Abstract
Background
Beta‐blockers refer to a mixed group of drugs with diverse pharmacodynamic and pharmacokinetic properties. They have shown long‐term beneficial effects on mortality and cardiovascular disease (CVD) when used in people with heart failure or acute myocardial infarction. Beta‐blockers were thought to have similar beneficial effects when used as first‐line therapy for hypertension. However, the benefit of beta‐blockers as first‐line therapy for hypertension without compelling indications is controversial. This review is an update of a Cochrane Review initially published in 2007 and updated in 2012.
Objectives
To assess the effects of beta‐blockers on morbidity and mortality endpoints in adults with hypertension.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to June 2016: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 6), MEDLINE (from 1946), Embase (from 1974), and ClinicalTrials.gov. We checked reference lists of relevant reviews, and reference lists of studies potentially eligible for inclusion in this review, and also searched the the World Health Organization International Clinical Trials Registry Platform on 06 July 2015.
Selection criteria
Randomised controlled trials (RCTs) of at least one year of duration, which assessed the effects of beta‐blockers compared to placebo or other drugs, as first‐line therapy for hypertension, on mortality and morbidity in adults.
Data collection and analysis
We selected studies and extracted data in duplicate, resolving discrepancies by consensus. We expressed study results as risk ratios (RR) with 95% confidence intervals (CI) and conducted fixed‐effect or random‐effects meta‐analyses, as appropriate. We also used GRADE to assess the certainty of the evidence. GRADE classifies the certainty of evidence as high (if we are confident that the true effect lies close to that of the estimate of effect), moderate (if the true effect is likely to be close to the estimate of effect), low (if the true effect may be substantially different from the estimate of effect), and very low (if we are very uncertain about the estimate of effect).
Main results
Thirteen RCTs met inclusion criteria. They compared beta‐blockers to placebo (4 RCTs, 23,613 participants), diuretics (5 RCTs, 18,241 participants), calcium‐channel blockers (CCBs: 4 RCTs, 44,825 participants), and renin‐angiotensin system (RAS) inhibitors (3 RCTs, 10,828 participants). These RCTs were conducted between the 1970s and 2000s and most of them had a high risk of bias resulting from limitations in study design, conduct, and data analysis. There were 40,245 participants taking beta‐blockers, three‐quarters of them taking atenolol. We found no outcome trials involving the newer vasodilating beta‐blockers (e.g. nebivolol).
There was no difference in all‐cause mortality between beta‐blockers and placebo (RR 0.99, 95% CI 0.88 to 1.11), diuretics or RAS inhibitors, but it was higher for beta‐blockers compared to CCBs (RR 1.07, 95% CI 1.00 to 1.14). The evidence on mortality was of moderate‐certainty for all comparisons.
Total CVD was lower for beta‐blockers compared to placebo (RR 0.88, 95% CI 0.79 to 0.97; low‐certainty evidence), a reflection of the decrease in stroke (RR 0.80, 95% CI 0.66 to 0.96; low‐certainty evidence) since there was no difference in coronary heart disease (CHD: RR 0.93, 95% CI 0.81 to 1.07; moderate‐certainty evidence). The effect of beta‐blockers on CVD was worse than that of CCBs (RR 1.18, 95% CI 1.08 to 1.29; moderate‐certainty evidence), but was not different from that of diuretics (moderate‐certainty) or RAS inhibitors (low‐certainty). In addition, there was an increase in stroke in beta‐blockers compared to CCBs (RR 1.24, 95% CI 1.11 to 1.40; moderate‐certainty evidence) and RAS inhibitors (RR 1.30, 95% CI 1.11 to 1.53; moderate‐certainty evidence). However, there was little or no difference in CHD between beta‐blockers and diuretics (low‐certainty evidence), CCBs (moderate‐certainty evidence) or RAS inhibitors (low‐certainty evidence). In the single trial involving participants aged 65 years and older, atenolol was associated with an increased CHD incidence compared to diuretics (RR 1.63, 95% CI 1.15 to 2.32). Participants taking beta‐blockers were more likely to discontinue treatment due to adverse events than participants taking RAS inhibitors (RR 1.41, 95% CI 1.29 to 1.54; moderate‐certainty evidence), but there was little or no difference with placebo, diuretics or CCBs (low‐certainty evidence).
Authors' conclusions
Most outcome RCTs on beta‐blockers as initial therapy for hypertension have high risk of bias. Atenolol was the beta‐blocker most used. Current evidence suggests that initiating treatment of hypertension with beta‐blockers leads to modest CVD reductions and little or no effects on mortality. These beta‐blocker effects are inferior to those of other antihypertensive drugs. Further research should be of high quality and should explore whether there are differences between different subtypes of beta‐blockers or whether beta‐blockers have differential effects on younger and older people.
Keywords: Adult, Aged, Humans, Middle Aged, Adrenergic beta-Antagonists, Adrenergic beta-Antagonists/adverse effects, Adrenergic beta-Antagonists/therapeutic use, Angiotensin Receptor Antagonists, Angiotensin Receptor Antagonists/therapeutic use, Antihypertensive Agents, Antihypertensive Agents/adverse effects, Antihypertensive Agents/therapeutic use, Atenolol, Atenolol/therapeutic use, Calcium Channel Blockers, Calcium Channel Blockers/therapeutic use, Coronary Disease, Coronary Disease/prevention & control, Diuretics, Diuretics/therapeutic use, Heart Arrest, Heart Arrest/prevention & control, Hypertension, Hypertension/drug therapy, Hypertension/mortality, Randomized Controlled Trials as Topic, Stroke, Stroke/prevention & control
Plain language summary
Beta‐blockers for hypertension
What is the aim of this review?
The aim of this Cochrane Review was to assess whether beta‐blockers decrease the number of deaths, strokes, and heart attacks associated with high blood pressure in adults. We collected and analysed all relevant studies to answer this question and found 13 relevant studies.
Are beta‐blockers as good as other medicines when used for treatment of adults with high blood pressure?
Beta‐blockers were not as good at preventing the number of deaths, strokes, and heart attacks as other classes of medicines such as diuretics, calcium‐channel blockers, and renin‐angiotensin system inhibitors. Most of these findings come from one type of beta‐blocker called atenolol. However, beta‐blockers are a diverse group of medicines with different properties, and we need more well‐conducted research in this area.
What was studied in the review?
Millions of people with high blood pressure have strokes, heart attacks, and other diseases, and many of them die. This situation could be prevented with appropriate treatment. Researchers have tried different medicines for treating high blood pressure.
What are the main results of the review?
We found 13 studies from high‐income countries, mainly Western Europe and North America. In the studies, the people receiving beta‐blockers were compared to people who received no treatment or other medicines. The studies showed the following.
Beta‐blockers probably make little or no difference in the number of deaths among people on treatment for high blood pressure. This effect appears to be similar to that of diuretics and renin‐angiotensin system inhibitors, but beta‐blockers are probably not as good at preventing deaths from high blood pressure as calcium‐channel blockers.
Beta‐blockers may reduce the number of strokes, an effect which appears to be similar to that of diuretics. However, beta‐blockers may not be as good at preventing strokes as renin‐angiotensin system inhibitors or calcium‐channel blockers.
Beta‐blockers may make little or no difference to the number of heart attacks among people with high blood pressure. The evidence suggests that this effect may not be different from that of diuretics, renin‐angiotensin system inhibitors, or calcium‐channel blockers. However, among people aged 65 years and older, the evidence suggests that beta‐blockers may not be as good at reducing heart attacks as diuretics.
People given beta‐blockers are more likely to have side effects and stop treatment than people taking renin‐angiotensin system inhibitors, but there may be little or no difference in side effects between beta‐blockers and diuretics or calcium‐channel blockers.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to June 2016.
Summary of findings
Summary of findings for the main comparison. Beta‐blockers versus placebo as first‐line therapy for hypertension.
| Beta‐blockers versus placebo as first‐line therapy for hypertension | |||||
| Participants: people with hypertension Settings: high‐income countries, mainly Western Europe and North America Intervention: beta‐blockers Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Beta‐blockers | ||||
| Total mortality | 52 per 1000 | 51 per 1000 (46 to 57) | RR 0.99 (0.88 to 1.11) | 23613 (4 studies) | ⊕⊕⊕⊝ Moderate1 |
| Total cardiovascular disease | 64 per 1000 | 57 per 1000 (51 to 63) | RR 0.88 (0.79 to 0.97) | 23613 (4 studies) | ⊕⊕⊝⊝ Low1,2 |
| Total stroke | 23 per 1000 | 18 per 1000 (15 to 22) | RR 0.80 (0.66 to 0.96) | 23613 (4 studies) | ⊕⊕⊝⊝ Low1,2 |
| Total coronary heart disease | 37 per 1000 | 34 per 1000 (30 to 40) | RR 0.93 (0.81 to 1.07) | 23613 (4 studies) | ⊕⊕⊕⊝ Moderate1 |
| Withdrawal due to adverse effect | 74 per 1000 | 249 per 1000 (60 to 1000) | RR 3.38 (0.82 to 13.95) | 22729 (3 studies) | ⊕⊕⊝⊝ Low3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 The two studies that contribute to the most weight of the pooled RR have high risk of bias (especially incomplete outcome reporting due to attrition bias): downgraded by 1 point. 2 The RR is too close to 1 and could easily include 1 if more trials are added: downgraded by 1 point. 3 Inconsistent results across studies (I2 = 100%): downgraded by 2 points.
Summary of findings 2. Beta‐blockers compared to diuretics as first‐line therapy for hypertension.
| Beta‐blockers compared to diuretics as first‐line therapy for hypertension | |||||
| Participants: people with hypertension Settings: high‐income countries, mainly Western Europe and North America Intervention: beta‐blockers Comparison: diuretics | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Diuretics | Beta‐blockers | ||||
| Total mortality | 41 per 1000 | 43 per 1000 (37 to 49) | RR 1.04 (0.91 to 1.19) | 18241 (5 studies) | ⊕⊕⊕⊝ Moderate1 |
| Total cardiovascular disease | 45 per 1000 | 51 per 1000 (45 to 58) | RR 1.13 (0.99 to 1.28) | 18135 (4 studies) | ⊕⊕⊕⊝ Moderate1 |
| Total stroke | 12 per 1000 | 14 per 1000 (8 to 25) | RR 1.17 (0.65 to 2.09) | 18135 (4 studies) | ⊕⊕⊝⊝ Low1,2 |
| Total coronary heart disease | 33 per 1000 | 37 per 1000 (27 to 50) | RR 1.12 (0.82 to 1.54) | 18135 (4 studies) | ⊕⊕⊝⊝ Low1,2 |
| Withdrawal due to adverse effect | 109 per 1000 | 184 per 1000 (104 to 327) | RR 1.69 (0.95 to 3.00) | 11566 (3 studies) | ⊕⊕⊝⊝ Low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 The two studies that contribute to the most weight of the pooled RR have high risk of bias (especially incomplete outcome reporting due to attrition bias): downgraded by 1 point. 2 Inconsistent results across studies (I2 = 73% for stroke, 66% for coronary heart disease, and 95% for adverse effects): downgraded by 1 point.
Summary of findings 3. Beta‐blockers compared to calcium‐channel blockers as first‐line therapy for hypertension.
| Beta‐blockers compared to calcium‐channel blockers as first‐line therapy for hypertension | |||||
| Participants: people with hypertension Settings: high‐income countries, mainly Western Europe and North America Intervention: beta‐blockers Comparison: calcium‐channel blockers | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Calcium‐channel blockers | Beta‐blockers | ||||
| Total mortality | 73 per 1000 | 78 per 1000 (73 to 83) | RR 1.07 (1.0 to 1.14) | 44825 (4 studies) | ⊕⊕⊕⊝ Moderate1 |
| Total cardiovascular disease | 81 per 1000 | 96 per 1000 (87 to 104) | RR 1.18 (1.08 to 1.29) | 19915 (2 studies) | ⊕⊕⊕⊝ Moderate2 |
| Total stroke | 23 per 1000 | 29 per 1000 (26 to 32) | RR 1.24 (1.11 to 1.4) | 44167 (3 studies) | ⊕⊕⊕⊝ Moderate3 |
| Total coronary heart disease | 39 per 1000 | 41 per 1000 (37 to 45) | RR 1.05 (0.96 to 1.15) | 44167 (3 studies) | ⊕⊕⊕⊝ Moderate3 |
| Withdrawal due to adverse effect | 33 per 1000 | 40 per 1000 (23 to 67) | RR 1.20 (0.71 to 2.04) | 21591 (2 studies) | ⊕⊕⊝⊝ Low2,4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 The RR is too close to 1 and could easily include 1 if more trials are added: downgraded by 1 point.
2 Only 2 hypertension trials comparing beta‐blockers to calcium‐channel blockers have reported data on this outcome: downgraded by 1 point. 3 Only 3 hypertension trials comparing beta‐blockers to calcium‐channel blockers have reported data on this outcome: downgraded by 1 point.
4 Inconsistent results across studies (I2 = 93%): downgraded by 1 point.
Summary of findings 4. Beta‐blockers compared to renin‐angiotensin system inhibitors as first‐line therapy for hypertension.
| Beta‐blockers compared to renin‐angiotensin system inhibitors as first‐line therapy for hypertension | |||||
| Participants: people with hypertension Settings: high‐income countries, mainly Western Europe and North America Intervention: beta‐blockers Comparison: renin‐angiotensin system inhibitors | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Renin‐angiotensin system inhibitors | Beta‐blockers | ||||
| Total mortality | 84 per 1000 | 92 per 1000 (82 to 104) | RR 1.10 (0.98 to 1.24) | 10828 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
| Total cardiovascular disease | 115 per 1000 | 115 per 1000 (83 to 159) | RR 1.0 (0.72 to 1.38) | 10828 (3 studies) | ⊕⊕⊝⊝ Low1,2 |
| Total stroke | 51 per 1000 | 66 per 1000 (56 to 77) | RR 1.30 (1.11 to 1.53) | 9951 (2 studies) | ⊕⊕⊕⊝ Moderate3 |
| Total coronary heart disease | 54 per 1000 | 49 per 1000 (41 to 57) | RR 0.90 (0.76 to 1.06) | 9951 (2 studies) | ⊕⊕⊝⊝ Low3,4 |
| Withdrawal due to adverse effect | 137 per 1000 | 194 per 1000 (177 to 211) | RR 1.41 (1.29 to 1.54) | 9951 (2 studies) | ⊕⊕⊕⊝ Moderate3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 Only 3 hypertension trials comparing beta‐blockers to RAS inhibitors have reported data on this outcome: downgraded by 1 point. 2 Inconsistent results across studies (I2 = 74%): downgraded by 1. 3 Only 2 hypertension trials comparing beta‐blockers to RAS inhibitors have reported data on this outcome: downgraded by 1 point.
4 Imprecise results, as the effect ranges from a clinically important benefit to a small increase in harm: downgraded by 1 point.
Background
Description of the condition
Hypertension is one of the leading causes of disability and premature deaths worldwide (GBD 2015). The rationale for treating hypertension achieved great impetus with the finding that even small reductions in blood pressure can significantly reduce associated morbidity and mortality risks (Collins 1990; Staessen 2003; Thomopoulos 2015). The major classes of drugs for treating hypertension include beta‐blockers, calcium‐channel blockers (CCBs), diuretics, and renin‐angiotensin system (RAS) inhibitors (Wiysonge 2013).
