Abstract
Introduction
Tyrosinemia Type 1 (HT1) is an autosomal recessive disorder caused by a defect in the enzyme fumarylacetoacetate hydroxylase in the tyrosine pathway. Implementation of nitisinone (NTBC) treatment has dramatically improved survival rate of individuals with HT1, yet recent reports on cognitive impairment in treated patients exist.
Aims
Describe long-term neurocognitive outcome individuals with HT1 treated with nitisinone and protein restricted diet.
Methodology
Twelve individuals with HT1 were analyzed with respect to psychomotor development and cognitive functioning using standardized psychometric tests. Plasma tyrosine and phenylalanine concentrations were also collected and analyzed, as part of the regular HT1 follow up program in our clinic.
Results
Delayed performance in Bayley scale mental developmental index (MDI) was identified in 29% to 38% of the patients assessed at different ages. At preschool age, mean full scale IQ (FSIQ) was 88 ± 16; six out of nine assessed children preformed within normal range, and one child presented with intellectual disability. At school age mean FSIQ was 79 ± 18, three out of nine children preformed within normal range and two showed intellectual disability. Repeated measures showed IQ decline over time in four out of eight patients, all of whom presented with symptoms in their first months of life. Patients that showed no progressive IQ decline were 8 months or older at diagnosis, with a mean age of 17 months. Significant correlation between Phe/Tyr ratio and FSIQ at school age was identified (r = − 0.689; p < 0.044).
Conclusion
Some patients with HT1 treated with nitisinone and protein restricted diet are at risk of presenting developmental delay and impaired cognitive functioning. Patients with early onset of symptoms could be at risk for progressive cognitive functioning decline over time.
Keywords: Tyrosinemia type 1, Nitisinone, NTBC, Tyrosine, Cognitive impairment
1. Introduction
Tyrosinemia Type 1 (HT1) is an autosomal recessive disorder caused by a defect in the fumarylacetoacetate hydroxylase enzyme catalyzing the last step of tyrosine breakdown. Toxic metabolites are formed including succinylacetone, maleylacetoacetate and fumarylacetoacetate that are responsible for the hepatic and renal manifestations of the disease. In 1992, the compound 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC, nitisinone) began to be used in the treatment of HT1, to prevent the accumulation of toxic metabolites. Implementation of nitisinone treatment has dramatically improved survival rate of individuals with HT1 [8].
Survival improvement is directly correlated to age at treatment initiation, especially when nitisinone is introduced during the first weeks of life [20] and hepatocellular carcinoma has not developed in the 5 first years of follow up. These findings explain the incorporation of HT1 to national newborn screening programs in several countries. With survival improvement, long-term complications such as cognitive impairment have been described.
Cognitive functioning in patients with HT1 has recently been studied with very diverse results. Masurel-Paulet et al. [9] first reported a high frequency of cognitive impairment causing schooling problems in a retrospective study of 46 patients with HT1 treated with nitisinone. In this sample, 35% of 23 school age children had schooling difficulties and 6 children had major cognitive disturbances. However, this retrospective study lacked specific psychometric testing.
Several single-center studies assessing intelligence quotient (IQ) in small groups reported an average IQ in HT1 patients below the normal range, but with important intragroup variability. None of these studies were able to identify a clear correlation between metabolic control and cognitive functioning [2], [4], [5]. A high frequency of variable dysfunction or retardation in language development was identified in one study [14].
Different results were reported by Pohorecka et al. [11] in a group of 9 Polish children with HT1. Children were assessed with WISC R and all performed within the normal range on all scales. In this group no emotional or behavioral problems were identified. Only attention difficulties were reported, which the authors considered might be associated with plasma tyrosine levels.
Patients with HT1 might be at risk of progressive cognitive deterioration over time. Bendadi et al. [4] reported results on 5 patients in which IQ tests were repeated at 2- to 3-year intervals. An average drop of 27 IQ points was observed. Also poorer executive functioning (working memory and cognitive flexibility) and social cognition compared to healthy controls has been reported [15].
The present study reports results on the long-term cognitive functioning in individuals with HT1 treated with nitisinone and protein restricted diet.
This is one of the longest and largest reports on neurocognitive functioning in patients with HT1 under NTBC treatment. Being a single-center study, all patients were treated and assessed using the same protocol.
