.389 | 86 | 3295.539 | |||||
| Total | 310450.364 | 87 | ||||||
| SGOT | Between Groups | (Combined) | 6816.917 | 1 | 6816.917 | 1.621 | 0.206 | |
| Linear Term | Unweighted | 6816.917 | 1 | 6816.917 | 1.621 | 0.206 | ||
| Weighted | 6816.917 | 1 | 6816.917 | 1.621 | 0.206 | |||
| Within Groups | 361706.674 | 86 | 4205.892 | |||||
| Total | 368523.591 | 87 | ||||||
| Total BILIRUBIN | Between Groups | (Combined) | 5.205 | 1 | 5.205 | 0.467 | 0.496 | |
| Linear Term | Unweighted | 5.205 | 1 | 5.205 | 0.467 | 0.496 | ||
| Weighted | 5.205 | 1 | 5.205 | 0.467 | 0.496 | |||
| Within Groups | 958.937 | 86 | 11.150 | |||||
| Total | 964.142 | 87 | ||||||
| Fasting plasma glucose level | Between Groups | (Combined) | 672.855 | 1 | 672.855 | 1.125 | 0.292 | |
| Linear Term | Unweighted | 672.855 | 1 | 672.855 | 1.125 | 0.292 | ||
| Weighted | 672.855 | 1 | 672.855 | 1.125 | 0.292 | |||
| Within Groups | 51421.589 | 86 | 597.925 | |||||
| Total | 52094.443 | 87 | ||||||
| TRIGLYCERIDE level | Between Groups | (Combined) | 39369.619 | 1 | 39369.619 | 20.760 | 0.000 | |
| Linear Term | Unweighted | 39369.619 | 1 | 39369.619 | 20.760 | 0.000 | ||
| Weighted | 39369.619 | 1 | 39369.619 | 20.760 | 0.000 | |||
| Within Groups | 163091.097 | 86 | 1896.408 | |||||
| Total | 202460.716 | 87 | ||||||
| Serum HDL | Between Groups | (Combined) | 9629.582 | 1 | 9629.582 | 12.966 | 0.001 | |
| Linear Term | Unweighted | 9629.582 | 1 | 9629.582 | 12.966 | 0.001 | ||
| Weighted | 9629.582 | 1 | 9629.582 | 12.966 | 0.001 | |||
| Within Groups | 63869.316 | 86 | 742.666 | |||||
| Total | 73498.898 | 87 | ||||||
Patients with presence of nafld were found to have deranged SGPT and SGOT(Means = 74.4182 and 75.7273) as compared to the ones with absent of nafld at a p value of 0.002 and 0.006 respectively. (Table 5, Table 6).
Table 5.
Analysis of presence OF NON alcoholic fatty liver disease status relation with SGPT,SGOT,BILIRUBIN,FBS,TRIGLYCERIDE and HDL.
| N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean |
Minimum | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Lower Bound | ||||||||
| SGPT | Yes | 55 | 74.4182 | 66.67602 | 8.99059 | 56.3931 | 92.4432 | 12.00 | 333.00 |
| No | 33 | 33.6667 | 33.60215 | 5.84938 | 21.7519 | 45.5815 | 7.00 | 200.00 | |
| Total | 88 | 59.1364 | 59.73604 | 6.36788 | 46.4795 | 71.7932 | 7.00 | 333.00 | |
| SGOT | Yes | 55 | 75.7273 | 75.44865 | 10.17349 | 55.3306 | 96.1239 | 9.00 | 333.00 |
| No | 33 | 36.6364 | 30.41979 | 5.29541 | 25.8500 | 47.4228 | 8.00 | 144.00 | |
| Total | 88 | 61.0682 | 65.08382 | 6.93796 | 47.2782 | 74.8581 | 8.00 | 333.00 | |
| Total BILIRUBIN | Yes | 55 | 1.7747 | 3.87796 | 0.52290 | 0.7264 | 2.8231 | 0.20 | 23.80 |
| No | 33 | 1.1027 | 2.11207 | 0.36766 | 0.3538 | 1.8516 | 0.10 | 12.43 | |
| Total | 88 | 1.5227 | 3.32898 | 0.35487 | 0.8174 | 2.2281 | 0.10 | 23.80 | |
| Fasting plasma glucose level(FBS) | Yes | 55 | 107.6545 | 28.66164 | 3.86473 | 99.9062 | 115.4029 | 75.00 | 200.00 |
| No | 33 | 98.3636 | 13.64006 | 2.37443 | 93.5271 | 103.2002 | 75.00 | 130.00 | |
| Total | 88 | 104.1705 | 24.47012 | 2.60852 | 98.9857 | 109.3552 | 75.00 | 200.00 | |
| TRIGLYCERIDE level | Yes | 55 | 130.5455 | 44.92785 | 6.05807 | 118.3998 | 142.6912 | 51.00 | 215.00 |