Description of the intervention
Beta‐blockers refer to a diverse group of drugs which block the action of endogenous catecholamines on beta‐adrenergic receptors, part of the autonomic (or sympathetic) nervous system (Wiysonge 2007a). The autonomic nervous system has been known to play a role in blood pressure control since 1949 (Smithwick 1949). The principal adrenergic receptors present in the human cardiovascular system are the β1, β2, and α1 receptors (Fergus 2015; Pucci 2016). Beta‐blockers vary in their β1/β2‐adrenergic receptor selectivity and vasodilatory properties, and this diversity has given rise to their classification into first, second, and third generation. First‐generation beta‐blockers exercise identical affinity for β1 and β2 receptors and are thus classified as non‐selective beta‐blockers (e.g. propranolol). Second‐generation beta‐blockers are more attracted to β1 than β2 receptors, and are thus termed selective beta‐blockers (e.g. atenolol). The third‐generation of beta‐blockers are known for their intrinsic vasodilatory properties (e.g. nebivolol) (Weber 2005).
How the intervention might work
Beta‐blockers have been used as first‐line therapy for hypertension since the late 1960s, apparently because activation of the sympathetic nervous system is important in the aetiology and maintenance of hypertension (Berglund 1981; JNC‐6 1997; Larochelle 2014; Philipp 1997; Psaty 1997; Ramsay 1999; Wiysonge 2013); but the robustness of the evidence for use of beta‐blockers as first‐line therapy for hypertension without compelling indications is controversial (Carlberg 2004; Khan 2006; Lindhom 2005; Messerli 2003; Opie 1997; Opie 2014; Wiysonge 2007a; Wright 2000). From 2004 to 2006, three meta‐analyses were published which found that beta‐blockers were less effective in reducing the incidence of stroke (Lindhom 2005), and the composite of major cardiovascular outcomes including stroke, myocardial infarction, and death (Khan 2006), compared to all drugs for treating hypertension. However, beta‐blockers might have different comparative outcomes versus the various other classes of drugs. For instance, several studies have claimed that CCBs are better than other antihypertensive agents in preventing stroke but less good at preventing coronary heart disease (CHD; Angeli 2004; Opie 2002; Verdecchia 2005). Thus, it is important to know to what extent the comparisons made by Lindholm and colleagues (Carlberg 2004; Lindhom 2005) and Khan and co‐authors (Khan 2006; Kuyper 2014) relate to beta‐blockers versus specific classes of antihypertensive drugs such as diuretics, CCBs, or RAS inhibitors. RAS inhibitors refer to angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and direct renin inhibitors (DRI). In general, beta‐blockers might be better or worse than one specific class of drugs for specific endpoints so that comparing beta‐blockers with all other classes could be misleading (Carlberg 2004; Lindhom 2005; Khan 2006). In addition, the safety of a medication is as important to the clinician and the person as is the effectiveness; but neither Lindholm and colleagues (Carlberg 2004; Lindhom 2005) nor Khan and co‐authors (Khan 2006; Kuyper 2014) provided data on this aspect when comparing beta‐blockers to other antihypertensive agents (see also Table 5).
1. Previous systematic reviews of beta‐blockers as first‐line hypertension therapy.
| Identification | Comparison | Trials included | Comments |
| Psaty 1997 | Beta‐blocker vs placebo | MRC 1985; MRCOA 1992; Coope 1986; STOP 1991 trials | STOP 1991 classified as beta‐blocker trial as 68% in active group were taking a beta‐blocker. |
| Messerli 1998 | Beta‐blocker vs placebo in older people | Coope 1986; MRCOA 1992 | The review concluded that beta‐blockers should not be used in elderly people with hypertension. |
| Wright 1999 | Beta‐blocker vs diuretic | Berglund 1981; HAPPHY 1987; MRC 1985; MRCOA 1992; VA COOP 1982 | IPPPSH not included because 67% of participants taking beta‐blocker were taking a diuretic. |
| Wright 2000 | Beta‐blocker vs placebo | MRC 1985; MRCOA 1992 | Coope 1986 and STOP excluded because of high use of diuretic. |
| Carlberg 2004 | Atenolol vs placebo, and atenolol vs other antihypertensive drugs | Placebo: Coope 1986; MRCOA 1992; Dutch TIA 1993; TEST 1995) Other antihypertensive drugs: HAPPHY 1987; MRCOA 1992; UKPDS‐39‐1998; LIFE 2002; ELSA 2002 |
Included trials in which only a proportion (> 50%) of participants were assigned to start treatment with atenolol. |
| NICE 2004 | Beta‐blockers vs placebo, thiazide diuretics, calcium‐channel blockers, ACE inhibitors, and angiotensin receptor blockers | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992; Dutch TIA 1993; TEST 1995; STOP‐2 1999 Thiazide diuretics: MRC 1985; HAPPHY 1987; MAPHY 1988; MRCOA 1992 Calcium‐channel blockers: CONVINCE 1998; STOP‐2 1999; NORDIL 2000; ELSA 2002; INVEST 2003 ACE inhibitors: CAPP 1999; STOP‐2 1999 Angiotensin receptor blockers: LIFE 2002 |
Included MAPPHY which is a subset of HAPPHY study. Included some studies in which only a proportion of participants were assigned to start treatment on a beta‐blocker. |
| Lindhom 2005 | Beta‐blocker vs placebo, and beta‐blocker vs other antihypertensive drugs | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992; Dutch TIA 1993; TEST 1995 Other antihypertensive drugs: Berglund 1981; MRC 1985; HAPPHY 1987; STOP 1991; MRCOA 1992; Yurenev 1992; UKPDS‐39‐1998; STOP‐2 1999; NORDIL 2000; LIFE 2002; ELSA 2002; CONVINCE 2003; ASCOT 2005 |
Included trials in which only a proportion (> 50%) of participants were assigned to start treatment with a beta‐blocker. |
| Bradley 2006 | Beta‐blocker vs placebo, diuretics, calcium‐channel blockers, and renin‐angiotensin system inhibitors | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992 Diuretics: Berglund 1981; VA COOP 1982; MRC 1985; HAPPHY 1987; MRCOA 1992 Calcium‐channel blockers: AASK 2002; ELSA 2002; INVEST 2003; ASCOT 2005 Renin‐angiotensin system inhibitors: UKPDS‐39‐1998; AASK 2002; LIFE 2002 |
Excluded Dutch TIA 1993 and TEST 1995 because not all participants in these 2 trials were had hypertension. |
| Khan 2006 | Beta‐blocker vs placebo, and beta‐blocker vs other antihypertensive drugs | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992; Dutch TIA 1993; TEST 1995 Other antihypertensive drugs: Berglund 1981; MRC 1985; HAPPHY 1987; STOP 1991; MRCOA 1992; Yurenev 1992; UKPDS‐39‐1998; STOP‐2 1999; CAPP 1999; NORDIL 2000; LIFE 2002; ELSA 2002; CONVINCE 2003; ASCOT 2005 |
Included trials in which only a proportion (> 50%) of participants were assigned to start treatment with a beta‐blocker. |
| NICE 2006 | Beta‐blockers vs thiazide diuretics, calcium‐channel blockers, ACE inhibitors, and angiotensin receptor blockers | Thiazide diuretics: MRC 1985; HAPPHY 1987; MRCOA 1992 Calcium‐channel blockers: ASCOT 2005; ELSA 1992; INVEST 2003 ACE inhibitors: no studies meeting criteria Angiotensin receptor blockers: LIFE 2002 |
Updated NICE 2004 review by evaluating head‐to‐head trials only. ASCOT new study added and excluded CONVINCE; NORDIL; and CAPP due to confounded use. |
| Dahlöf 2007 | Beta‐blockers with or without diuretics vs placebo or no treatment | Coope 1986; MRC 1985; MRCOA 1992; STOP 1991; UKPDS‐39 | IPPPSH 1985 not included. STOP 1991 included because > 85% of participants on active treatment received beta‐blocker as first‐line or second‐line therapy. Regarded the 'control group' in the UKPDS‐39 as placebo, even though the group permitted antihypertensive therapy (other than ACE inhibitors and beta‐blockers), because the target for blood pressure reduction was not as low as in the beta‐blocker group. |
| Wright 2009 | Beta‐blocker vs placebo | MRC 1985; MRCOA 1992; Dutch TIA 1993; TEST 1995; UKPDS‐39 1998 | IPPPSH 1985 and Coope 1986 excluded because of high use of diuretics in beta‐blocker group. UKPDS‐39 included using 'less tight control group' as placebo, but participants took antihypertensive treatments for 57% of total person‐years. |
| Wiysonge 2012 | Beta‐blocker vs placebo, diuretics, calcium‐channel blockers, and renin‐angiotensin system inhibitors | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992 Diuretics (Berglund 1981; VA COOP 1982; MRC 1985; HAPPHY 1987; MRCOA 1992 Calcium‐channel blockers: AASK 2002; ELSA 2002; INVEST 2003; ASCOT 2005 Renin‐angiotensin system inhibitors: UKPDS‐39‐1998; AASK 2002; LIFE 2002 |
Previously published version of this systematic review |
| Kuyper 2014 | Beta‐blocker vs placebo, and beta‐blocker vs other antihypertensive drugs | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; STOP 1991; MRCOA 1992; Dutch TIA 1993; TEST 1995 Other antihypertensive drugs: Berglund 1981; MRC 1985; HAPPHY 1987; STOP 1991; MRCOA 1992; Yurenev 1992; UKPDS‐39‐1998; STOP‐2 1999; CAPP 1999; NORDIL 2000; LIFE 2002; ELSA 2002; CONVINCE 2003; ASCOT 2005 |
Compared the efficacy of atenolol vs non‐atenolol beta‐blockers in clinical trials enrolling young (aged < 60 years) and older people with hypertension. The review concluded that atenolol should not be used in older people with hypertension but class effect uncertain, and beta‐blockers reasonable option for the young. |
| Wiysonge 2017 | Beta‐blocker vs placebo, diuretics, calcium‐channel blockers, and renin‐angiotensin system inhibitors | Placebo: IPPPSH 1985; MRC 1985; Coope 1986; MRCOA 1992 Diuretics: Berglund 1981; VA COOP 1982; MRC 1985; HAPPHY 1987; MRCOA 1992 Calcium‐channel blockers: AASK 2002; ELSA 2002; INVEST 2003; ASCOT 2005 Renin‐angiotensin system inhibitors: UKPDS‐39‐1998; AASK 2002; LIFE 2002 |
Current systematic review |
ACE: angiotensin‐converting enzyme.
Why it is important to do this review
Proper understanding of the evidence for beta‐blocker therapy in hypertension requires a regularly updated systematic, comprehensive, and appropriate analysis of all currently available data. In 2007, we published a Cochrane Review which re‐assessed the place of beta‐blockers as first‐line therapy for hypertension relative to each of the other major classes of antihypertensive drugs. An update of the review was published in 2012. The current review is an update of the 2012 review.
Objectives
To assess the effects of beta‐blockers on morbidity and mortality endpoints in adults with hypertension.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a duration of one year or more.
Types of participants
Men and non‐pregnant women, aged 18 years and over, with hypertension as defined by cut‐off points operating at the time of the study under consideration.
Types of interventions
The treatment group must have received a beta‐blocker drug either as monotherapy or as a first‐line drug in a stepped‐care approach. The control group could have been a placebo, no treatment, or another antihypertensive drug (including a different beta‐blocker or the same beta‐blocker at a different dose).
Types of outcome measures
Primary outcomes
Mortality.
Secondary outcomes
Total (i.e. fatal and non‐fatal) stroke.
Total coronary heart disease (myocardial infarction, sudden death).
Total cardiovascular disease (CVD: i.e. fatal and non‐fatal CHD, stroke, congestive heart failure, and transient ischaemic attacks).
Adverse events leading to discontinuation of allocated treatment.
Degree of reduction in systolic and diastolic blood pressure achieved by beta‐blocker therapy in relation to each comparator treatment.
We used the definitions employed by the investigators of the study under consideration.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomised controlled trials without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 14 June 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 6) via the Cochrane Register of Studies (CRS‐Web) (searched 14 June 2016);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 14 June 2016);
Embase Ovid (searched 14 June 2016);
ClinicalTrials.gov (www.clinicaltrials.gov) searched 14 June 2016);
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies from 19 January 2015 are found in Appendix 1. Search strategies for all major databases are provided in Appendix 2.
Searches for previous versions of the review were conducted in June 2006, May 2011, December 2011, and November 2012 (Bradley 2006; Wiysonge 2007b; Wiysonge 2012; Wiysonge 2013). In the previous search conducted in June 2006 (Bradley 2006; Wiysonge 2007b), we searched PubMed, Embase, Cochrane Database of Systematic Reviews, and the York Database of Abstracts of Reviews of Effectiveness for previous reviews and meta‐analyses of antihypertensive treatments that included beta‐blockers. Reports of relevant trials referred to in these reviews were obtained. We then carried out an exhaustive search for eligible RCTs in MEDLINE (for the period 1966 to June 2006) using the terms "adrenergic beta‐antagonists" [MESH], "beta (blockers)" and exp "hypertension" [MESH] combined with the optimally sensitive strategy for identifying RCTs recommended by Cochrane (Higgins 2011); Embase (for the period 1980 to June 2006) using a search strategy similar to that used for MEDLINE; and CENTRAL (the Cochrane Library, 2016, Issue 2). Finally, experts in the field of hypertension and drug companies manufacturing beta‐blockers were contacted for unpublished trials. After reaching consensus on the search strategy for each electronic database, the information specialist of the South African Cochrane Centre conducted the respective electronic searches.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
We did not perform a separate search for adverse effects of interventions used for the treatment of hypertension. We considered adverse effects described in included studies only.
We also screened the reference lists of 41 potentially eligible studies and 25 relevant reviews and guidelines (Balamuthusamy 2009; Bangalore 2007; Bangalore 2008; Bath 2014; Carlberg 2004; Chen 2010; Dahlöf 2007; ESH‐ESC 2013; Gradman 2010; Howlett 2014; James 2014; Jennings 2013; Khan 2006; Kuyper 2014; Larochelle 2014; NICE 2006; Poirier 2014; Pucci 2016; Ripley 2014; Sander 2011; Sciarretta 2011; Thomopoulos 2015; Wong 2014a; Wong 2014b; Wright 2009). In addition, we searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) using the terms (beta‐blocker OR beta‐blockers) AND hypertension on 06 July 2015.
Data collection and analysis
For the current update, two review authors (CSW and HB) independently examined the eligibility of all titles and abstracts of studies identified by electronic or bibliographic scanning. The two review authors then independently assessed the risk of bias within included studies and extracted data. At each stage, the they resolved differences by discussion and consensus. If any discrepancies had persisted, JV would have arbitrated.
We assessed the risk of bias by addressing seven specific domains, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The seven domains were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias'. For each included study, we described what the study authors reported that they did for each domain and then made a decision relating to the risk of bias for that domain; by assigning a judgement of 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias.
The data extracted for each study were: methods, including means of assigning participants to trial interventions, blinding of those receiving and providing care and outcome assessors, losses to follow‐up and how they were handled, and length of trial follow‐up; participant characteristics, including gender, ethnicity and comorbid conditions; interventions, including type and dose of beta‐blocker and other medications used; outcome measures, including morbidity and mortality endpoints, and adverse events.