2. Material and methods
2.1. Subjects
The present is a retrospective, single center study. At INTA, 17 patients have been diagnosed and treated for HT1. Of the total group, one patient died due to hepatocellular carcinoma before a liver transplant could be performed and another died from complications after liver transplant. Two successful liver transplants have been performed; one patient who successfully received a liver transplant was excluded because she did not receive nitisinone and was lost to follow-up. Informed consent was not obtained for one of the patients and one patient who was recently diagnosed was not included because psychometric evaluation had not yet been performed. Previously most of the HT1 patients were included in an international multicenter research project on nitisinone [21].
Data collected between 1996 and 2015 on twelve individuals with HT1 treated with nitisinone and a protein-restricted diet were analyzed in the present study (Table 1). All had been clinically diagnosed, with a confirmed diagnosis at a mean of 9.8 months of age. Nitisinone treatment was initiated between 2 and 42 months of age, with a mean of 27 months. One of the patients required a liver transplant, but this patient's cognitive functioning data are from prior to the transplant.
Table 1.
Characteristics of the individuals.
| Patient | Age at time of study | Sex | Age at diagnosis (months) |
Age at start of NTBC | Education | NTBC serum μmol/L |
Mean Tyra μmol/L |
Mean Phea μmol/L |
Relevant information | Psychomotor and cognitive functioning | Symptoms at diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 y, 7 m | Female | 2 m | 3 m | Regular | 39 ± 20 | 587 ± 220 | 75 ± 21 | Borderline IQ | Acute hepatic failure | |
| 2 | 17 y, 4 m | Female | 36 m | 42 m | Regular | 48 ± 19 | 656 ± 71 | 80 ± 20 | Borderline IQ | Rickets-hepatosplenomegaly | |
| 3 | 14 y, 2 m | Male | 2 m | 4 m | Special | 38 ± 10 | 267 ± 128 | 65 ± 28 | History of child neglect | Intellectual disability | Hepatosplenomegaly-nephrocalcinosis rickets |
| 4 | 12 y, 5 m | Male | 11 m | 11 m | Regular | 38 ± 11 | 381 ± 163 | 80 ± 31 | Normal IQ | Rickets-hepatosplenomegaly | |
| 5 | 12 y, 5 m | Male | 9 m | 10 m | Regular | 42 ± 18 | 428 ± 247 | 67 ± 28 | Normal IQ | Acute hepatic failure | |
| 6 | 12 y, 7 m | Female | 8 m | 9 m | Regular | 38 ± 12 | 533 ± 245 | 85 ± 32 | Normal IQ | Acute hepatic failure, Rickets | |
| 7 | 10 y, 9 m | Female | 3 m | 4 m | Regular | 37 ± 13 | 583 ± 303 | 93 ± 26 | Borderline IQ | Acute hepatic failure, Coagulopathy | |
| 8 | 9 y, 9 m | Male | 1 m | 1 m | Regular | 30 ± 8 | 444 ± 155 | 88 ± 26 | ADD | Intellectual disability | Acute hepatic failure |
| 9 | 7 y, 6 m | Female | 6 m | 8 m | Regular | 41 ± 14 | 381 ± 159 | 107 ± 14 | Epilepsy, Ulcerative colitis | Borderline IQ | Hepatomegaly |
| 10 | 5 y, 5 m | Female | 4 m | 6 m | Regular | 22,6 ± 5 | 236 ± 122 | 95 ± 56 | Pre-term birth | Normal IQ | Hepatosplenomegaly - Acute hepatic failure |
| 11 | 3 y, 8 m | Male | 11 m | 12 m | 40 ± 14 | 187 ± 100 | 93 ± 31 | Normal motor and mental development | Rickets hepatosplenomegaly | ||
| 12 | 1 y, 8 m | Male | 10 m | 10 m | b | b | b | Neonatal asphyxia | Developmental delay | Acute hepatic failure, Hepatosplenomegaly, hypoglycemia |
From diagnose to moment to last assessment.
Data not included due to limited number of samples due to age.
Treatment was initiated immediately after diagnosis in all patients. Nitisinone was administered at a 1 mg/kg/day bodyweight dose. The targeted NTBC serum concentration was between 30 and 60 μmol/L [6]. NTBC dose was adjusted according bodyweight, and seeking to maintain succinylacetone in plasma complete suppression.