| No | 33 | 126.2727 | 53.93415 | 9.38873 | 107.1485 | 145.3969 | 51.00 | 210.00 | |
| Total | 88 | 128.9432 | 48.24038 | 5.14244 | 118.7220 | 139.1643 | 51.00 | 215.00 | |
| Serum HDL | Yes | 55 | 113.2727 | 30.01818 | 4.04765 | 105.1577 | 121.3878 | 32.00 | 175.00 |
| No | 33 | 117.7879 | 27.62444 | 4.80880 | 107.9927 | 127.5831 | 50.00 | 160.00 | |
| Total | 88 | 114.9659 | 29.06570 | 3.09841 | 108.8075 | 121.1243 | 32.00 | 175.00 | |
Table 6.
Anova for NON alcoholic fatty liver disease status WITH,SGOT,BILIRUBIN,FBS,TRIGLYCERIDE HDL.
| ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | Sig. | ||||
| SGPT | Between Groups | (Combined) | 34251.648 | 1 | 34251.648 | 10.665 | 0.002 | |
| Linear Term | Unweighted | 34251.648 | 1 | 34251.648 | 10.665 | 0.002 | ||
| Weighted | 34251.648 | 1 | 34251.648 | 10.665 | 0.002 | |||
| Within Groups | 276198.715 | 86 | 3211.613 | |||||
| Total | 310450.364 | 87 | ||||||
| SGOT | Between Groups | (Combined) | 31517.045 | 1 | 31517.045 | 8.043 | 0.006 | |
| Linear Term | Unweighted | 31517.045 | 1 | 31517.045 | 8.043 | 0.006 | ||
| Weighted | 31517.045 | 1 | 31517.045 | 8.043 | 0.006 | |||
| Within Groups | 337006.545 | 86 | 3918.681 | |||||
| Total | 368523.591 | 87 | ||||||
| Total BILIRUBIN | Between Groups | (Combined) | 9.314 | 1 | 9.314 | 0.839 | 0.362 | |
| Linear Term | Unweighted | 9.314 | 1 | 9.314 | 0.839 | 0.362 | ||
| Weighted | 9.314 | 1 | 9.314 | 0.839 | 0.362 | |||
| Within Groups | 954.828 | 86 | 11.103 | |||||
| Total | 964.142 | 87 | ||||||
| Fasting plasma glucose level | Between Groups | (Combined) | 1780.370 | 1 | 1780.370 | 3.043 | 0.085 | |
| Linear Term | Unweighted | 1780.370 | 1 | 1780.370 | 3.043 | 0.085 | ||
| Weighted | 1780.370 | 1 | 1780.370 | 3.043 | 0.085 | |||
| Within Groups | 50314.073 | 86 | 585.047 | |||||
| Total | 52094.443 | 87 | ||||||
| TRIGLYCERIDE level | Between Groups | (Combined) | 376.534 | 1 | 376.534 | 0.160 | 0.690 | |
| Linear Term | Unweighted | 376.534 | 1 | 376.534 | 0.160 | 0.690 | ||
| Weighted | 376.534 | 1 | 376.534 | 0.160 | 0.690 | |||
| Within Groups | 202084.182 | 86 | 2349.816 | |||||
| Total | 202460.716 | 87 | ||||||
| Serum HDL | Between Groups | (Combined) | 420.473 | 1 | 420.473 | 0.495 | 0.484 | |
| Linear Term | Unweighted | 420.473 | 1 | 420.473 | 0.495 | 0.484 | ||
| Weighted | 420.473 | 1 | 420.473 | 0.495 | 0.484 | |||
| Within Groups | 73078.424 | 86 | 849.749 | |||||
| Total | 73498.898 | 87 | ||||||
5. Discussion
A nation wide survey conducted in America revealed a very high prevalence of cholelithiasis i.e. 13.9%–26.7% in women and 5.3%–8.9% in men under the domain of third National Health and Nutrition Examination Survey [20]. In Pakistan, the prevalence of gallstone is still not known. However in India which is the neighbouring country to Pakistan with similar geography and ethnicity had a study on 5100 and 1448 people with and without symptoms of gallstones. They had gallbladder ultrasonography that showed noteworthy figures of prevalence of 5.59% in women and 1.99% in males [21]. Our study showed this trend as well, as most (62.5%) of the gallstone patients were females. There is a general conjecture that sex hormones and cholesterol metabolism have possible interrelation [16]. This makes a point