We conducted quantitative analyses according to standard Cochrane guidelines (Higgins 2011). We analysed trial participants in groups to which they were randomised, regardless of which or how much treatment they actually received, and expressed study results as risk ratios (RR) with 95% confidence intervals (CI). We assessed heterogeneity between studies by graphical inspection of results and, more formally followed by, the Chi2 test of homogeneity. In the absence of significant statistical heterogeneity between studies (P > 0.1), we performed meta‐analysis using a fixed‐effect method (Breslow 1980; Mantel 1959). When there was significant heterogeneity between study results, we used the random‐effects method (DerSimonian 1986), and investigated the cause of heterogeneity by stratified analysis with reference to the characteristics of the studies included in the meta‐analysis. The study characteristics considered in the subgroup analyses were age (less than 65 years versus 65 years and older), type of beta‐blockade (cardioselective versus non‐selective), control group (placebo versus no treatment), and risk of bias (high versus low risk of bias). In addition, we used the I2 statistic to describe the percentage of between‐study variability in effect estimates (for each outcome) attributable to true heterogeneity rather than chance (Higgins 2003).
Various related reviews differ from ours in their inclusion or exclusion of various studies (Carlberg 2004; Dahlöf 2007; Khan 2006; Lindhom 2005; Wright 2009). We conducted sensitivity analyses to confirm that those different decisions did not lead to different conclusions.
Results
Description of studies
Figure 1 shows the search and selection of studies for this review, in line with the statement of preferred reporting items for systematic reviews and meta‐analyses (Moher 2009).
1.
PRISMA flow diagram showing the search and selection of studies.
Results of the search
We obtained 4453 records from the search conducted in January 2015; including 696 duplicates. Of the remaining 3757 records, 1263 were new records. We screened these and found no potentially eligible studies. The search conducted on 6 July 2015 found 450 studies in Clinicaltrials.gov and 283 records of 257 studies in the WHO International Clinical Trials Registry Platform. None of these 'ongoing' studies was potentially eligible. Finally, the search conducted in June 2016 yielded 2716 records, with 596 being duplicates. We screened the remaining 2120 records (of which 1551 were new records) and found no potentially eligible studies.
From the search conducted in June 2006, we identified 21 potentially eligible RCTs (AASK 2002; ASCOT 2005; Berglund 1981; Coope 1986; ELSA 2002; HAPPHY 1987; INVEST 2003; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; UKPDS‐39‐1998; VA COOP 1982; CAPP 1999; CONVINCE 1998; Dutch TIA 1993; MAPHY 1988; NORDIL 2000; STOP 1991; STOP‐2 1999; TEST 1995), from which we excluded eight. In five of the six RCTs, participants in the 'beta‐blocker' group were not randomly allocated to a beta‐blocker at baseline but to conventional therapy, which referred to either a beta‐blocker or a diuretic (CAPP 1999; CONVINCE 1998; NORDIL 2000; STOP 1991; STOP‐2 1999). None of the five RCTs reported data separately for the participants taking beta‐blockers and participants taking diuretics. We excluded two studies because not all participants had hypertension at baseline (Dutch TIA 1993; TEST 1995). We excluded the eighth RCT (MAPHY 1988), because it was a subset of an included RCT (HAPPHY 1987).
The remaining 13 RCTs with 91,561 participants meet our inclusion criteria (AASK 2002; ASCOT 2005; Berglund 1981; Coope 1986; ELSA 2002; HAPPHY 1987; INVEST 2003; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; UKPDS‐39‐1998; VA COOP 1982), and we included them in the previous review (Bradley 2006; Wiysonge 2007b).
The May 2011 search yielded 1566 records from the electronic databases (after removing duplicates), which we screened and identified 19 potentially eligible studies (ACCORD 2010; ADaPT 2008; APSIS 2006; CAPRICORN 2001; CARDHIAC 2008; CHHIPS 2009; CIBIS‐II 1999; COMET 2003; COPE 2005; COPERNICUS 2004; COSMOS 2010; Dietz 2008; GEMINI 2008; IMPACT‐HF 2004; MERIT‐HF 2002; Nilsson 2007; REASON 2009; RESOLVD 2000; SENIORS 2005). Following review of the full‐text articles of the 19 studies, we found that none of them met our inclusion criteria.
Finally, we obtained 508 abstracts from the December 2011 search; with one potentially eligible study (Marazzi 2011). This study did not met our inclusion criteria and was excluded.
Included studies
The 13 included RCTs compared a beta‐blocker to a placebo or no treatment (Coope 1986; IPPPSH 1985; MRC 1985; MRCOA 1992), a diuretic (Berglund 1981; HAPPHY 1987; MRC 1985; MRCOA 1992; VA COOP 1982), a CCB (AASK 2002; ASCOT 2005; ELSA 2002; INVEST 2003), an ACE inhibitor (AASK 2002; UKPDS‐39‐1998), or an ARB (LIFE 2002).
Unlike two related reviews (Dahlöf 2007; Wright 2009), we did not consider the UKPDS‐39‐1998 as a placebo‐controlled trial because participants in the 'less tight control group' (which these reviews consider as placebo) took antihypertensive treatment for 57% of total person‐years.
Ten RCTs recruited participants of both sexes (AASK 2002; ASCOT 2005; Coope 1986; ELSA 2002; INVEST 2003; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; UKPDS‐39‐1998). Six RCTs included participants up to the age of 65 years (Berglund 1981; HAPPHY 1987; IPPPSH 1985; MRC 1985; UKPDS‐39‐1998; VA COOP 1982), and the rest included participants aged 18 to 70 years (AASK 2002), 40 to 79 years (ASCOT 2005), 45 to 75 years (ELSA 2002), more than 50 years (INVEST 2003), 55 to 80 years (LIFE 2002), 60 to 79 years (Coope 1986), and 65 to 74 years (MRCOA 1992).
All 13 studies were conducted in industrialised countries, mainly Western Europe and North America. Nine RCTs provided information on race or ethnicity: AASK 2002 (0% white), INVEST 2003 (44% white), VA COOP 1982 (48% white), UKPDS‐39‐1998 (86% white), IPPPSH 1985 (92% white), LIFE 2002 (92% white), ASCOT 2005 (95.0% white), ELSA 2002 (98.2% white), and HAPPHY 1987 (more than 99% white).
We have described the 13 RCTs included in this review in detail in the Characteristics of included studies table, and summarised their main features below:
AASK 2002. This RCT compared the effects of an ACE inhibitor (ramipril), a CCB (amlodipine), and a beta‐blocker (metoprolol) on hypertensive renal disease progression in African American people aged 18 to 70 years. Additional antihypertensive agents were added sequentially to achieve blood pressure goals. Cardiovascular events, cardiovascular mortality. and all‐cause mortality were reported. The trial followed 1094 participants for a mean duration of 4.1 years.
ASCOT 2005. The participants were randomised to a CCB (amlodipine) adding an ACE inhibitor (perindopril) as required to reach blood pressure targets or a beta‐blocker (atenolol) adding a diuretic (bendroflumethiazide) as required. The participants were men and women with hypertension aged 40 to 79 years. The main outcome measure was combined non‐fatal myocardial infarction and fatal CHD, and secondary endpoints included all‐cause mortality, cardiovascular mortality, and total stroke. At the end of the trial, 78% of participants were taking at least two antihypertensive medications and only 15% were taking amlodipine and 9% were taking atenolol monotherapy. The study enrolled 19,257 participants and followed them for a median duration of 5.5 years.
Berglund 1981. This RCT evaluated the long‐term effects of a thiazide diuretic (bendroflumethiazide) compared to a beta‐blocker (propranolol) in men with hypertension aged 47 to 54 years. Hydralazine and other antihypertensive medications were added to achieve blood pressure goals. The investigators reported total mortality. At the end of the trial, 70% of participants taking diuretic and 74% taking beta‐blockers were on assigned treatment and 40% of participants taking diuretic and 42% taking beta‐blocker were on monotherapy. The study enrolled 106 participants and the study lasted 10 years.
Coope 1986. The trial was designed to determine whether the treatment of hypertension using beta‐blocker therapy (atenolol) in a stepped‐care approach compared to no treatment reduced the incidence of stroke, CHD, cardiovascular death, or all‐cause mortality. Step one was monotherapy with atenolol, step two added a thiazide diuretic (bendrofluazide), and steps three and four added other antihypertensive agents. At the end of the trial, 70% of participants in the beta‐blocker group were taking assigned treatment, 17% were taking atenolol alone, and 53% were taking atenolol plus bendrofluazide. The trial followed up 884 participants aged 60 to 79 years for a mean duration of 4.4 years.
ELSA 2002. The trial was designed to compare the effects of a beta‐blocker (atenolol) and a CCB (lacidipine) on the change in mean maximum intima‐media thickness and plaque number in men and women with hypertension. The investigators also reported data on fatal and non‐fatal cardiovascular events and total mortality. If satisfactory blood pressure control was not achieved, trial medication could be increased, and when necessary open‐label hydrochlorothiazide was added. At the end of the trial, 85% of participants in the beta‐blocker group and 78% in the CCB group were known to be on assigned treatment. The participants on monotherapy at the end of the trial were 43% in the beta‐blocker group and 42% in the CCB group. The trial followed up 2334 participants aged 45 to 75 years for a mean duration of 3.75 years.
HAPPHY 1987. The trial was designed to compare the effects of beta‐blockers (mainly atenolol, 1599 participants or metoprolol, 1631 participants) and thiazide diuretics (bendroflumethiazide or hydrochlorothiazide) on the incidence of non‐fatal myocardial infarction, CHD mortality, and total mortality in men with mild to moderate hypertension. Other drugs were added to reduce blood pressure as necessary. At the end of the trial, 86% of participants in the beta‐blocker group and 83% in the diuretic group were on assigned treatment. More participants in the beta‐blocker group (68%) than in the diuretic group (62%) were on monotherapy. The trial followed up 6569 participants aged 40 to 64 years for a mean duration of 45.1 months.
INVEST 2003. The trial was designed to compare the effect of a CCB (verapamil sustained release, SR), and a beta‐blocker (atenolol) in hypertensive participants with documented coronary artery disease, on all‐cause and cardiovascular death, and various non‐fatal cardiovascular events. Other drugs, mainly trandolapril (to the verapamil SR group) and hydrochlorothiazide (to the atenolol group), were added to achieve blood pressure control as required. At two years, 77.5% of participants in the beta‐blocker group and 81.5% in the CCB group were on the assigned treatment (18.1% taking beta‐blocker and 17.4% taking CCB monotherapy). The trial followed up 22,576 participants aged 50 years and older for a mean duration of 2.7 years.
IPPPSH 1985. The trial was designed to evaluate the effect of antihypertensive therapy with a beta‐blocker (oxprenolol) on the incidence of cardiac events (myocardial infarction and sudden death) and cerebrovascular accidents. Trial medication could be increased or other non‐beta‐blocker antihypertensive drugs added according to predefined recommendations, as necessary, to reduce blood pressure. During the trial, 30% of participants remained on beta‐blocker monotherapy while 15% remained on placebo only. The trial followed up 6357 participants aged 40 to 64 years for three to five years.
LIFE 2002. The trial was designed to evaluate the effects of an ARB (losartan) compared to a beta‐blocker (atenolol) in people with hypertension with documented left ventricular hypertrophy on the combined incidence of cardiovascular mortality and morbidity. Other drugs were added to reduce blood pressure as necessary. At the end of the trial, 63% of participants in the beta‐blocker group and 67% in the ARB group were on assigned treatment; 11% of participants were on monotherapy in each group. The trial followed up 9193 participants aged 55 to 80 years for a mean duration of 4.8 years.
MRC 1985. The trial was designed to determine whether drug treatment of mild hypertension reduced the rates of fatal and non‐fatal stroke and of coronary events. Participants were randomised to active treatment (propranolol or bendrofluazide) or placebo. At the end of the study, the proportion of participants on assigned treatment in the beta‐blocker group was 59%, in the diuretic group was 62%, and placebo group was 56%. The trial followed up 17,354 participants aged 35 to 64 years for a mean duration of 4.9 years.
MRCOA 1992. The trial was designed to establish whether treatment of hypertension in older adults reduced the risk of stroke, CHD, and death from all causes. Participants were randomised to a beta‐blocker (atenolol), a diuretic (amiloride and hydrochlorothiazide), or placebo. Other drugs were added as necessary. At five years, 52% of participants assigned to beta‐blockers required supplementary drugs compared to 38% in the diuretic group. At the end of the study, 37% of participants in the beta‐blocker group, 52% in the diuretic group, and 47% in the placebo group were on the assigned treatment. The trial followed up 4396 participants aged 65 to 74 years for 5.8 years.
UKPDS‐39‐1998. The trial was designed to determine whether tight control of blood pressure with either a beta‐blocker (atenolol) or an ACE inhibitor (captopril) prevents macrovascular and microvascular complications in participants with type 2 diabetes. Participants were randomised to study drugs, with other drugs added as required. At the end of the trial, 65% of participants in the beta‐blocker group and 78% in the ACE inhibitor group were on assigned treatment. The trial followed up 758 participants aged 25 to 65 years for 8.4 years.
VA COOP 1982. This trial compared a beta‐blocker (propranolol) and a diuretic (hydrochlorothiazide) for the initial treatment of hypertension in men aged 21 to 65 years. During treatment, fewer participants receiving hydrochlorothiazide required termination as compared with men receiving propranolol. A total of 683 men were recruited. During the initial 10 weeks (i.e. dose‐finding period), the clinic staff titrated the blinded drug upward until the target blood pressure was reached. Participants were withdrawn from the study if, on any follow‐up visit, diastolic blood pressure was 120 mmHg or more. The trial lasted one year.
Excluded studies
We excluded 28 potentially eligible studies because of the very short duration of relevant interventions (CHHIPS 2009; Dietz 2008), a beta‐blocker was not given as monotherapy or first‐line therapy (ACCORD 2010; CAPP 1999; CAPRICORN 2001; CARDHIAC 2008; CIBIS‐II 1999; CONVINCE 1998; COPE 2005; GEMINI 2008; Marazzi 2011; NORDIL 2000; STOP 1991; STOP‐2 1999), the study was not an RCT (ADaPT 2008), the study was a subset of an included RCT (MAPHY 1988), the study has not reported data on mortality or hard cardiovascular endpoints (COSMOS 2010; Nilsson 2007), or not all enrolled participants had hypertension (APSIS 2006; CIBIS‐II 1999; CAPRICORN 2001; COMET 2003; COPERNICUS 2004; Dutch TIA 1993; IMPACT‐HF 2004; MERIT‐HF 2002; RESOLVD 2000; SENIORS 2005; TEST 1995).The trials where not all enrolled participants had hypertension were of beta‐blockers in people with heart failure (CIBIS‐II 1999; COMET 2003; COPERNICUS 2004; IMPACT‐HF 2004; Marazzi 2011; MERIT‐HF 2002; RESOLVD 2000; SENIORS 2005), angina pectoris (APSIS 2006), post‐myocardial infarction (CAPRICORN 2001), or transient ischaemic attack or stroke (Dutch TIA 1993; TEST 1995).
We have described each of the 28 excluded studies in greater detail in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias in included studies is summarised in Figure 2 and Figure 3.
2.