All children were prescribed a low-phenylalanine (PHE), low-tyrosine (TYR) diet designed to meet their needs for growth without providing excesses of these amino acids, according to RDI [6] Supplementation with mixture of amino acids free of TYR and PHE was prescribed for all patients. All though during some periods, children did not follow the indication, due to the high cost of the formula. None of the patients received phenylalanine supplementation due to phenylalanine concentrations below the lower target limit (< 35 μmol/L).
2.2. Instruments
Psychomotor development was assessed with Bayley-II during infancy, and cognitive performance at preschool and school age with Wechsler age appropriate scales. Mean plasma tyrosine and phenylalanine levels during first three years of life and the complete treatment period were analyzed. All patients had their routine blood analysis done every three months, in the same dates.
-
–
The Bayley [3] scales of Infant Development Second Edition assesses psychomotor development from the first month of life until 42 months of age. Standard scores are derived for a mental development index (MDI) and a motor or performance development index (PDI) with a mean of 100 (SD = 15).
-
–
The Wechsler Preschool and Primary Scale of Intelligence (WPPSI) (1996) provides a verbal IQ (VIQ), performance IQ (PIQ) and full scale IQ (FSIQ) for children 4 to 6 years of age, with standard score means of 100 (SD = 15) [18].
-
–
The Wechsler Intelligence Scale for Children, Revised and third edition (WISC-R or WISC-III), which also provides a VIQ, PIQ and full scale IQ (FSIQ; mean = 100, SD = 15) were administered to children 6.5 to 16 years [19].
2.3. Statistical analysis
The data were analyzed and are presented as mean ± SD or median (interquartile range), depending on the normality of the results. To determine the correlation between infant development and metabolic control, data on Tyr and Phe plasma concentrations, Tyr/Phe ratio and child mental development index (MDI) at age 30–36 months were analyzed using the Spearman correlation test. The same statistical analysis was used to determine correlation between data on life-long Tyr and Phe plasma concentrations and Tyr/Phe ratio and school age FSIQ. To evaluate if the IQ decline over time was significant in the group of patients with declining IQ, a paired t-test was performed. Statistical significance was considered when p < 0.05. Statistical analysis was performed with GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Psychomotor development and cognitive functioning during childhood
Psychomotor development between 6 and 16 months of age showed a mean MDI in the low normal range, with two patients having mental developmental delay (Table 2). Mean PDI was diminished, showing a higher frequency of delay in the motor area.
Table 2.
Mental and motor performance scores as assessed with Bayley's scales of Infant Development in HT1 at 6 to 42 months of age.
| Age at testing | |||
|---|---|---|---|
| Test | 6–16 months | 16–26 months | 28–42 months |
| (N = 6) | (N = 7) | (N = 8) | |
| MDI | 84 ± 23 | 80 ± 17 | 77 ± 20 |
| PDI | 69 ± 17 | 74 ± 16 | 85 ± 20 |
| MDI < 70 | 2 (33%) | 2 (29%) | 3 (38%) |
MDI: mental development index; PDI: performance development index (mean ± SD).
Between 16 and 26 months mean MDI was maintained in the same range and better results were seen in the PDI. Between ages 28 and 42 months mean MDI was diminished, with three out of eight patients having mental developmental delay. During this period, the mean PDI was in the low normal range, showing improvement regarding previous periods (Table 2).
Not all the same patients participated in each of the three assessment periods; therefore, results are described, but not compared between ages.
Between 4 and 6 years of age, mean full scale IQ (FSIQ) was low average, with similar results in verbal and performance areas (Table 3). One of the nine patients presented with mental retardation (FSIQ 65), two had borderline IQ (FSIQ 78–79), three had low average IQ (FSIQ 82–85), and three patients had normal average IQ (FSIQ 104–114).
Table 3.
Intellectual performance of HT1 preschool and school ages, assessed with WPPSI and WISC-R or WISC III.
| Age at testing | ||
|---|---|---|
| Test | 4 to 6 years of age | 6 to 10 years of age |
| (N = 9) | (N = 9) | |
| VIQ | 89 ± 15 | 81 ± 19.6 |
| PIQ | 90 ± 15 | 80 ± 15 |
| FSIQ | 88 ± 16 | 79 ± 18 |
| FSIQ 70–79 | 2 (22%) | 4 (44%) |
| FSIQ < 70 | 1 (11%) | 2 (22%) |
VIQ: verbal intellectual quotient, PIQ: performance intellectual quotient, FSIQ: full-scale intellectual quotient (mean ± SD).