to ruminate on how sex is implicated in cholesterol stones formation. Most of the studies suggest female predominance when it comes to the prevalence of gallstones along with it the likelihood of being at risk for stone formation [20], [21], [22]. On contrary, Liu CM et al. denied gender relation to gallstones [23]. . The mean age of the participants was 36.40 ± 12.97 and this was in line with the results of study by Lirussi F. et al. that indicated an existence of high age relation with cholelithiasis more astounded in women [22]. Liu CM et al. explained since people with high age had been more exposed to a pile of chronic factors like alcohol intake, hyperlipidemia and diabetes mellitus (DM), hence they are more prone to gallstone development due to manifestation of decreased motility of gallbladder [23].
Chen LY et al. [16] while discussing gallstone formation phenomenon also favored the theory of hyperlipidemia, decreased motility of gall bladder being overweight and insulin resistance as culprit factors of cholelithiasis pathology. According to him separation of crystals of cholesterol from supersaturated bile is the prime event. Phospholipids and bile acids makes cholesterol more soluble and prevents its precipitation. Phospholipid transfer protein (PLTP) transfers lipids from low-density lipoproteins to high-density lipoproteins. In hyperlipidemia, haptoglobin inhibits PLTP and results in reversal of cholesterol transport. Cholesterol transporter ABCG5-G8 and phospholipid floppase ABCB4 polymorphisms makes one vulnerable to gallstone risk [16]. Protein kinase(PKCβ)is also considered as a major physiological regulator of lipids [16].
A huge percentage (45.5%) of our participants had habit of addiction. Of all 5% had smoking addiction. Diehl et al. [24] argued about role of smoking in stone formation as nicotine would probably raises the lithogeneity of the bile. Beatle nut chewing was very commonly seen in our study and 40.5% had its addiction which is much higher than another study which reported a figure of 16.6% in the neighbouring country India [25].
None of our study participant had alcoholism habit. Alcohol consumption counts as a contributing factor for gallstone formation [11], [12]. In oppose to this there is a study that says that intake of small to moderate amount of alcohol lowers the biliary cholesterol saturation index and thus act as protective [26].
In this study 39% used to drink un-boiled water. Unisa S et al. also noticed the drinking of water from unsafe source was common in gallstone patients [21]. 38.77% reported taking oral contraceptive pills which is a known risk factor for cholelithiasis [11], [12].
Our study results were also in coherence with another series in matter of educational status as most of the participants were found to have low education [21]. As per Table 2 most of the study participants had inactive lifestyle. Physical inactiveness is a well known factor accounted for gallstones [13].
About 38.6% had abdominal surgery in the past. A with retrospective study design showed that major abdominal surgery can result in acceleration of stone formation. However this needs to be confirmed through a prospective research so that prophylactic measures could be taken [27].