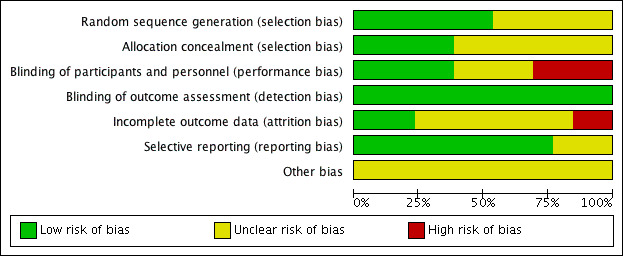
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
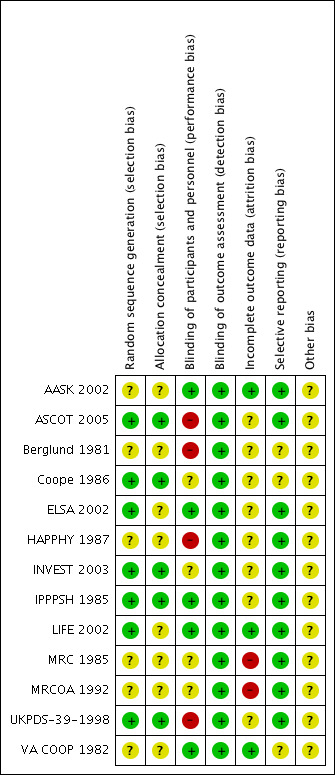
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven trials reported the method used to generate the randomisation sequence adequately (ASCOT 2005; Coope 1986; ELSA 2002; INVEST 2003; IPPPSH 1985; LIFE 2002; UKPDS‐39‐1998). It was unclear in the remaining six (AASK 2002; Berglund 1981; HAPPHY 1987; MRC 1985; MRCOA 1992; VA COOP 1982).
Five trials had adequate allocation concealment (ASCOT 2005; Coope 1986; INVEST 2003; IPPPSH 1985; UKPDS‐39‐1998), while in the remaining eight, the information provided was insufficient to assess this aspect of risk of bias (AASK 2002; Berglund 1981; ELSA 2002; HAPPHY 1987; LIFE 2002; MRC 1985; MRCOA 1992; VA COOP 1982).
Blinding
Outcome assessors were blinded in 11 studies (AASK 2002; ASCOT 2005; Coope 1986; ELSA 2002; HAPPHY 1987; INVEST 2003; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; VA COOP 1982), and two trials were completely unblinded (Berglund 1981; UKPDS‐39‐1998). However, in the Berglund 1981 study, the outcome assessed (i.e. death) is unlikely to be influenced by lack of blinding.
Participants were also blinded in seven trials (AASK 2002; ELSA 2002; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; VA COOP 1982), but healthcare workers were only blinded in five trials (AASK 2002; ELSA 2002; IPPPSH 1985; LIFE 2002; VA COOP 1982) .
Incomplete outcome data
Loss to follow‐up was negligible in AASK 2002 (0%), ASCOT 2005 (0.3%), IPPPSH 1985 (0.6%), HAPPHY 1987 (1%), LIFE 2002 (2%), INVEST 2003 (2.5%), ELSA 2002 (4%), UKPDS‐39‐1998 (4%), Berglund 1981 (7%), and VA COOP 1982 (8%), but high in MRC 1985 (19%) and MRCOA 1992 (25%) trials. Coope 1986 did not report loss to follow‐up.
The following trials stated the proportions of participants taking assigned beta‐blocker treatment at the end of the trial: HAPPHY 1987 (86%), ELSA 2002 (85%), Berglund 1981 (74%), Coope 1986 (70%), UKPDS‐39‐1998 (65%), LIFE 2002 (63%), MRC 1985 (59%), VA COOP 1982 (39%), MRCOA 1992 (37%), and IPPPSH 1985 (30%).
Selective reporting
Ten studies reported outcomes as stated in the respective study protocols (AASK 2002; ASCOT 2005; ELSA 2002; HAPPHY 1987; INVEST 2003; IPPPSH 1985; LIFE 2002; MRC 1985; MRCOA 1992; UKPDS‐39‐1998). We did not have access to the study protocols of the remaining studies (Berglund 1981; Coope 1986; VA COOP 1982).
Other potential sources of bias
All the studies added other antihypertensive drugs to the first‐line treatment to help achieve the blood pressure goals. The observed effects may equally have resulted from the additional drugs used. In addition, two studies were stopped early for data‐dependent reasons (AASK 2002; ASCOT 2005).
The high risk of bias in most of the included studies limits our confidence in the effect estimates for beta‐blockers as first‐line therapy for hypertension (Balshem 2011; Guyatt 2011), as shown in the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Due to the small number of participants in trials with ACE inhibitors (2 trials with 1635 participants (AASK 2002; UKPDS‐39‐1998)) and ARBs (1 trial with 9193 participants (LIFE 2002)), we combined data for the two classes of RAS inhibitors. We excluded the trial that compared the effects of atenolol and aliskiren, the first DRI to be approved for the treatment of hypertension (Dietz 2008), because of the very short duration (12 weeks) of relevant interventions.
Mortality
The effect of beta‐blocker therapy on total mortality was not significantly different from that of placebo (4 trials, 23,613 participants: RR 0.99, 95% CI 0.88 to 1.11; I2 = 0%; moderate certainty evidence).
Apart from the four studies included in our placebo comparison, previous related reviews included four other studies (Dutch TIA 1993; STOP 1991; TEST 1995; UKPDS‐39‐1998). When we added these studies in a sensitivity analysis, there was still no evidence of a significant effect of beta‐blockers on mortality (8 trials, 28,181 participants: RR 0.93, 95% CI 0.85 to 1.02, I2 = 39%).
In addition, total mortality was not significantly different between beta‐blockers and diuretics (5 trials, 18,241 participants: RR 1.04, 95% CI 0.91 to 1.19, I2 = 0%; moderate certainty evidence), and beta‐blockers and RAS inhibitors (3 trials, 10,828 participants: RR 1.10, 95% CI 0.98 to 1.24, I2 = 54%; moderate certainty evidence).
Total mortality was significantly higher for beta‐blockers compared to CCBs (4 trials, 44,825 participants: RR 1.07, 95% CI 1.00 to 1.14, I2 = 2%; moderate certainty evidence) corresponding to an absolute risk increase (ARI) of 0.5% and number of participants needed to treat for an additional harmful outcome (NNTH) with a beta‐blocker rather than a CCB treated for five years of 200.
Total stroke
Participants treated with a beta‐blocker had a significantly lower risk of developing a stroke than participants taking placebo (4 trials, 23,613 participants: RR 0.80, 95% CI 0.66 to 0.96, I2 = 0%; low certainty evidence). A sensitivity analysis adding the four studies included in related reviews yielded similar results (8 trials, 28,181 participants: RR 0.79, 95% CI 0.70 to 0.90, I2 = 31%).
Expressed as absolute risk reduction (ARR), beta‐blockers reduced the risk of stroke by 0.5% (compared to placebo). The corresponding number of participants needed to treat for an additional beneficial outcome (NNTB) with a beta‐blocker for approximately five years to prevent one stroke was 200.
We found no statistically significant difference in stroke events between participants treated with a beta‐blocker and participants treated with a diuretic (4 trials, 18,135 participants: RR (random effects) 1.17, 95% CI 0.65 to 2.09, I2 = 73%; moderate certainty evidence). However, participants treated with a beta‐blocker (atenolol) had more stroke events than participants treated with a CCB (3 trials, 44,167 participants: RR 1.24, 95% CI 1.11 to 1.40, I2 = 0%; ARI = 0.6%, NNTH 180; moderate certainty evidence) or an RAS inhibitor (2 trials, 9951 participants: RR 1.30, 95% CI 1.11 to 1.53, I2 = 29%; ARI = 1.5%, NNTH 65; moderate certainty evidence).
The heterogeneity among trials comparing beta‐blockers to diuretics may be related to the type of beta‐blockade (I2 = 73%, P = 0.01). There was an increase in the risk of stroke with the non‐selective beta‐blocker, propranolol, in the MRC 1985 trial (RR 2.28, 95% CI 1.31 to 3.95) with an ARI of 0.5% and NNTH with a beta‐blocker for approximately five years of 200; but no difference with the cardio‐selective beta‐blockers, atenolol or metoprolol (RR 1.00, 95% CI 0.74 to 1.33, I2 = 60).
Total coronary heart disease
The effect of beta‐blocker therapy on CHD was not significantly different from that of a placebo (4 trials, 23,613 participants: RR 0.93, 95% CI 0.81 to 1.07, I2 = 0%; moderate certainty evidence). A sensitivity analysis adding the four studies included in related reviews yielded similar results (8 trials, 28,181 participants: RR 0.91, 95% CI 0.81 to 1.02, I2 = 0%).
The beta‐blocker effect was similar to that of a diuretic (4 trials, 18,135 participants: RR (random effects) 1.12, 95% CI 0.82 to 1.54, I2 = 66%; low certainty evidence), a CCB (3 trials, 44,167 participants: RR 1.05, 95% CI 0.96 to 1.15, I2 = 32%; moderate certainty evidence), or a RAS inhibitor (2 trials, 9951 participants: RR 0.90, 95% CI 0.76 to 1.06, I2 = 42%; low certainty evidence).
There was significant statistical heterogeneity between trials comparing beta‐blockers to diuretics (I2 = 66%, P = 0.03), which may be explained by differences in age. The pooled RR in the trials whose participants were less than 65 years of age was 0.97 (95% CI 0.81 to 1.17, I2 = 5%, P = 0.35), while in the single trial involving participants aged 65 years and older atenolol was associated with an increased CHD incidence (RR 1.63, 95% CI 1.15 to 2.32) (MRCOA 1992). The difference between the subgroups was statistically significant (test for subgroup differences: Chi2 = 6.70, degrees of freedom (df) = 1, P = 0.01, I2 = 85.1%).
Total cardiovascular disease
Compared to participants taking placebo, participants taking beta‐blockers had a significantly reduced risk of having a cardiovascular event (4 trials, 23,613 participants: RR 0.88, 95% CI 0.79 to 0.97, I2 = 21%; ARR 0.7%, NNTB 140 for 5 years; low certainty evidence). A sensitivity analysis adding studies included in related reviews yielded similar results.
The effect of beta‐blockers on total cardiovascular events was not significantly different from that of diuretics (4 trials, 18,135 participants: RR 1.13, 95% CI 0.99 to 1.28, I2 = 45%; moderate certainty evidence) and RAS inhibitors (3 trials, 10,828 participants: RR (random effects) 1.00, 95% CI 0.72 to 1.38, I2 = 74%; low certainty evidence). Beta‐blockers increased total cardiovascular disease as compared to CCBs (2 trials, 19,915 participants: RR 1.18, 95% CI 1.08 to 1.29, I2 = 0%; ARI = 1.3%, NNTH 80; moderate certainty evidence).
The significant heterogeneity of effect on total cardiovascular disease between beta‐blockers and RAS inhibitors (I2 = 74%, P = 0.02) was explained by the effect of beta‐blockers being similar to that of ACE inhibitors (2 trials, 635 participants: RR 0.82, 95% CI 0.64 to 1.05, I2 = 0%) but worse than that of an ARB (1 trial, 9193 participants: RR 1.16, 95% CI 1.04 to 1.30) with an ARI of 1.8% and NNTH of 56.
Adverse events leading to discontinuation of allocated treatment
We analysed data on the rate of withdrawal from randomly assigned treatment due to any adverse events, and also report on the frequency of specific adverse events including depression, fatigue, and sexual dysfunction.
Trial participants on a beta‐blocker were no more likely than participants receiving a placebo to discontinue treatment due to adverse events (3 trials, 22,729 participants: RR (random effects) 3.38, 95% CI 0.82 to 13.95; low certainty evidence). However, there was significant heterogeneity of effect between the trials (I2 = 100%, P < 0.00001); with no difference in the likelihood of discontinuing treatment with oxprenolol (1 trial, 6357 participants: RR 0.95, 95% CI 0.87 to 1.04) and an increased likelihood with propranolol or atenolol (2 trials, 16,372; RR (random effects) 6.35, 95% CI 3.94 to 10.22, I2 = 91%). A sensitivity analysis adding studies included in related reviews also revealed significant heterogeneity of effect (I2 = 99%, P < 0.00001).
Participants taking a beta‐blocker were more likely to discontinue treatment due to adverse events than participants taking a RAS inhibitor (2 trials, 9951 participants: RR 1.41, 95% CI 1.29 to 1.54, I2 = 12%; ARI 5.5%, NNTH 18; low certainty evidence), but there was no significant difference with a diuretic (3 trials, 11,566 participants: RR (random effects) 1.69, 95% CI 0.95 to 3.00, I2 = 95%; low certainty evidence) or a CCB (2 trials, 21,591 participants: RR (random effects) 1.20, 95% CI 0.71 to 2.04, I2 = 93%; low certainty evidence).
There was no significant difference in the incidence of depressive symptoms between beta‐blockers and placebo (2 trials, 7082 participants: RR (random effects) 1.03, 95% CI 0.65 to 1.63, I2 = 83.0) or RAS inhibitors (1 trial, 758 participants: RR 1.12, 95% CI 0.07 to 17.80).
Beta‐blockers did not increase the risk of fatigue compared to placebo or no treatment (2 trials, 13,782 participants: RR (random effects) 4.35, 95% CI 0.17 to 108.74, I2 = 99.0%). However, trial participants taking a beta‐blocker were more likely to develop fatigue than participants taking a diuretic (1 trial, 8700 participants: RR 2.48, 95% CI 1.73 to 3.54), a CCB (1 trial, 19,257 participants: RR 1.99, 95% CI 1.84 to 2.16), or a RAS inhibitor (2 trials, 9951 participants: RR 1.17, 95% CI 1.06 to 1.28, I2 = 0%).
The risk of sexual dysfunction was not different between beta‐blockers and placebo (2 trials, 19,414 participants: RR (random effects) 1.95, 95% CI 0.33 to 11.59, I2 = 97.5%). However, beta‐blockers decreased the risk of sexual dysfunction when compared to diuretics (1 trial, 8700 participants: RR 0.50, 95% CI 0.36 to 0.70); but increased the risk relative to CCBs (1 trial, 19,257 participants: RR 1.27, 95% CI 1.14 to 1.42) and RAS inhibitors (2 trials, 9951 participants: RR 1.34, 95% CI 1.10 to 1.63, I2 = 56.2%).
Degree of reduction in systolic and diastolic blood pressure achieved by beta‐blocker therapy in relation to each comparator treatment
Compared to placebo, first‐line beta‐blockers plus supplementary antihypertensive drugs reduced systolic blood pressure by about 11 mmHg and diastolic blood pressures by about 6 mmHg (Table 6). However, compared to diuretics, CCBs, or RAS inhibitors, the mean systolic and diastolic blood pressures at the end of the trials were 0 to 2 mmHg higher in the beta‐blocker group (Table 6).