At school age, mean FSIQ, VIQ and PIQ were on the dividing line between borderline and low average, showing similar functioning between verbal and performance scales (Table 3). Of the nine patients, three performed within the normal range (FSIQ 89–111) and four within the borderline range (FSIQ 72–78). Two children had mental retardation (FSIQ 50–66). The child who performed most poorly was under child protection service care and had a history of severe neglect and child abuse. The other child with mental retardation was diagnosed with attentional deficit disorder and had interrupted pharmacological treatment at the time the cognitive assessment took place, which might have partially influenced the results.
Of the three children with severe developmental delay according to the mental development index at age 28–42 months, at school age one had a normal IQ, one had borderline IQ and one had intellectual disability. Of the two patients who had mild developmental delay at 24 months, at school age one had a normal IQ and the other a borderline IQ. Of the three patients who had normal development in mental scale at 24 months, all had normal IQs at school age.
3.2. Long-term cognitive performance
To evaluate long-term cognitive functioning, data were analyzed in 8 patients who had two or more IQ assessments available in a minimum of a two-year period. Mean IQ in the first evaluation available was 85.8 (range 65–111). The mean IQ in the last evaluation was 78.5 (range 40–111). The difference between first and last IQ assessment was not statistically significant.
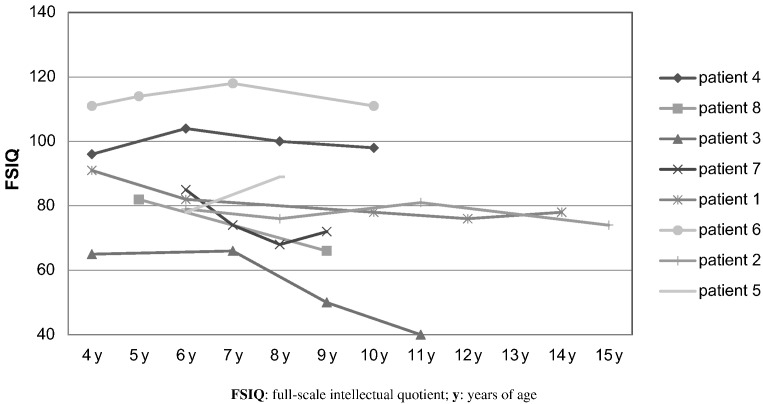
Four of the eight patients had progressive IQ declines, with an average loss of 16.75 points of IQ (range 13–25 points SD 5.7). The IQ decline in these four patients was significant (p > 0,001). The four children in this group presented with their first symptoms between 1 and 3 months of age and started treatment at a mean age of 2 months (Fig. 1).
Fig. 1.
Longitudinal IQ scores in patients with Tyr1 under nitisinone treatment. FSIQ: full-scale intellectual quotient; y: years of age.
Three patients performed within the same range over time and one increased 11 points, going from borderline to low average IQ. The four children who showed no FSIQ decline were diagnosed between 8 and 47 months of age with a mean age of 17 months (Fig. 1).
3.3. Metabolic control and psychomotor development and cognitive functioning
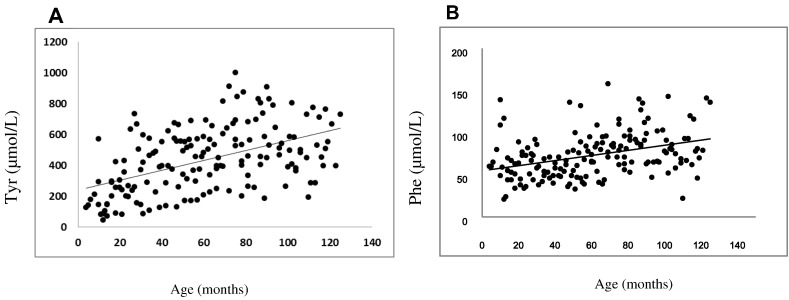
To assess the impact of metabolic control on psychomotor development, historical levels of phenylalanine (Phe), tyrosine (Tyr) and the Phe/Tyr ratio were analyzed. Phe (r = 0.145; p < 0.0034) and Tyr (r = 0.454; p < 0.0001) concentration were significantly correlated with age, increasing through time (Fig. 2). No correlation was found between tyrosine, phenylalanine or the Phe/Tyr ratio during the first three years of life and Bayley's mental development index at 30 months of age.