Majority (40.93%) of our participants had a BMI greater than 25(Fig. 1), nevertheless the mean of abdominal circumference came out to be greater as well i.e. 87.5 ± 5.8. Our results in terms of BMI are quite different from another study that reported 80% of patients had normal BMI and that was no different than control group [25]. Obese women are slightly higher risk of stone formation as compared to women with ideal BMI especially when talking about cholesterol gallstone [24]. The observation of Trotman has brought the fact into light that pigment GS and with cholesterol GS patients had no differences in their BMIs [28].
Our results show 46.6% of the first degree relatives of the patients also had cholelithiasis and this trend was also in assertion as proved by another study [29]. This point leads to contemplate a strong reason to screen the first degree relatives for gallstones.
Out of all 90.73% our participants were multipara. Parity has been accounted as one of the significant factors for GS(14). Sarin et al. [30] mentioned about 94% of patients were multiparous in his study outcome.
A previous study made the fact apparent that the obese patients with an Asian lineage had raised diastolic blood pressure. Our study out came in the same harmony, as diasystolic BP was greater than 85 in 56.69%. Moreover the Systolic BP was also greater than 130 in 55.30%. Reason being people with hypertension may have more sympathetic nerve activities that may probably decrease bowel movements [31].
Patients with gallstones have associated NAFLD, with concurrent metabolic syndrome and these ailments share similar factors for example obesity, hypertriglyceridemia and diabetes mellitus [8]. The patients with metabolic syndrome had a co morbid of hypertention and diabeties at a p-values of 0.001. and 0.00, respectively which were highly significant. The patient with NAFLD had hypertention and diabetes as a co morbid at a p-values of 0.89 and 0.336, respectively.
The existing association between cholilithiasis and metabolic syndrome has never been studied before 2005. Afterwards Mendez-Sanchez et al. [32] presented the first report which showed a very strong association among the two entities. The author was so much fascinated my that association that leaded him to conclude “ …. gallstone disease may be a part of metabolic syndrome.”The established association was later on got confirmed by studies with a large sample sizes [16], [33]. Ata N et al. indicated prophylactic surgery in patients with metabolic syndrome for gallstones [33]. In our study 28.4% had metabolic syndrome.
Metabolic syndrome has the prevalence of 25% in Europe [34] and 10–19% in Asia [35]. The contributing factors for metabolic syndrome include sedentary lifestyle, genetic predisposition Specific macronutrients, and higher intake of total energy. Pakistan has prevalence of 18%–46%. About 46%–75% diabetic patients suffers metabolic syndrome [35].
I-Ching Lin et al. reported difference in the means of HDL-C, BMI, TG, systolic B.P, diastolic B.P, fasting blood glucose and age between gallstone and non gallstone groups [36]. These facts are also evident by our results more in patients with metabolic syndrome (Table 3, Table 4). The mean TG was high i.e 162.52 with standard deviation of ± 37.79 in patients with metabolic syndrome than with no metabolic syndrome at very high significant p-vale of 0.00. Similar differences were also seen in HDL among the two groups with a significant p value of 0.001(Table 4).
Apart from gall stone development there is other various concerns with metabolic syndrome as some studies have reported its association with diabetes, cardiovascular disease chronic kidney disease and nonalcoholic fatty liver disease [37], [38]. So, metabolic syndrome hovers as a gigantic health issue globally.
Non alcoholic fatty liver disease (NAFLD) has the prevalence of 15%–20% [1]. It stands among the most common chronic liver conditions and is becoming the center of concern in the world of medicine [39]. During the recent years its occurrence has been shown rising because of dramatically increased rates in obesity, metabolic syndrome and diabetes [1].
The prevalence of NAFLD among outpatients is well established and described in various studies [1], [39] while the proportion for hospitalized patients not known [39]. Further more the association of NAFL with other gastrointestinal conditions has not been into talk [39].
In our study NAFLD was present in 62.5%. Moreover patients with NAFLD had a deranged and raised SGPT and SGOT levels (Table 5, Table 6). The means were raised in NAFLD patients i.e 74.41 and 75.72 respectively as compared to the ones with no NAFLD. The P-values were significant i.e. 0.002 and 0.006 respectively. Our results are in accordance with another study with similar objective [29]. However presence of normal ranges of liver enzymes does not exclude NAFLD as rectified by some other series [19], [40]. Mofrad et al. as reported a NAFLD spectrums with normal values of ALT in retrospective series. The result of one series showed about 10% of the gallstone patients at the time of diagnosis had NAFLD progressed to fibrosis which was biopsy proven.