2. Effect of beta‐blockers on lowering of blood pressure.
| Trial identification | Beta‐blocker | Comparison drug | Baseline BP (SBP/DBP; mmHg) | Mean BP difference (SBP/DBP)* |
| Beta‐blocker vs placebo/no treatment | ||||
| Coope 1986 | Atenolol | No treatment | 196.7/99.7 | ‐18.0/‐11.0 |
| MRCOA 1992 | Atenolol | Placebo | 184.0/91.0 | ‐13.0/‐7.0 |
| MRC 1985 | Propranolol | Placebo | 162.0/98.5 | ‐9.5/‐5.0 |
| IPPPSH 1985 | Oxprenolol | Placebo | 173.2/107.9 | ‐4.1/‐1.5 |
| Beta‐blocker vs diuretic | ||||
| MRCOA 1992 | Atenolol | Diuretic | 184.0/91.0 | +1.0/‐0.5 |
| HAPPHY 1987 | Atenolol or metoprolol or propranolol | Diuretic | 166.0/107.9 | 0.0/‐1.0 |
| Berglund 1981 | Propranolol | Diuretic | 174.0/105.5 | ‐4.0/+2.0 |
| VA COOP 1982 | Propranolol | Diuretic | 146.3/101.5 | +7.0/+1.6 |
| MRC 1985 | Propranolol | Diuretic | 162.0/98.5 | +3.5/+1.0 |
| Beta‐blocker vs calcium‐channel blocker | ||||
| ELSA 2002 | Atenolol | Calcium‐channel blocker | 163.1/101.3 | +0.2/‐0.1 |
| INVEST 2003 | Atenolol | Calcium‐channel blocker | 150.8/87.2 | +0.3/+0.2 |
| ASCOT 2005 | Atenolol | Calcium‐channel blocker | 164.0/94.7 | +1.6/+1.8 |
| AASK 2002 | Metoprolol | Calcium‐channel blocker | 150.0/96.0 | +2.0/0.0 |
| Beta‐blocker vs renin‐angiotensin system inhibitor | ||||
| UKPDS‐39‐1998 | Atenolol | Renin‐angiotensin system inhibitor (ACE inhibitor) | 159.0/93.0 | ‐1.0/‐1.0 |
| LIFE 2002 | Atenolol | Renin‐angiotensin system inhibitor (ARB) | 174.5/97.7 | +1.1/‐0.2 |
| AASK 2002 | Metoprolol | Renin‐angiotensin system inhibitor (ACE inhibitor) | 150.0/96.0 | 0.0/‐1.0 |
* 'Minus sign' means beta‐blocker group had lower BP, and 'plus sign' means beta‐blocker group had higher BP than control group.
ACE: angiotensin‐converting enzyme; ARB: angiotensin receptor blocker; BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Discussion
Summary of main results
We included 13 eligible RCTs, which compared beta‐blockers to placebo, diuretics, CCBs, and RAS inhibitors. These RCTs generally had a high risk of bias resulting from limitations in study design, conduct, and data analysis.
We found little or no difference in all‐cause mortality between beta‐blockers and placebo, diuretics or RAS inhibitors, but all‐cause mortality was higher for beta‐blockers compared to CCBs. The evidence on mortality was of moderate‐certainty for all comparisons. Total cardiovascular disease was lower for beta‐blockers compared to placebo, which is a reflection of the significant decrease in stroke, since there was little or no difference in CHD between beta‐blockers and placebo. There were no significant differences between beta‐blockers and placebo in adverse events leading to withdrawal from assigned treatment (low‐certainty evidence).
The effect of beta‐blockers on cardiovascular disease was worse than that of CCBs (moderate‐certainty evidence), but was not different from that of diuretics (moderate‐certainty evidence) or RAS inhibitors (low‐certainty evidence). In addition, there was an increase in stroke with beta‐blockers compared to CCBs (moderate‐certainty evidence) and RAS inhibitors (moderate‐certainty evidence). However, there was little or no difference in CHD between beta‐blockers and diuretics (low‐certainty evidence), CCBs (moderate‐certainty evidence), or RAS inhibitors (low‐certainty evidence). Participants taking beta‐blockers were more likely to discontinue treatment due to adverse events than participants taking RAS inhibitors (moderate‐certainty evidence), but there was no significant difference with diuretics (low‐certainty evidence) or CCBs (low certainty evidence).
We demonstrated a high degree of homogeneity of effect for the comparisons of beta‐blockers versus CCBs for all‐cause mortality (I2 = 2%), stroke (I2 = 0%), and total cardiovascular events (I2 = 0%) but with less homogeneity for CHD (I2 = 32%). For the comparison of beta‐blockers versus RAS inhibitors, the I2 values for stroke and withdrawal rates also demonstrate a high degree of consistency across the studies making our conclusions more secure (Higgins 2003; Higgins 2011). For the comparison with diuretics, there were no statistically significant differences in any morbidity or mortality outcome.
Overall completeness and applicability of evidence
Though beta‐blockers are a heterogeneous group of pharmacological agents, differing in beta‐adrenergic receptor selectivity, intrinsic sympathomimetic activity, and vasodilatory capabilities (Kamp 2010; Pedersen 2007; Polónia 2010), we found no outcome trials with head‐to‐head comparisons between beta‐blockers for the treatment of hypertension (Poirier 2014). Of the 40,245 participants using beta‐blockers in this review, atenolol was used by 30,150 participants (75%). Due to the paucity of data using beta‐blockers other than atenolol, it is not possible to say whether the (lack of) effectiveness and (in)tolerability of beta‐blockers seen in this review is a property of atenolol or is a class effect of beta‐blockers. From this review, we cannot support the claim by Lindhom and colleagues that cardioselective beta‐blockers may be inferior to non‐selective beta‐blockers in the treatment of hypertension (Carlberg 2004).
A limitation of both previous reviews and ours is the absence of trials assessing the effects of the new vasodilating beta‐blockers (e.g. carvedilol, bucindolol, and nebivolol) on mortality and hard cardiovascular outcomes. Possible mechanisms to explain the poor ability of beta‐blockers to reduce stroke include a propensity to cause diabetes (Opie 2004), a failure to decrease central aortic pressure as much as brachial pressure, and others. Diabetes likely requires years to develop cardiovascular complications (Verdecchia 2004), so we favour the mechanism involving lesser reduction of central aortic pressure by beta‐blockers. Vasodilating beta‐blockers (Broeders 2000; Kalinowski 2003; Pucci 2016) have been shown to reduce central pressures better than conventional beta‐blockers (Kamp 2010; Polónia 2010); most probably because vasodilatation favourably alters the pattern of the pressure wave reflecting back from the periphery, thereby lowering the central pressure. Nonetheless, carvedilol and nebivolol also cause bradycardia, which is thought to be the principal mechanism whereby atenolol with or without thiazide may be less able to lower the central pressure than amlodipine with or without perindopril (Williams 2006). At any rate, high‐quality outcome studies are required to show that hard cardiovascular endpoints such as stroke and CHD are significantly reduced by beta‐blockers not studied in this review.
Information reported in the trials considered in this review was insufficient to explore the effect of race or ethnicity, as most trial participants were white (Park 2007). However, the finding that beta‐blockers are less effective than diuretics in older people, is most likely to be applicable to older black people as well (Materson 1993).
Quality of the evidence
The certainty of the evidence on the effects of beta‐blockers was generally moderate to low (Balshem 2011). In the GRADE system, RCTs without important limitations constitute high‐certainty evidence. However, the system considers five factors that can lower the certainty of the evidence: study limitations, heterogeneity, indirectness, imprecision, and publication bias. Overall, the GRADE system classifies research evidence into high‐, moderate‐, low‐, or very low‐certainty. Low‐certainty evidence implies that the "true effect is likely to be different from the estimate of effect" found in the review.
Our major concern with the evidence related to inherent shortcomings in the included primary studies. The emphasis was often on the results with the first drug used, whereas most studies used stepped‐up therapy to help achieve the blood pressure goals. Thus poorer outcomes with first‐line beta‐blockers may equally have resulted from the use of other drugs; explaining why other authors restricted their systematic reviews of beta‐blocker therapy to trials where confounding supplementary drug classes were administered to less than half of participants (Wright 1999; Wright 2000; Wright 2009). Although we were less restrictive than Wright and colleagues (Wright 1999; Wright 2000), we included only trials in which all the participants in one group received a beta‐blocker at baseline, whether or not other antihypertensive drug classes were later added to achieve blood pressure targets. This requirement was in contrast to other systematic reviews (Carlberg 2004; Dahlöf 2007; Khan 2006; Lindhom 2005). The dropout rates were high in two of the studies of diuretics, potentially introducing attrition bias (MRC 1985; MRCOA 1992).
It may be that only people with complicated hypertension or advanced disease are included in most studies, thereby ignoring the possible differing benefits of different antihypertensive medications on different organs and on different stages of disease development (Zanchetti 2005). A further problem is that in the two groups of the studies we analysed, and especially in the case of the comparison with diuretics, there were discrepancies between the achieved blood pressure levels (Table 6), and even small blood pressure differences may be linked to significant differences in outcomes (Collins 1990; Staessen 2003). However, there were no consistent differences in the blood pressure reduction between beta‐blockers and the other agents used to explain the outcome differences we found (Table 6). Yet another limitation is that (due to the scarcity of relevant trials) we combined the potentially different classes of RAS inhibitors (i.e. ACE inhibitors (captopril and lisinopril) and ARB (losartan). However, we believe that the similarities between these agents as antihypertensive drugs outweigh any potential differences.
Potential biases in the review process
We minimised potential biases in the review process by adhering to the Cochrane guidelines (Higgins 2011). We conducted a comprehensive search for eligible studies, without limiting the search to a specific language. Two review authors independently assessed study eligibility, extracted data, and assessed the risk of bias in each included study.
Agreements and disagreements with other studies or reviews
We showed that beta‐blockers are inferior to various CCBs for all‐cause mortality, stroke, and total cardiovascular events, and to RAS inhibition for stroke. By comparing beta‐blockers with all other therapies, Lindholm and colleagues were only able to show an inferiority of beta‐blockade on stroke reduction (Carlberg 2004; Lindhom 2005). In a similar meta‐analysis, Khan and McAlister found beta‐blockers to be inferior to all other therapies in effects on a composite outcome of major cardiovascular events (stroke, myocardial infarction, and death) and stroke for older people with hypertension but found no difference in effects for younger people (Khan 2006). The claim by Khan 2006 that the defects of beta‐blockade are limited to older people relies heavily on the Medical Research Council trial in older people with hypertension in which the beta‐blocker was atenolol and where the dropout rate was 25% (MRCOA 1992). In addition, Khan 2006 classified trials which enrolled participants as young as 40 (ASCOT 2005), 45 (ELSA 2002), and 50 (INVEST 2003) years as trials of older people with hypertension. At present, there are insufficient data to make a valid comparison of beta‐blocker effects on younger versus older people, although this is an important hypothesis.
We used the I2 statistic to evaluate the consistency in study results (Higgins 2003; Higgins 2011). In our meta‐analyses, heterogeneity was very low for the outcomes of beta‐blockers versus placebo or no treatment. We found a modest 20% relative reduction in stroke by beta‐blockers compared to placebo with six studies, which is similar to the relative reduction reported by Lindholm and colleagues using seven studies (Lindhom 2005). With their wider inclusion criteria, Lindholm and colleagues included three studies not considered by us (Dutch TIA 1993; STOP 1991; TEST 1995), which resulted in significant heterogeneity of effect in their findings. By contrast, there was excellent homogeneity of effect with the four studies included in our comparison of beta‐blockers to placebo as shown by an I2 value of 0% (Coope 1986; IPPPSH 1985; MRC 1985; MRCOA 1992). Thus, we were able to give additional validation to one of the crucial findings of Lindholm and colleagues (Lindhom 2005), namely that stroke reduction by beta‐blockade is suboptimal.
Two other reviews also differed from ours in their inclusion or exclusion of various studies (Dahlöf 2007; Wright 2009). Both considered the UKPDS‐39‐1998 as a placebo‐controlled trial and excluded IPPPSH 1985. In addition, Wright 2009 excluded Coope 1986 because of high use of diuretics in the beta‐blocker group while Dahlöf 2007 included STOP 1991 because more than 85% of participants on active treatment received beta‐blocker as first‐line or second‐line therapy. Both reviews considered the "less tight control group" in UKPDS‐39‐1998 as "placebo" because the target for blood pressure reduction in this group was not as low as in the beta‐blocker group. However, participants in this control group took antihypertensive treatment for 57% of their total person‐years in the UKPDS‐39‐1998 trial.
We combined trials of low‐dose and high‐dose thiazide diuretics because of the paucity of trials comparing beta‐blockers to diuretics (Berglund 1981; HAPPHY 1987; MRC 1985; MRCOA 1992; VA COOP 1982). This may be the reason for the lack of a statistical difference between beta‐blockers and diuretics in our review, since Wright and Musini have shown that first‐line low‐dose thiazides reduce stroke, CHD, and mortality outcomes while first‐line high‐dose thiazides have no significant effects on mortality and CHD (Wright 2009).
We conducted sensitivity analyses and found our results to be consistent with those of the related reviews, despite differences in inclusion and exclusion criteria (Effects of interventions). Overall, despite a variation in the studies included in other beta‐blocker reviews arising from different interpretations of inclusion criteria, all the reviews arrived at similar conclusions that the available evidence does not support the use of beta‐blockers as first‐line drugs in the treatment of hypertension.
Authors' conclusions
Implications for practice.
First‐line beta‐blockers in people with hypertension lead to modest reductions in stroke and have no significant effects on total mortality and coronary heart disease. In addition, beta‐blockers are inferior to calcium‐channel blockers and renin‐angiotensin system inhibitors for various important outcomes. Most of this evidence is considered to be of low quality according to the GRADE system, implying that further research is likely to change our confidence in the estimate of these effects. However, the evidence comes mainly from trials that used atenolol. Our findings extend the results of previous meta‐analyses suggesting that beta‐blockers are inferior first‐line choices when compared to diuretics, renin‐angiotensin system inhibitors, and calcium‐channel blockers.
Implications for research.
More randomised controlled trials studying the use of beta‐blockers for elevated blood pressure are required. Such hypertension trials must measure clearly defined morbidity and mortality endpoints, including coronary heart disease, heart failure, and stroke. These trials should be used to define differences between beta‐blockers and other classes of antihypertensive drugs and between the different subclasses of beta‐blockers. In addition, the possible differential effect of beta‐blockers on younger and older people needs to be assessed in future hypertension trials.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2017 | New search has been performed | Up to date search. No new studies met the inclusion criteria. |
| 12 January 2017 | New citation required but conclusions have not changed | Conclusions have been reworded and there is a change in authorship and author affiliations. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 16 November 2012 | Amended | New search from December 2011 to November 2012. |
| 27 August 2012 | Amended | updated author affiliations |
| 9 July 2012 | New search has been performed | New search from June 2006 to December 2011. No new studies met the inclusion criteria. The Risk of Bias table has been updated for all included studies and 4 Summary of findings tables have been added to the updated review. In the 2007 version there were unintended errors in the data entered for withdrawals due to side effects for the two UK Medical Research Council trials (MRC 1985,MRCOA 1992), which led to the erroneous conclusion that patients on beta‐blockers were more likely to discontinue treatment due to side effects than those on diuretics. The corrected data, in this update, show no significant differences in withdrawals due to side effects between beta‐blockers and diuretics. The overall message in the conclusions has not changed. |
| 9 July 2012 | New citation required but conclusions have not changed | New citation due to update |
| 13 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the staff of the Cochrane Hypertension Group for assistance during the update of this systematic review. We acknowledge the significant contributions of Professor Anthony Mbewu and Dr Roy Maroney to the protocol and previous versions of the review. Professor Mbewu and Dr Maroney did not contribute to and have neither read nor approved the current review.