Fig. 2.
Tyrosine and phenylalanine plasma concentrations in patients under nitisinone treatment. A. Plasma tyrosine level by age. B. Plasma phenylalanine level by age.
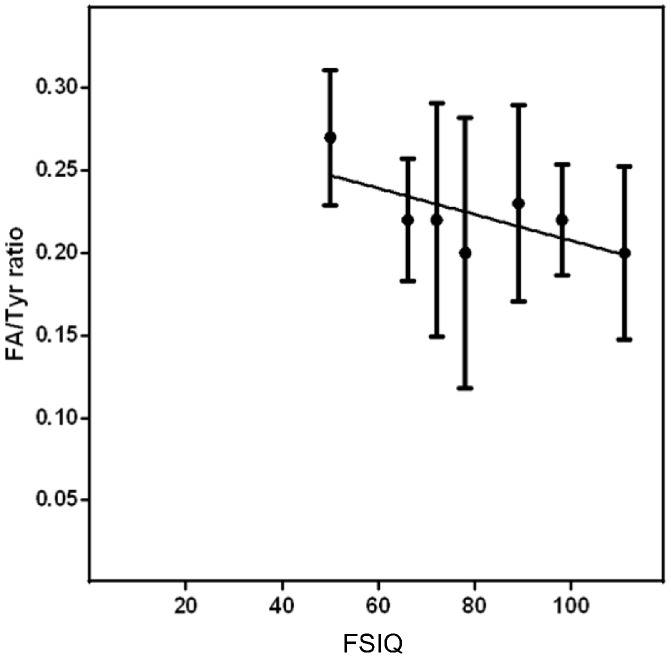
When analyzing life-long metabolic control, no significant correlation was found between tyrosine or phenylalanine plasma concentrations and IQ at school age. Only a significant correlation between Phe/Tyr ratio and IQ was identified (r = − 0.689; p < 0.0436) (Fig. 3).
Fig. 3.
Tyrosine and phenylalanine ratio correlation with FSIQ at school age in patients with HT1 receiving nitisinone.
4. Discussion
At the Institute of Nutrition and Food Technology (INTA), University of Chile, the national referral center for inborn errors of metabolism, 17 patients have been diagnosed and treated for HT1 in the last 20 years. Since 2012, all children and adults diagnosed with HT1 in Chile can obtain nitisinone treatment at no charge through a government subsidy. The follow-up program for HT1 patients includes regular measurements of phenylalanine and tyrosine levels, as well as specialized assessments by a multidisciplinary team of pediatricians, dietitians, neurologists, and psychologists.
The results obtained in the study of the present cohort are consistent with those reported in previous studies [2], [4], [5], [14] showing that a high number of patients with HT1 perform below normal in the assessment of psychomotor development and intellectual functioning.
It is also worth noting that these results differ from those reported by Pohorecka et al. [11] in which all children had cognitive functioning within the normal range. Similarly, Robaey [12] in the Tyrosinemia 2015 International Symposium presented the long- term follow up of 41 patients in Canada, of which only 2% of the patients had IQs under 70.
One limitation of the present report was molecular study was not available for any of the subjects. Future studies should analyze how genotype might affect phenotype in individuals with HT1, and if this is one of the variables that might explain the differences in cognitive functioning among different existing reports.
It is also interesting how results demonstrated that a psychomotor delay was not a clear indicator of future cognitive functioning. Yet, children who had normal psychomotor development at different assessment ages were less likely to later present IQ scores below the normal range. Psychomotor development can be influenced by long periods of hospitalization and lack of proper stimulation by caregivers. Also, the only child with moderate mental retardation had a complex history of child abuse and neglect, suggesting that his lower functioning might not be only attributed to HT1, but also other factors impacting his development. In future studies a more detailed analysis should be done to assess the impact of age of symptom onset and environmental impact on psychomotor development in this group of patients.
Regarding cognitive functioning over time, four out of eight individuals showed a decrease in IQ scores. Unexpectedly, children who showed decreases in IQ scores had an earlier onset of symptoms and treatment initiation (between months 1 to 3). Using the groups defined by Van Spronsen et al. [16], they could be categorized as very early and early patients. As for all children without decreases in IQ scores over time would be late presenting forms (between 8 and 40 months). These classifications for which groups differed in prognosis (regarding survival rate prior to the introduction of nitisinone), might also be useful regarding the prognosis on IQ evolution over time, and might be consistent with the hypothesis that the form of presentation of the disease might be impacting the neurocognitive functioning over time and not necessarily the treatment. Still taking into account the limited size of the group, future research in larger samples is necessary to assess if this correlation really exists.