NAFLD contributes as the commonest cause that leads to cirrhosis, portal hypertension and hepatic cancer [5] and it has been estimated that approximately 22% of NAFLD progresses to cirrhosis [5]. One of the study justified for performing liver biopsy for the detection of NAFLD during cholecystectomy and that series captured more NAFLD in patients who undergone biopsies than those with simple ultrasound findings and liver function tests [19]. We would like to mention here that this case series was done according to PROCESS Guidelines [41].
6. Conclusion
Non alcoholic fatty liver was highly prevalent in our study subjects. Based on our study results we recommend health education and implementation of lifestyle modification when patients present with gall stone disease requiring cholecystectomy as many of them could have NAFLD which may eventually progress to cirrhosis. We also found association of metabolic syndrome with gallstones and NAFLD. A huge percentage of first degree relatives of gall stone patients had gallstones and this relation was more pronounced patients who had associated NAFLD. A huge percentage of first degree relatives of gall stone patients had gallstones and this relation was more pronounced patients who had associated NAFLD.
Strengths and limitations of this study
The study helped to enlighten the possible link between gallstone disease, NAFLD and MS which are top-line discussion topics nowadays. Many pertinent evidences were discussed along with the possible theories behind as their explanations. Not only this, we also discussed how outcomes of the study can help in future interventions. We also discussed if we could control MS and NAFLD occurrences we can minimize gallstone disease and respective surgery. And this all can help in decreasing the morbidity, mortality of gallstone and the complications due to its surgery.
Ethical approval
Yes.
IRB-748/DUHS/Approval/2016/240.
Sources of funding
None.
Author contribution
Dr.Qamaruddin Baloch and Zahid Ali Memon did all the surgeries.
Dr.Farah and Iqra did data collection.
Everybody contributed in writing and thinking process.
Conflicts of interest
None.
Guarantor
Professor Qamaruddin Baloch, Farah Ahmed.
Research registration unique identifying number (UIN)
It was an observational study.
References
- 1.Ashtari Sara, Amin Pourhoseingholi Mohamad, Zali Mohamad Reza. Non-alcohol fatty liver disease in Asia: prevention and planning. World J. Hepatol. 2015 Jul 8;7(13):1788–1796. doi: 10.4254/wjh.v7.i13.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E., Leone N., Vanni E., Marchesini G., Brunello F. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 5.Browning J., Szczepaniak L., Dobbins R. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Browning J.D., Horton J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque M., Sanyal A.J. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best. Pract. Res. Clin. Gastroenterol. 2002;16:709–731. doi: 10.1053/bega.2002.0325. [DOI] [PubMed] [Google Scholar]
- 8.Fracanzani Anna Ludovica, Valenti Luca, Russello Maurizio, Miele Luca, Bertelli Cristina, Bellia Alessandro . Gallstone disease is associated with more severe liver damage in patients with non-alcoholic fatty liver disease. PLoS One. 2012;7(7):e41183. doi: 10.1371/journal.pone.0041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naeem Muhammad, Ali Rahimnajjad Nasir, KazimRahimnajjad Muhammad, MadihaKhurshid, Ahmed QaziJalaluddin, Mariam Shahid Syed, Khawar Faiza, Najjar MolhamMustafa. Assessment of characteristics of patients with cholelithiasis from economically deprived rural Karachi. Pakistan. 2012 Jun 28 doi: 10.1186/1756-0500-5-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S.K., Shukla V.K. Silent gallstones: a therapeutic dilemma. Trop. Gastroenterol. 2004 Apr-Jun;25(2):65–68. [PubMed] [Google Scholar]