Professor CS Wiysonge's work is partly supported by the South African Medical Research Council, the National Research Foundation of South Africa, and the Effective Health Care Research Consortium (Grant: 5242).
Appendices
Appendix 1. 2015 search strategy
Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 19 January 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic beta‐antagonists/ (76928) 2 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp. (73611) 3 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (86331) 4 or/1‐3 (139776) 5 hypertension/ (192862) 6 hypertens$.tw. (304808) 7 exp blood pressure/ (247717) 8 (blood pressure or blood pressure).mp. (350302) 9 or/5‐8 (589677) 10 randomized controlled trial.pt. (381216) 11 controlled clinical trial.pt. (88387) 12 randomi?ed.ab. (334664) 13 placebo.ab. (147683) 14 drug therapy.fs. (1727364) 15 randomly.ab. (198880) 16 trial.ab. (288170) 17 groups.ab. (1274045) 18 or/10‐17 (3261120) 19 animals/ not (humans/ and animals/) (3879559) 20 18 not 19 (2775676) 21 4 and 9 and 20 (19415) 22 21 and (2013$ or 2014$ or 2015$).ed. (674) 23 remove duplicates from 22 (663)
Embase <1974 to 2015 January 16> Search Date: 19 January 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp beta adrenergic receptor blocking agent/ (243970) 2 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp. (178474) 3 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (104425) 4 or/1‐3 (294052) 5 exp hypertension/ (510805) 6 hypertens$.tw. (448067) 7 exp blood pressure/ (413025) 8 blood pressure o bloodpressure.mp. (0) 9 or/5‐8 (911302) 10 randomized controlled trial/ (358482) 11 crossover procedure/ (41032) 12 double‐blind procedure/ (119385) 13 (randomi?ed or randomly).tw. (749012) 14 (crossover$ or cross‐over$).tw. (73500) 15 placebo$.ab. (204404) 16 (doubl$ adj blind$).tw. (152473) 17 assign$.ab. (245912) 18 allocat$.ab. (86645) 19 or/10‐18 (1145599) 20 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5518138) 21 19 not 20 (995733) 22 4 and 9 and 21 (11880) 23 22 and (2013$ or 2014$ or 2015$).em. (1164) 24 remove duplicates from 23 (1150)
Cochrane Central Register of Controlled Trials on Wiley <Issue 1, 2015> via Cochrane Register of Studies Online Search Date: 19 January 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1:(adrenergic beta‐antagonist*) ‐ 3953 #2: (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) ‐ 14056 #3: beta near2 (adrenergic* or antagonist* or block* or receptor*) ‐ 11011 #4: #1 OR #2 OR #3 ‐ 18403 #5: antihypertens* or hypertens* ‐ 35486 #6: ("blood pressure" or bloodpressure) ‐ 46400 #7: #5 OR #6 ‐ 63228 #8: #4 AND #7 ‐ 9332 #9: 01/10/2013 TO 19/01/2015:CD ‐ 123974 #10: #8 AND #9 ‐ 793 ***************************
Hypertension Group Specialised Register Search Date: 19 January 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (adrenergic beta‐antagonist*) 2 (beta blocker*) 3 (beta adrenergic block*) 4 (adrenergic beta receptor block*) 5 (beta adrenergic receptor block*) 6 #1 OR #2 OR #3 OR #4 OR #5 7 (hypertens*) 8 #6 AND #7 9 #8 AND (RCT OR Review OR Meta‐Analysis) (1782) ***************************
ClinicalTrials.gov (via Cochrane Register of Studies) Search Date: 19 January 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: randomized Study type: Interventional Conditions: hypertension Interventions: "adrenergic beta‐antagonist" OR "adrenergic beta‐antagonists" OR "beta blocker" OR "beta blockers" Outcome Measures: blood pressure First received: 1/10/2013 to 19/1/2015 (9) ***************************
Appendix 2. 2016 Search strategy
Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 14 June 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic beta‐antagonists/ (79179) 2 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp. (75673) 3 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (90482) 4 or/1‐3 (145660) 5 hypertension/ (210798) 6 hypertens$.tw. (330792) 7 exp blood pressure/ (264762) 8 (blood pressure or blood pressure).mp. (373969) 9 or/5‐8 (633729) 10 randomized controlled trial.pt. (420851) 11 controlled clinical trial.pt. (91010) 12 randomi?ed.ab. (379711) 13 placebo.ab. (159968) 14 drug therapy.fs. (1873762) 15 randomly.ab. (223574) 16 trial.ab. (328035) 17 groups.ab. (1409370) 18 or/10‐17 (3572728) 19 animals/ not (humans/ and animals/) (4231241) 20 18 not 19 (3046252) 21 4 and 9 and 20 (20003) 22 21 and (2015$ or 2016$).ed. (528) 23 remove duplicates from 22 (498) ***************************
Cochrane Central Register of Controlled Trials on Wiley <2016, Issue 6> via Cochrane Register of Studies Online Search Date: 14 June 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1MESH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL TREES9429 #2adrenergic beta‐antagonist*4072 #3(acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol)14950 #4beta near2 (adrenergic* or antagonist* or block* or receptor*)12693 #5#1 OR #2 OR #3 OR #420606 #6antihypertens* or hypertens*40964 #7blood pressure or bloodpressure52553 #8#6 OR #772648 #9#5 AND #810268 #1001/01/2015 TO 14/06/2016:CD AND 01/01/2015 TO 14/06/2016:CD107219 #11#9 AND #10558 ***************************
Embase <1974 to 2016 June 13> Search Date: 14 June 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp beta adrenergic receptor blocking agent/ (257952) 2 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp. (186549) 3 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (112699) 4 or/1‐3 (312536) 5 exp hypertension/ (577705) 6 hypertens$.tw. (508421) 7 exp blood pressure/ (464921) 8 blood pressure o bloodpressure.mp. (0) 9 or/5‐8 (1027859) 10 randomized controlled trial/ (408424) 11 crossover procedure/ (47399) 12 double‐blind procedure/ (131405) 13 (randomi?ed or randomly).tw. (880104) 14 (crossover$ or cross‐over$).tw. (82444) 15 placebo$.ab. (231893) 16 (doubl$ adj blind$).tw. (169466) 17 assign$.ab. (283020) 18 allocat$.ab. (102246) 19 or/10‐18 (1321527) 20 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5874427) 21 19 not 20 (1153534) 22 4 and 9 and 21 (12623) 23 22 and (2015$ or 2016$).em. (818) 24 remove duplicates from 23 (795) ***************************
Cochrane Hypertension Specialised Register Search Date: Search Date: 14 June 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (adrenergic beta‐antagonist*) (1506) #2 (beta blocker*) (2211) #3 (beta adrenergic block*) (247) #4 (adrenergic beta receptor block*) (13) #5 (beta adrenergic receptor block*) (1141) #6 #1 OR #2 OR #3 OR #4 OR #5 (3838) #7 RCT:DE (22671) #8 (Review or Meta‐Analysis):MISC2 (1147) #9 #6 AND (#7 OR #8) (2176) #10 (#9) AND (1/1/2015 TO 14/6/2016:CRSMODIFIED) (398) ***************************
ClinicalTrials.gov Search Date: 14 June 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: randomized Study type: Interventional Conditions: hypertension Interventions: "adrenergic beta‐antagonist" OR "adrenergic beta‐antagonists" OR "beta blocker" OR "beta blockers" Outcome Measures: blood pressure (95) ***************************
Data and analyses
Comparison 1. Beta‐blocker versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 23613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 2 Total stroke | 4 | 23613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.96] |
| 3 Total coronary heart disease | 4 | 23613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 4 Cardiovascular death | 4 | 23613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.09] |
| 5 Total cardiovascular disease | 4 | 23613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| 6 Withdrawal due to adverse effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Oxprenolol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Propranolol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Atenolol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
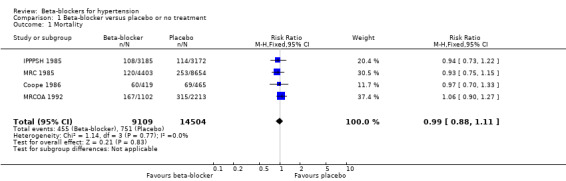
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 1 Mortality.
1.2. Analysis.
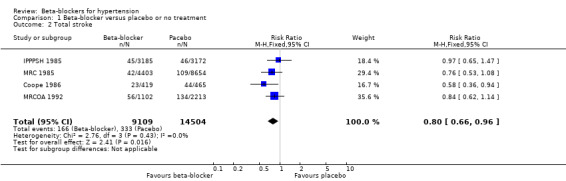
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 2 Total stroke.
1.3. Analysis.
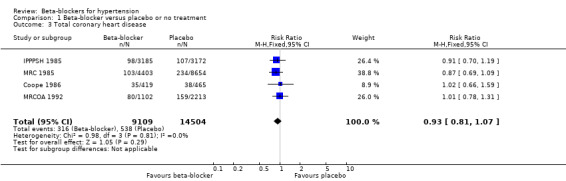
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 3 Total coronary heart disease.
1.4. Analysis.
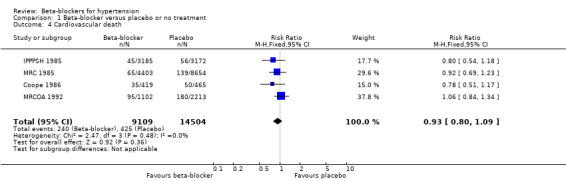
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 4 Cardiovascular death.
1.5. Analysis.
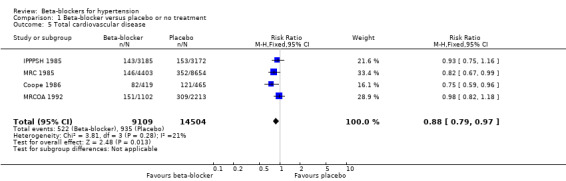
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 5 Total cardiovascular disease.
1.6. Analysis.
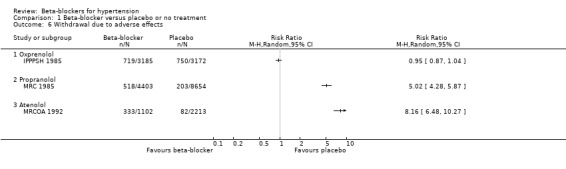
Comparison 1 Beta‐blocker versus placebo or no treatment, Outcome 6 Withdrawal due to adverse effects.
Comparison 2. Beta‐blocker versus diuretic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 18241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.19] |
| 2 Total stroke | 4 | 18135 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.65, 2.09] |
| 2.1 Cardio‐selective beta‐blocker | 3 | 9435 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.55, 1.54] |
| 2.2 Non‐selective beta‐blocker | 1 | 8700 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.31, 3.95] |
| 3 Total coronary heart disease | 4 | 18135 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.82, 1.54] |
| 3.1 Aged < 65 years | 3 | 15952 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.17] |
| 3.2 Aged > 65 years | 1 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.15, 2.32] |
| 4 Cardiovascular death | 3 | 17452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.32] |
| 5 Total cardiovascular disease | 4 | 18135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.28] |
| 6 Withdrawal due to adverse effects | 3 | 11566 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.95, 3.00] |
2.1. Analysis.
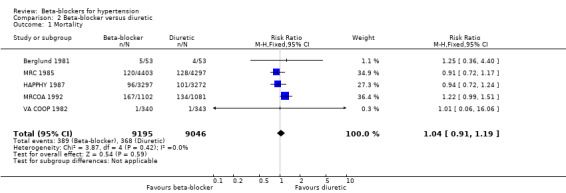
Comparison 2 Beta‐blocker versus diuretic, Outcome 1 Mortality.
2.2. Analysis.
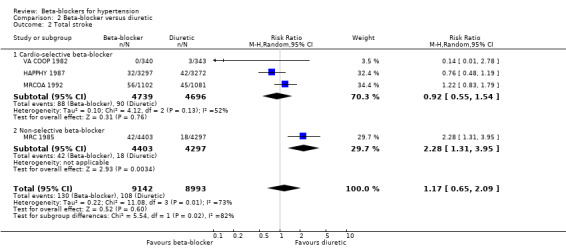
Comparison 2 Beta‐blocker versus diuretic, Outcome 2 Total stroke.
2.3. Analysis.
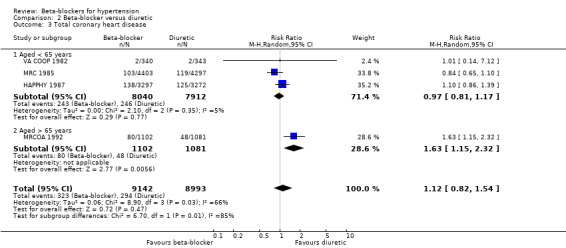
Comparison 2 Beta‐blocker versus diuretic, Outcome 3 Total coronary heart disease.
2.4. Analysis.
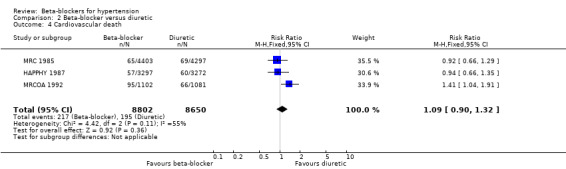
Comparison 2 Beta‐blocker versus diuretic, Outcome 4 Cardiovascular death.
2.5. Analysis.
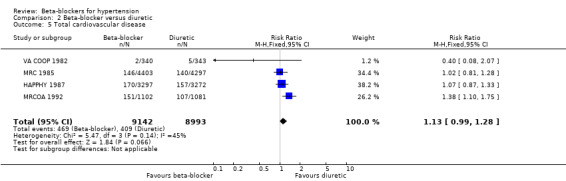
Comparison 2 Beta‐blocker versus diuretic, Outcome 5 Total cardiovascular disease.
2.6. Analysis.
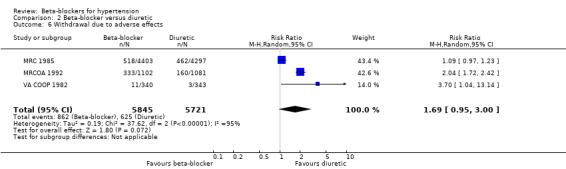
Comparison 2 Beta‐blocker versus diuretic, Outcome 6 Withdrawal due to adverse effects.
Comparison 3. Beta‐blocker versus calcium‐channel blocker (CCB).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 44825 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.14] |
| 2 Total stroke | 3 | 44167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.11, 1.40] |
| 3 Total coronary heart disease | 3 | 44167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 4 Cardiovascular death | 4 | 44825 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.92, 1.46] |
| 5 Total cardiovascular disease | 2 | 19915 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.08, 1.29] |
| 6 Withdrawal due to adverse effects | 2 | 21591 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.71, 2.04] |
3.1. Analysis.
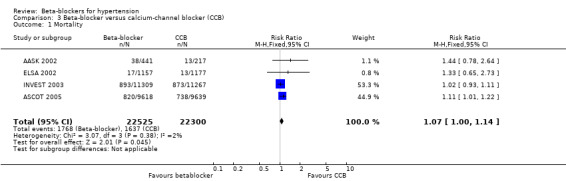
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 1 Mortality.