The mechanism to explain suboptimal neuropsychological outcome in HT1 patients remains unclear. High tyrosine levels have been implicated in central nervous system toxicity in an experimental study performed in animals models of tyrosinemia type II disease. Chronic and acute exogenous administration of l-tyrosine induces impairment in energy metabolism showing particular damage at striatum, cerebral cortex and hippocampus [7].
Nitisinone treatment can lead to higher tyrosine levels due the metabolic block. However, the association of neurocognitive decline with higher plasma tyrosine levels in patients under chronic nitisinone treatment has been explored in previous papers with no clear association [4], [14].
High blood tyrosine concentrations may cause low CSF serotonin levels by blocking the transport of its metabolic precursor, large neutral amino acids tryptophan, across the blood-brain barrier. At the same time, high blood tyrosine concentrations may cause a high influx of tyrosine into the brain causing higher-than-normal CSF dopamine levels [13].
Very low phenylalanine levels in HT1 patients could be important as well. Association between low phenylalanine concentrations and delayed psychomotor development and skin problems, and responsiveness to Phe supplementation at early stages of development, have been reported [17]. Thus, both high tyrosine and low phenylalanine concentrations may be important in the cascade ultimately leading to brain dysfunction in HT1.
Results also show that tyrosine concentration increased with age. Progressive deterioration in metabolic control has previously been reported in PKU patients and is related to the challenges associated with a protein-restricted diet [1], [10].
It is fundamental to wait for a larger series of patients comparing newborn screening with the clinical diagnosed to address some of these questions.
Financial disclosures
The study was not funded by any grant. The study was supported by the Laboratory of Genetics and Metabolic Diseases, INTA, University of Chile.
Compliance with ethics guidelines
Conflict of interest
-
–
María Ignacia García declares that she has no conflict of interest.
-
–
Carolina Arias declares that she has no conflict of interest.
-
–
Alicia de la Parra declares that she has no conflict of interest.
-
–
Miguel Arredondo declares that he has no conflict of interest.
-
–
Juan Francisco Cabello declares that he has no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the Institute of Nutrition and Food Technologies IRB, which reviewed and approved the study. Informed consent was obtained for all participants.
Acknowledgements
The authors would like to acknowledge all the children that have participated of this study, and the medical, nutritionist and laboratory staff of the LabGEM of INTA, University of Chile, for their assistance. Also would like acknowledge Dr. Philip Anderson and Dr.Verónica Valdés for all their support and editing.
References
- 1.Ahring K., Bélanger-Quintana A., Dokoupil K., Gokmen-Ozel H., Lammardo A.M., MacDonald A., Motzfeldt K., Nowacka M., Robert M., van Rijn M. Blood phenylalanine control in phenylketonuria: a survey of 10 European centers. Eur. J. Clin. Nutr. 2011;65(2):275–278. doi: 10.1038/ejcn.2010.258. [DOI] [PubMed] [Google Scholar]
- 2.Aktuglu Zeybek C., Kiykim E., Soyucen E., Cansever S., Altay S., Zubarioglu T., Erkan T., Aydin A. Hereditary tyrosinemia type 1 in Turkey: twenty year single-center experience. Pediatr. Int. 2015;57:281–289. doi: 10.1111/ped.12503. [DOI] [PubMed] [Google Scholar]
- 3.Bayley N. The psychological corporation; San Antonio, Texas: 1993. Bayley Scales of Infant Development. [Google Scholar]
- 4.Bendadi F., de Koning T., Visser G., Prinsen H., de Sain M., Verhoeven-Duif N., Van Sponsen F., van Hasselt P. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J. Pediatr. 2014;164:398–401. doi: 10.1016/j.jpeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.De Laet C., Terrones V., Jaeken J., Francois B., Carton D., Sokal E., Dan B., Goyens P. Neuropsychological outcome of NTBC-treated patients with tyrosinemia type 1. Dev. Med. Child Neurol. 2011;53:962–964. doi: 10.1111/j.1469-8749.2011.04048.x. [DOI] [PubMed] [Google Scholar]