- 11.Kratzer W., Kachele V., Mason R.A., Muche R., Hay B., Wiesneth M., Hill V., Beckh K., Adler G. Gallstone prevalence in relation to smoking, alcohol, coffee consumption, and nutrition. The Ulm Gallstone Study. Scand. J. Gastroenterol. 1997;32:953–958. doi: 10.3109/00365529709011208. [DOI] [PubMed] [Google Scholar]
- 12.Amigo L., Zanlungo S., Mendoza H., Miquel J.F., Nervi F. Risk factors and pathogenesis of cholesterol gallstones: state of the art. Eur. Rev. Med. Pharmacol. Sci. 1999;3:241–246. [PubMed] [Google Scholar]
- 13.Völzkea Henry, Baumeistera Sebastian E., Altea Dietrich, Hoffmannb Wolfgang, Schwahna Christian, Simon Peter, Johna Ulrich, Lerchc Markus M. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 14.Mousavi E., Gharipour M., Tavassoli A., Sadri G.H., Sarrafzadegan N. Multiparity and risk of metabolic syndrome: Isfahan healthy heart program. MetabSyndrRelatDisord. 2009 Dec;7(6):519–524. doi: 10.1089/met.2008.0076. [DOI] [PubMed] [Google Scholar]
- 15.Sharma Rajani, Kumar Ujwal, Jha Nawal Kisore, Sachan Shashwati Ghosh, Sharma Shubha Rani. 2014. Consumption of Heme Iron: a Major Risk Factor Is Pigment Gallstone Formation. IJBR.Vol-5 No 1. [Google Scholar]
- 16.Chen Li-Ying, Qiao Qiao-Hua, Zhang Shan-Chun, Chen Yu-Hao, Chao Guan-Qun, Fang Li-Zheng. Metabolic syndrome and gallstone disease. World J. Gastroenterol. 2012;21(18(31)):4215–4220. doi: 10.3748/wjg.v18.i31.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai T.Y., Cheng J.F., Lai Y.M. Prevalence of metabolic syndrome and related factors in Taiwanese high-tech industry workers. Clinics. 2011;66:1531–1535. doi: 10.1590/S1807-59322011000900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ATP III At-a-glance: Quick Desk Reference. Available at :http://www.nhlbi.nih.gov/health-pro/guidelines/current/cholesterol-guidelines/quick-desk-reference-html.
- 19.Oktay Yener, Fikret Aksoy, Mustafa Dem‹r, Alp Özçel‹k, Canan Erengül. Gallstones associated with nonalcoholic steatohepatitis (NASH) and metabolic syndrome. Turk J. Gastroenterol. 2010;21(4):411–415. doi: 10.4318/tjg.2010.0128. [DOI] [PubMed] [Google Scholar]
- 20.Everhart J.E., Khare M., Hill M., Maurer K.R. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999 Sep;117(3):632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 21.Unisa Sayeed, Jagannath Palepu, Dhir Vinay, Khandelwal Chiranjeeva, Sarangi Lalatendu, Roy Tarun Kumar. Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB Oxf. 2011 Feb;13(2):117–125. doi: 10.1111/j.1477-2574.2010.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lirussi F., Nassuato G., Passera D., Toso S., Zalunardo B., Monica F., Virgilio C., Frasson F., Okolicsanyi L. Gallstone disease in an elderly population: the Silea study. Eur. J. Gastroenterol. Hepatol. 1999;11:485–491. doi: 10.1097/00042737-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Liu C.M., Tung T.H., Chou P., Chen V.T., Hsu C.T., Chien W.S., Lin Y.T., Lu H.F., Shih H.C., Liu J.H. Clinical correlation of gallstone disease in a Chinese population in Taiwan: experience at Cheng Hsin General Hospital. World J. Gastroenterol. 2006;12:1281–1286. doi: 10.3748/wjg.v12.i8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diehl A.K., Schwesinger W.H., Holleman D.R., Jr., Chapman J.B., Kurtin W.E. Clinical correlates of gallstone composition: Distinguishing pigment from cholesterol stones. Am. J. Gastroenterol. 1995;90:967–972. [PubMed] [Google Scholar]