3.2. Analysis.
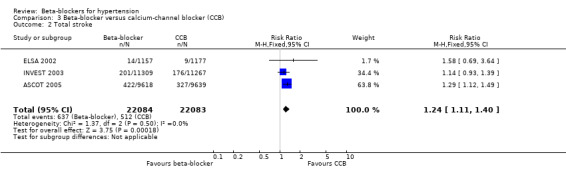
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 2 Total stroke.
3.3. Analysis.
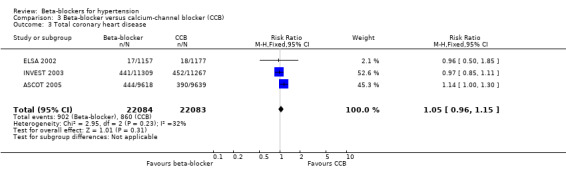
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 3 Total coronary heart disease.
3.4. Analysis.
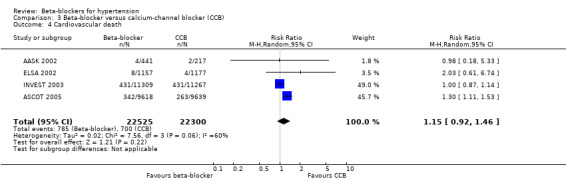
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 4 Cardiovascular death.
3.5. Analysis.
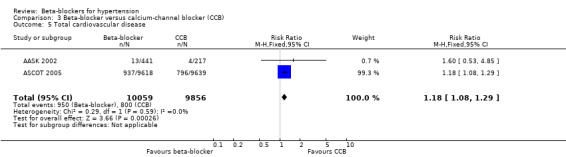
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 5 Total cardiovascular disease.
3.6. Analysis.
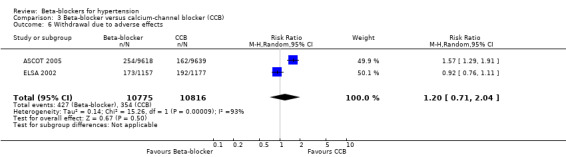
Comparison 3 Beta‐blocker versus calcium‐channel blocker (CCB), Outcome 6 Withdrawal due to adverse effects.
Comparison 4. Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 10828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.24] |
| 2 Total stroke | 2 | 9951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.53] |
| 3 Total coronary heart disease | 2 | 9951 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 4 Cardiovascular death | 3 | 10828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 5 Total cardiovascular disease | 3 | 10828 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.72, 1.38] |
| 5.1 Angiotensin‐converting enzyme inhibitors | 2 | 1635 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 5.2 Angiotensin receptor blockers | 1 | 9193 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.04, 1.30] |
| 6 Withdrawal due to adverse effects | 2 | 9951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.29, 1.54] |
4.1. Analysis.
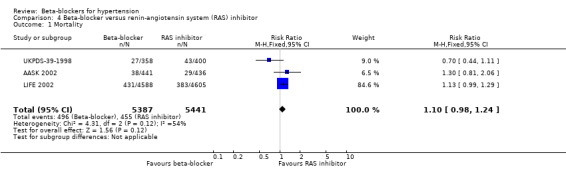
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 1 Mortality.
4.2. Analysis.
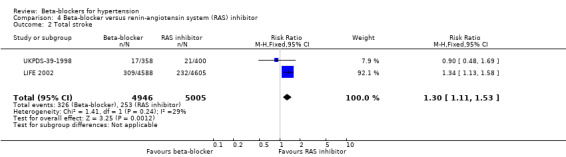
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 2 Total stroke.
4.3. Analysis.
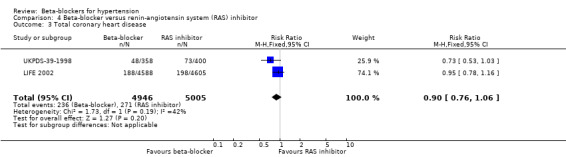
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 3 Total coronary heart disease.
4.4. Analysis.
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 4 Cardiovascular death.
4.5. Analysis.
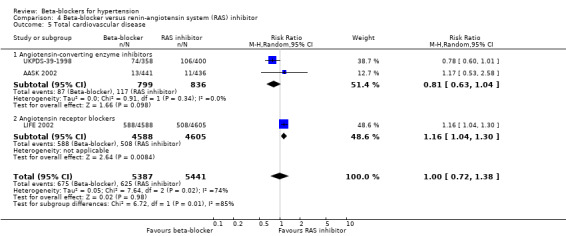
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 5 Total cardiovascular disease.
4.6. Analysis.
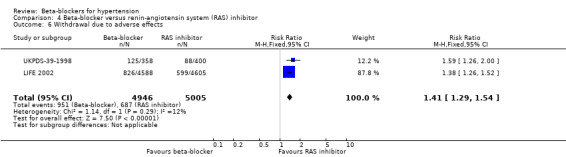
Comparison 4 Beta‐blocker versus renin‐angiotensin system (RAS) inhibitor, Outcome 6 Withdrawal due to adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AASK 2002.
| Methods | Multicentre study Randomisation: described as randomised controlled trial, but method of allocating participants to treatment was not described. Blinding: participants, providers, and outcome assessors blinded Loss to follow‐up: 0% Mean duration of follow‐up: 4.1 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic location: USA Study setting: hospital Number of participants: 1094 (61.2% men) Age range: 18 to 70 years (mean: 54 years) Entry criteria: DBP ≥ 95 mmHg (mean BP 150/96 mmHg) and glomerular filtration rate 20 mL/minute/1.73 m2 to 65 mL/minute/1.73 m2 and no other identified causes of renal insufficiency Race: all African Americans Exclusion criteria: DBP < 95 mmHg, known history of diabetes mellitus, urinary protein to creatinine ratio > 2.5, accelerated or malignant hypertension within 6 months, secondary hypertension, non‐BP‐related causes of kidney disease, serious systemic disease, clinical CHF, or specific (contra)indication for a study drug or procedure |
|
| Interventions |
Beta‐blocker group: Metoprolol 50 mg/day to 200 mg/day ACE inhibitor group: Ramipril 2.5 mg/day to 10 mg/day Calcium‐channel blocker group: Amlodipine 5 mg/day to 10 mg/day If the BP goal could not be achieved by the randomly allocated drug, additional open‐labelled antihypertensive drugs were added sequentially. |
|
| Outcomes | Cardiovascular events Cardiovascular mortality All‐cause mortality |
|
| Notes | A formal stopping rule was constructed based on the primary renal function analysis with separate O'Brien‐Fleming boundaries for the chronic and total mean slopes for each of the 3 primary treatment group comparisons. The stopping rule stipulated that a treatment group should be discontinued at 1 of the study's annual interim analyses if the stopping boundaries indicating faster progression were crossed in the same direction for both the chronic and total mean slopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Described as "randomly allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 0% Participants withdrawing from the study were accounted for in an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes as stated in protocol |
| Other bias | Unclear risk | Amlodipine group terminated early at recommendation of Data and Safety Monitoring Board, according to predetermined stopping rules. Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
ASCOT 2005.
| Methods | Multicentre study Randomisation: computer‐generated, using separate lists for each co‐ordinating centre. Participating physicians called the co‐ordinating centre to obtain the treatment allocation for each participant. Open treatment and blinded endpoint evaluation (PROBE) design. Loss to follow‐up: 0.3% withdrew consent and 0.3% lost to follow‐up Median duration of follow‐up: 5.5 years Analyses: by intension‐to‐treat |
|
| Participants | Geographic location: UK, Ireland, Denmark, Finland, Iceland, Norway, and Sweden Study setting: hospital and primary care Number of participants: 19,257 (76.6% men) Age range: 40 to 79 years (mean: 63 years) Entry criteria: sitting SBP ≥ 160 with or without DBP ≨ 100 mmHg (for people with untreated hypertension) OR SBP ≥ 140 with or without DBP ≥ 90 mmHg (for people taking antihypertensive treatment), and 3 CHD risk factors. Race: 95% white Exclusion criteria: previous MI, current angina, cerebrovascular event in previous 3 months, fasting triglycerides > 4.5 mmol/L, heart failure, uncontrolled arrhythmias, or any clinically important haematological or biochemical abnormality on routine screening Comorbid conditions: current smoking (33%), LVH (22%), type 2 diabetes (27%); peripheral arterial disease (6%), previous stroke or TIA (11%), microalbuminuria, obesity, hyperlipidaemia |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 50 mg Step 2: atenolol 100 mg Step 3: atenolol 100 mg + bendroflumethiazide 1.25 mg + potassium Step 4: atenolol 100 mg + bendroflumethiazide 2.5 mg + potassium Step 5: atenolol 100 mg + bendroflumethiazide 2.5 mg + potassium + doxazosin gastrointestinal transport system 4 mg Step 6: atenolol 100 mg + bendroflumethiazide 2.5 mg + potassium + doxazosin gastrointestinal transport system 8 mg Further treatment to achieve BP goal added, as required Calcium‐channel blocker group: Step 1: amlodipine 5 mg Step 2: amlodipine 10 mg Step 3: amlodipine 10 mg + perindopril 4 mg Step 4: amlodipine 10 mg + perindopril 8 mg (2 × 4 mg) Step 5: amlodipine 10 mg + perindopril 8 mg (2 × 4 mg) + doxazosin gastrointestinal transport system 4 mg Step 6: amlodipine 10 mg + perindopril 8 mg (2 × 4 mg) + doxazosin gastrointestinal transport system 8 mg Further treatment to BP goal added, as required. On average, of total time, 79% were taking atenolol and 83% were taking amlodipine. At the end of the study, 9% were taking atenolol monotherapy and 15% taking amlodipine monotherapy. |
|
| Outcomes | Primary outcome: combined endpoint of non‐fatal MI (including silent MI) and fatal CHD Secondary outcomes: all‐cause mortality, total stroke, primary endpoint minus silent MI, all coronary events, total cardiovascular events and procedures, cardiovascular mortality, and non‐fatal and fatal heart failure Tertiary outcomes: silent MI, unstable angina, chronic stable angina, peripheral arterial disease, life‐threatening arrhythmias, development of diabetes, development of renal impairment, and the effects on the primary endpoint and on total cardiovascular events and procedures among prespecified subgroups |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 0.3% withdrew consent and 0.3% were lost to follow‐up. Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes as stated in protocol |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
Berglund 1981.
| Methods | Single‐centre study Randomisation: described as randomised controlled trial, but method of allocating participants to treatment was not described. Blinding: not known if participants, providers, or assessors blinded Loss to follow‐up: 7% Mean duration of follow‐up: 10 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic region: Sweden Study setting: hospital Number of participants: 106 (all men) Age range: 47 to 54 years (mean: 50.8 years) Race: not reported BP at entry: > 170/105 mmHg Comorbid conditions: not mentioned |
|
| Interventions |
Beta‐blocker group: Step 1: propranolol 80 mg twice daily Step 2: propranolol 160 mg twice daily Step 3: propranolol 160 mg twice daily + hydralazine 25 mg to 50 mg twice daily Step 4: propranolol 160 mg twice daily + hydralazine 25 mg to 50 mg twice daily + other antihypertensive drugs Diuretic group: Step 1: bendroflumethiazide 2.5 mg once daily Step 2: bendroflumethiazide 5 mg once daily Step 3: bendroflumethiazide 5 mg once daily + hydralazine 25 mg to 50 mg twice daily Step 4: bendroflumethiazide 5 mg once daily + hydralazine 25 mg to 50 mg twice daily + other antihypertensive drugs At the end of trial, 74% were taking propranolol and 70% were taking bendroflumethiazide; with 42% taking propranolol and 40% taking bendroflumethiazide monotherapy. |
|
| Outcomes | Total mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised controlled trial, but method of allocating participants to treatment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Completely unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding of outcome assessment, but the outcome assessed (i.e. death) is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 7%. Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Unclear risk | No access to the protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
Coope 1986.
| Methods | Multicentre study Randomisation: participants were randomised on a 50:50 basis without stratification using random number tables. Opaque envelopes were supplied in sequence from the trial administrative centre that gave instructions for allocation to treatment or control group. Loss to follow‐up: not stated Mean duration of follow‐up: 4.4 years |
|
| Participants | Geographic region: England and Wales Study setting: primary care Number of participants: 884 (31% men) Age range: 60 to 79 years (mean: 65 years) Race: not stated Exclusion criteria: atrial fibrillation, A‐V heart block, ventricular failure, bronchial asthma, diabetes mellitus (needing pharmacological treatment) or any serious concomitant disease, and untreated hypertension with levels persistently > 280 mmHg for SBP or 120 mmHg for DBP or people already being treated for hypertension (within 3 months) Mean BP at entry: 196.4/98.8 mmHg BP entry criteria: not stated Comorbid conditions: smoking 215 (24%) |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 100 mg/day Step 2: bendrofluazide 5 mg/day Step 3: methyldopa 500 mg/day Step 4: any other recognised therapy such as nifedipine retard 20 mg twice daily Control group: No treatment Proportion on assigned treatment at end of study: beta‐blocker group: 70% |
|
| Outcomes | Total mortality CHD mortality: fatal MI, sudden death CHD morbidity: non‐fatal MI Cerebrovascular mortality: fatal stroke Cerebrovascular morbidity: non‐fatal stroke Cardiovascular mortality: fatal stroke, MI, sudden death, ventricular failure, ruptured aneurysm, hypertensive nephropathy Cardiovascular morbidity: non‐fatal stroke, MI, non‐fatal ventricular failure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table |
| Allocation concealment (selection bias) | Low risk | Used opaque sequentially numbered envelopes supplied by the trial administrative centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not indicated whether reasons for missing outcome data were similar across treatment groups |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
ELSA 2002.
| Methods | Multicentre study Randomisation: computer‐generated, using separate lists for each centre with a block size of 4. Participants and study personnel, excluding the Safety Committee, were blinded to treatment assignment for study duration Loss to follow‐up: 3.9% Mean duration of follow‐up: 3.75 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic location: France, Germany, Greece, Italy, Spain, Sweden, UK Study setting: 410 clinical units Number of participants: 2334 (54.8% men) Age range: 45 to 75 years (mean: 56 years) Entry criteria: sitting SBP 150 mmHg to 210 mmHg and DBP 95 mmHg to 115 mmHg, fasting serum total cholesterol concentration ≤ 320 mg/dL, fasting serum triglyceride concentration ≤ 300 mg/dL, and serum creatinine concentration ≤ 1.7 mg/dL Race: 98.2% white Main exclusion criteria: recent MI or stroke and insulin‐dependent diabetes mellitus Mean BP at entry: 163.5/101.3 mmHg Comorbid conditions: current smoking (20.5%), ≥ 1 plaque (64%), previous antihypertensive therapy (63%), diabetes, hyperlipidaemia |
|
| Interventions |
Beta‐blocker group: Atenolol 50 mg once daily Calcium‐channel blocker group: Lacidipine 4 mg once daily If satisfactory BP control was not achieved, lacidipine could be increased to 6 mg and atenolol to 100 mg (month 1), with open‐label hydrochlorothiazide added (12.5 mg/day (month 3) and 25 mg/day (month 6)) |
|
| Outcomes | Change in mean maximum intima‐media thickness Plaque number Fatal and non‐fatal cardiovascular events Total mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 3.9%. Not indicated whether reasons for missing outcome data were similar across treatment groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
HAPPHY 1987.