- 6.De Laet C., Dionisi-Vici C., Leonard J.V., McKiernan P., Mitchell G., Monti L., de Baulny H.O., Pintos-Morell G., Spiekerkötter U. Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis. 2013;11(8):8. doi: 10.1186/1750-1172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira G., Carvalho-Silva M., Gomes L., Scaini G., Teixeira L., Mota I., Schuck P., Ferreira G., Streck E. The characterization of neuroenergetic effects of chronic l-tyrosine administration in young rats: evidence for striatal susceptibility. Metab. Brain Dis. 2015;30(1):215–221. doi: 10.1007/s11011-014-9615-3. [DOI] [PubMed] [Google Scholar]
- 8.Lindstedt S., Holme E., Lock E.A., Hjalmarson O., Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;3:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 9.Masurel-Paulet A., Poggi-Bach J., Rolland M.O., Bernard O., Guffon N., Bobbelaere D., Sarles J., Ogier de Baulny H., Touate G. NTBC treatment in tyrosinemia type I: long-term outcome in French patients. J. Inherit. Metab. Dis. 2008;31:81–87. doi: 10.1007/s10545-008-0793-1. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald A., van Rijn M., Feillet F., Lund A.M., Bernstein L., Bosch A.M., Gizewska M., van Spronsen F. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann. Nutr. Metab. 2012;61(4):289–295. doi: 10.1159/000342256. [DOI] [PubMed] [Google Scholar]
- 11.Pohorecka M1., Biernacka M., Jakubowska-Winecka A., Biernacki M., Kuśmierska K., Kowalik A., Sykut-Cegielska J. Behavioral and intellectual functioning in patients with tyrosinemia type I. Pediatr. Endocrinol. Diabetes Metab. 2012;18(3):96–100. [PubMed] [Google Scholar]
- 12.Robaey P. St Pierre & Bröijersén (Copresidents) in the Tyresinemia 2015 International symposium. 2015. Psychologie et développement. (Québec) [Google Scholar]
- 13.Thimm E., Herebian D., Assmann B., Klee D., Mayatepek E., Spierkerkoetter U. Increase of CSF tyrosine and impaired serotonin turnover in tyrosinemia type I. Mol. Genet. Metab. 2011;102:122–125. doi: 10.1016/j.ymgme.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Thimm E., Richter-Werkle R., Kamp G., Molke B., Herebian D., Klee D., Mayatepek E., Spiekerkoetter U. Neurocognitive outcome in patients with hypertyrosinemia type 1 after long term treatment with NTBC. J. Inherit. Metab. Dis. 2012;35:263–268. doi: 10.1007/s10545-011-9394-5. [DOI] [PubMed] [Google Scholar]
- 15.Van Ginkel W., Jahja R., Huijbregts S., Daly A., MacDonald A., De Laet C., Cassiman D., Eyskens F., Körver-Keularts I., Goyens P., McKiernan P., Van Spronsen F. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J. Rare Dis. 2016;11:87. doi: 10.1186/s13023-016-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Spronsen F.J., Thomasse Y., Smit G.P., Leonard J.V., Clayton P.T., Fidler V., Berger R., Heymans H.S. Hereditary tyrosinemia type I: a new clinical classification with difference in prognosis in diet treatment. Hepatology. 1994;20(5):1187–1191. [PubMed] [Google Scholar]
- 17.Van Vliet D., Van Dam E., van Rijn M., Derks T., Venema-Liefaard G., Hitzert M., Lunsing R., Heiner-Fokkema R., van Spronsen F. JIMD Reports. Vol. 18. 2014. Infants with tyrosinemia type 1: should phenylalanine be supplemented? pp. 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler D. TEA Ediciones; Madrid: 1994. Escala de inteligencia de Wechsler para niños escolares revisada (WISC-R) [Google Scholar]
- 19.Wechsler D. TEA Ediciones; Madrid: 1969. Test de inteligencia de Wechsler para preescolares y educacion primaria (WPPSI) [Google Scholar]
- 20.Larochelle J., Alvarez F., Bussières J.F., Chevalier I., Dallaire L., Dubois J.…Holme E. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol. Gen. Metab. 2012;107(1):49–54. doi: 10.1016/j.ymgme.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Holme E., Lindstedt S. Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1, 3-cyclohexanedione) J. Inherit. Metab. Dis. 1998;21(5):507–517. doi: 10.1023/a:1005410820201. [DOI] [PubMed] [Google Scholar]