- 25.Jayanthi V., Prashanthi R., Sivakumar G., Surendran R., Srinivas U., Mathew S., Rajakumar S., Palanivelu C., Ramesh A., Prabhakar K., Subramanian G., Ramathilakam B., Vijaya S. Epidimiology of gallstone disease – Top line findings. Bombay Hosp. J. 1999;41:494–502. [Google Scholar]
- 26.Scragg R.K.R., McMichael A.J., Baghurst P.A. Diet, alcohol and relative weight in gallstone disease : a case control study. Br. J. Med. 1984;288:1113–1119. doi: 10.1136/bmj.288.6424.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little J.M., Avramovic J. Gallstone formation after major abdominal surgery. Lancet. 1991 May 11;337(8750):1135–1137. doi: 10.1016/0140-6736(91)92796-5. [DOI] [PubMed] [Google Scholar]
- 28.Trotman B.W., Ostrow J.D., Soloway R.D. Pigment vs cholesterol cholelithiasis : comparison of stone and bile composition. Am. J. Dig. Dis. 1974;19:585–590. doi: 10.1007/BF01073011. [DOI] [PubMed] [Google Scholar]
- 29.Sarin S.K., Negi V.S., Dewan R., Sasan S., Saraya A. High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology. 1995 Jul;22(1):138–141. [PubMed] [Google Scholar]
- 30.Sarin S.K., Kapur B.M.L., Tandon R.K. Cholesterol and pigment gallstones in northern India. A prospective analysis. Dig. Dis. Sci. 1986;31:1041–1045. doi: 10.1007/BF01300256. [DOI] [PubMed] [Google Scholar]
- 31.Liew P.L., Wang W., Lee Y.C., Huang M.T., Lin Y.C., Lee W.J. Gallbladder disease among obese patients in Taiwan. Obes. Surg. 2007;17:383–390. doi: 10.1007/s11695-007-9068-4. [DOI] [PubMed] [Google Scholar]
- 32.Méndez-Sánchez N., Chavez-Tapia N.C., Motola-Kuba D., Sanchez-Lara K., Ponciano-Rodríguez G., Baptista H., Ramos M.H., Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J. Gastroenterol. 2005 Mar 21;11(11):1653–1657. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ata N., Kucukazman M., Yavuz B., Bulus H., Dal K., Ertugrul D.T., Yalcin A.A., Polat M., Varol N., Akin K.O., Karabag A. Nazligul Y Can. J. Gastroenterol. 2011 May;25(5):274–276. doi: 10.1155/2011/356761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panayiotou A.G., Griffin M., Kouis P., Tyllis T., Georgiou N., Bond D. Association between presence of the metabolic syndrome & its components with carotid intima-media thickness & carotid and femoral plaque area: a population study. Diabetol. Metab. Syndr. 2013;5(1):44. doi: 10.1186/1758-5996-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basit A., Shera A.S. Prevalence of Metabolic syndrome in Pakistan. Metab. Syndr. Relat. Disord. 2008;6(3):171–175. doi: 10.1089/met.2008.0005. [DOI] [PubMed] [Google Scholar]
- 36.Lin I-Ching, Yang Yu-Wen, Wu Mei-Feng, Yeh Yi-Hui, Liou Jenn-Chang, Lin Ying-Li, Chiang Chih-Hsiang. The association of metabolic syndrome and its factors with gallstone disease. BMC Fam. Pract. 2014;15:138. doi: 10.1186/1471-2296-15-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattar N., McConnachie A., Shaper A.G., Blauw G.J., Buckley B.M. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal V., Shah A., Rice C., Franklin B.A., McCullough P.A. Impact of treating the metabolic syndrome on chronic kidney disease. Nat. Rev. Nephrol. 2009;5:520–528. doi: 10.1038/nrneph.2009.114. ([PubMed]) [DOI] [PubMed] [Google Scholar]
- 39.Reddy S.K., Zhan M., Alexander H.R., El-Kamary S.S. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World J. Gastroenterol. 2013 Dec 7;19(45):8301–8311. doi: 10.3748/wjg.v19.i45.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mofrad P., Contos M.J., Haque M. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003 Jun;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 41.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P., the PROCESS Group The PROCESS Statement: Preferred Reporting of Case Series in Surgery. Int. J. Surg. 2016;36(Pt A):319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]