| Methods | Multicentre study Randomisation: participants were divided into 3 groups according to predicted CHD risk based on a serum cholesterol, smoking habits, and SBP. Each risk group was divided into 3 age strata and participants in the 9 groups were allocated to treatment at random. Allocation method not described. Blinding: participants and providers not blinded, assessors blinded Loss to follow‐up: 1% Mean duration of follow‐up: 45.1 months Analyses: by intention‐to‐treat |
|
| Participants | Geographic region (% participant‐years): Belgium (0.8%), Canada (4.8%), Czechoslovakia (1.9%), Denmark (0.6%), Finland (14.0%), France (1.0%), Germany (3.3%), Greece (0.3%), Iceland (3.6%), Italy (2.7%), the Netherlands (1.6%), Norway (1.8%), Sweden (39.4%), UK (15.6%), USA (8.4%) Study setting: primary care Number of participants: 6569 (100% men) Age range: 40 to 64 years (mean: 52.2 years) Race: > 99% white Exclusion criteria: history of MI, angina pectoris, stroke, malignant or secondary hypertension, malignant disease, liver cirrhosis, alcoholism or other serious diseases; people with absolute or relative contraindications to beta‐blockers (chronic obstructive lung disease) or thiazide diuretics (diabetes mellitus or gout); and people with other non‐hypertensive conditions requiring treatment with beta‐blockers or diuretics. Mean BP at entry: 166/107 mmHg BP entry criteria: diastolic BP 100 mmHg to 130 mmHg Comorbid conditions: smoking 2266 (34.5%) |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 100 mg/day or metoprolol 200 mg/day; (until 1981) ‐ atenolol 200 mg/day or metoprolol 400 mg/day. Propranolol 160 mg/day given to 46 participants in 1 centre. Diuretic group: Step 1: bendroflumethiazide 5 mg/day or hydrochlorothiazide 50 mg/day; (until 1981) ‐ bendroflumethiazide 10 mg/day or hydrochlorothiazide 100 mg/day. Additional treatment for both groups: Step 2: hydralazine 75 mg/day Step 3: hydralazine 150 mg/day Step 4: step 3 + spironolactone 75 mg/day Step 5: step 3 + spironolactone 150 mg/day Step 6: step 5 + optional drug Percentage on assigned treatment at end of study: beta‐blocker group: 85.9% (68% as monotherapy); diuretic group: 83.4% (62% as monotherapy) |
|
| Outcomes | Total mortality ‐ death from any cause CHD mortality ‐ fatal MI, sudden death CHD morbidity ‐ non‐fatal MI Cerebrovascular mortality ‐ fatal stroke Cerebrovascular morbidity ‐ non‐fatal stroke Cardiovascular mortality ‐ fatal stroke, MI Cardiovascular morbidity ‐ non‐fatal stroke, non‐fatal MI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 1%. Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
INVEST 2003.
| Methods | Multicentre study Randomisation: Internet‐based management system automatically randomised each participant to a treatment strategy. Randomisation scheme used a standard C routine and blocked by site using randomly permuted block sizes of 4 and 6. Randomisation result was automatically stored in the central database as part of the participant's record and was also returned to the site investigator for electronic signature of strategy drugs in accordance with the protocol. Blinding: not clear whether participants were blinded; provider not blinded; assessor blinded Mean duration of follow‐up: 2.7 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic location: Australia, Canada, Cuba, Dominican Republic, El Salvador, Germany, Guatemala, Hungary, Italy, Mexico, New Zealand, Panama, Turkey, US Study setting: primary care Number of participants: 22,576 (47.9% men) Age: ≥ 50 years (mean 66.1 years) Entry criteria: sitting BP > 140/90 mmHg and documented coronary artery disease; mean entry BP 149.5/86.3 mmHg (SD 19.7/12.0). Race: 48.4% white, 13.4% black, 35.6% Hispanic, 0.7% Asian Exclusion criteria: people taking beta‐blockers within 2 weeks of randomisation or taking beta‐blockers for an MI that occurred in the previous 12 months Comorbid conditions: current smokers (12.4%), hypercholesterolaemia (55.8%), diabetes (28.3%), prior MI or abnormal angiogram (53.0%), previous stroke (5.1%), LVH (21.9%) |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 50 mg/day (added trandolapril 2 mg/day for participants with diabetes, renal impairment, or heart failure) Step 2: atenolol 50 mg/day + hydrochlorothiazide 25 mg/day Step 3: atenolol 50 mg twice day + hydrochlorothiazide 25 mg twice daily Step 4: atenolol 50 mg twice day + hydrochlorothiazide 25 mg twice daily + trandolapril 2 mg/day Step 5: maximum tolerated or add non‐study antihypertensive medication, or both. Titration ranges: atenolol 25 mg/day to 200 mg/day, hydrochlorothiazide 12.5 mg/day to 100 mg/day, trandolapril 1 mg/day to 8 mg/day, verapamil SR 120 mg/day to 480 mg/day Calcium‐channel blocker group: Step 1: verapamil SR 240 mg/day (added trandolapril 2 mg/day for participants with diabetes, renal impairment, or heart failure) Step 2: verapamil SR 240 mg/day + trandolapril 2 mg/day Step 3: verapamil SR 180 mg twice daily + trandolapril 2 mg twice daily Step 4: verapamil SR 180 mg twice daily + trandolapril 2 mg twice daily + hydrochlorothiazide 25 mg/day Step 5: maximum tolerated or add non‐study antihypertensive medication, or both. Titration ranges: atenolol 25 mg/day to 200 mg/day, hydrochlorothiazide 12.5 mg/day to 100 mg/day, trandolapril 1 mg/day to 8 mg/day, verapamil SR 120 mg/day to 480 mg/day Percentage on assigned treatment at end of study: beta‐blocker group: 77.5% (18.1% as monotherapy); calcium‐channel blocker group: 81.5% (17.4% as monotherapy) |
|
| Outcomes | Primary: first occurrence of death from any cause, non‐fatal MI, or non‐fatal stroke Secondary: all‐cause death, non‐fatal MI, non‐fatal stroke, cardiovascular death, angina, cardiovascular hospitalisations, BP control, cancer, Alzheimer's disease, Parkinson's disease, gastrointestinal bleeding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (assumed to be computer‐generated, because it is a blocked randomisation with varying block sizes) |
| Allocation concealment (selection bias) | Low risk | Central allocation (web‐based randomisation: an Internet‐based management system automatically randomised each participant to a treatment strategy). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether participants were blinded; provider not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
IPPPSH 1985.
| Methods | Multicentre study Randomisation: random allocation of participants was achieved by providing to the investigating centres participant numbers randomised into balanced blocks each having 6 numbers. Sealed envelopes containing the treatment code were provided to each investigator. Loss to follow‐up: 0.6% Duration of follow‐up: 3 to 5 years (mean 4 years) |
|
| Participants | Geographic region: UK (36.4%), Canada (12.0%), the Netherlands (3.6%), Israel (20.9%), Italy (11.7%), Federal Republic of Germany (15.4%) Number of participants: 6357 (50.2% men) Age range: 40 to 64 years (mean age: 52.2 years) Entry BP criteria: diastolic BP of 100 mmHg to 125 mmHg (Korotkoff Phase V) measured in seated position using standard mercury sphygmomanometer; mean SBP at entry 173 mmHg (SD 18.4) Race: Exclusion criteria: past or present history of angina pectoris or MI; heart failure; relevant cardiac valvular disease; atrio‐ventricular blocks grades II and III or sick sinus syndrome; bradycardia (< 50 beats per minute); intermittent claudication; previous cerebrovascular accident; insulin‐dependent diabetes; pregnancy; obstructive airways disease or history of bronchial asthma; renal, hepatic, gastrointestinal or any other severe disease Comorbid conditions: current smokers (29.1%) |
|
| Interventions |
Beta‐blocker group: Step 1: oxprenolol slow release 160 mg/day Control group: Step 1: film‐coated placebo of identical appearance Additional treatment for both groups: Step 2: diuretic or sympatholytic or vasodilator Step 3: diuretic + sympatholytic, or diuretic + vasodilator, or sympatholytic + vasodilator Step 4: diuretic + sympatholytic + vasodilator During study, 30% of participants remained on beta‐blocker only while 15% remained placebo only. Total diuretic use was 67% in the beta‐blocker group and 82% in the placebo group. |
|
| Outcomes | CHD mortality: fatal MI, sudden death CHD morbidity ‐ non‐fatal MI Cerebrovascular mortality ‐ fatal stroke Cerebrovascular morbidity ‐ non‐fatal stroke Cardiovascular mortality Cardiovascular morbidity Total mortality Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used so assumed to be computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Random allocation of participants was achieved by providing to the investigating centres participant numbers randomised into balanced blocks each having 6 numbers. Sealed envelopes containing the treatment code were provided to each investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
LIFE 2002.
| Methods | Multicentre study. 2‐week run‐in placebo period Randomisation: allocation numbers assigned with treatment groups using a computer‐generated allocation schedule; participants were classed as assigned to a group when they had received an allocation number. All participants received masked losartan and masked atenolol, 1 active and 1 placebo tablet. Blinding: participants, providers, and outcome assessors blinded Mean duration of follow‐up: 4.8 years (SD 0.9) Analyses: by intention‐to‐treat |
|
| Participants | Geographic region: Scandinavia, UK and USA Study setting: 945 clinical centres, mostly primary care except in Denmark where most participants were referred to hospital‐based centres. 9222 randomised but 29 participants at 1 centre excluded for irregularities. 9193 (46% men): Denmark (15%), Finland (16%), Iceland (1%), Norway (15%), Sweden (24%), UK (9%), USA (19%) Age range: 55 to 80 years BP entry criteria: DBP 95 mmHg to 115 mmHg or SBP 160 mmHg to 200 mmHg Race: 92% white, 6% black Exclusion criteria: secondary hypertension, MI or stroke within the previous 6 months; angina pectoris requiring treatment with beta‐blockers or calcium‐channel blockers; heart failure or left ventricular ejection fraction of ≤ 40%; a disorder requiring treatment with angiotensin‐II antagonist, beta‐blocker, hydrochlorothiazide, or ACE inhibitor Comorbid conditions: LVH (100%), smoking (16%), diabetes (13%), previous MI (16%), previous stroke (8%), atrial fibrillation (4%), peripheral vascular disease (6%) |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 50 mg/day and losartan placebo daily Angiotensin‐II antagonist group: Step 2: losartan 50 mg/day and atenolol placebo daily Additional treatment for both groups: Step 2: add hydrochlorothiazide 12.5 mg/day Step 3: double dose of Step 1 therapy, atenolol 100 mg/day or losartan 100 mg/day + hydrochlorothiazide 12.5 mg/day Step 4: add other antihypertensive drugs excluding ACE inhibitors, angiotensin‐II antagonists and beta‐blockers Participants on assigned treatment at end of follow‐up: losartan group: 84%, atenolol group: 80% |
|
| Outcomes | Primary: CVD mortality and mortality (composite endpoint of cardiovascular death, MI, and stroke) Secondary: total mortality, angina pectoris, or CHF requiring hospital admission |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs were added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
MRC 1985.
| Methods | Multicentre study Randomisation: stratified blocks of 8 within each sex, 10‐year age group and clinic Blinding: participants and outcome assessors blinded, providers not blinded Loss to follow‐up: 19% Mean duration of follow‐up: 4.9 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic region: England, Scotland, and Wales Study setting: primary care Number of participants: 17,354 (52% men) Age range: 35 to 64 years (mean: 52 years) Race: not stated Exclusion criteria: secondary hypertension; taking antihypertensive treatment; normally accepted indications for antihypertensive treatment (such as congestive cardiac failure) present; MI or stroke within the previous 3 months; presence of angina, intermittent claudication, diabetes, gout, bronchial asthma, serious intercurrent disease, or pregnancy Mean BP at entry: 162/98 mmHg BP entry criteria: SBP < 200 mmHg and DBP 90 to 109 mmHg Comorbid conditions: smoking 29% |
|
| Interventions |
Control: Matching placebo Beta‐blocker group: Propranolol up to 240 mg Supplementary drug: methyldopa (guanethidine used initially) Diuretic group: Bendrofluazide 10 mg/day Supplementary drug: methyldopa Percentage on assigned therapy at study end: beta‐blocker group: 59%, diuretic group: 61.8%, placebo group: 56.3% |
|
| Outcomes | Total mortality: death from any cause CHD mortality ‐ fatal MI, sudden death CHD morbidity ‐ non‐fatal MI Cerebrovascular mortality ‐ fatal stroke Cerebrovascular morbidity ‐ non‐fatal stroke Cardiovascular mortality ‐ fatal stroke, MI, sudden death Cardiovascular morbidity ‐ non‐fatal stroke, MI, ruptured aneurysms, and others |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Method not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded, but providers not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up (19%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
MRCOA 1992.
| Methods | Multicentre study Randomisation: stratified blocks of 8 within each sex and clinic Blinding: participants and outcome assessors blinded, providers not blinded Loss to follow‐up: 25% Mean duration of follow‐up: 5.8 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic region: England, Scotland, and Wales Study setting: primary care Number of participants: 4396 (42% men) Age range: 65 to 74 years (mean: 70.3 years) Race: not reported Exclusion criteria: known or suspected secondary hypertension; taking antihypertensive drugs; cardiac failure or any other accepted indication for antihypertensive treatment; receiving treatment for angina pectoris; history of MI or stroke within the preceding 3 months; impaired renal function; diabetic asthma; serious intercurrent disease, including malignancy known to be present at time of examination; serum potassium concentration ≤ 3.4 mmol/L or > 5.0 mmol/L Mean BP at entry: 184/91 mmHg BP entry criteria: SBP 160 mmHg to 209 mmHg and DBP < 115 mmHg Comorbid conditions: smoking: 17.5% |
|
| Interventions |
Control group: Matching placebo Beta‐blocker group: Step 1: atenolol 50 mg/day, may be increased to 100 mg/day Step 2: amiloride 2.5 mg/day + hydrochlorothiazide 25 mg/day or amiloride 5 mg/day + hydrochlorothiazide 50 mg/day Step 3: nifedipine up to 20 mg/day Step 4: other drugs Diuretic group: Step 1: amiloride 2.5 mg/day + hydrochlorothiazide 25 mg/day or amiloride 5 mg/day + hydrochlorothiazide 50 mg/day Step 2: atenolol 50 mg/day Step 3: nifedipine up to 20 mg/day Step 4: other drugs Percentage on assigned treatment at end of study: beta‐blocker group: 37%; diuretic group: 52%; placebo group: 47% |
|
| Outcomes | Total mortality: death from any cause CHD mortality ‐ fatal MI, sudden death CHD morbidity ‐ non‐fatal MI Cerebrovascular mortality ‐ fatal stroke Cerebrovascular morbidity ‐ non‐fatal stroke Cardiovascular mortality ‐ fatal stroke, MI, sudden death Cardiovascular morbidity ‐ non‐fatal stroke, MI, CHF, TIAs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Method not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded, but providers not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up (25%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
UKPDS‐39‐1998.
| Methods | Multicentre study Randomisation: included participants were part of the UKPDS involving allocation at random to 1 of 3 therapeutic groups: less tight control (avoid beta‐blockers and ACE inhibitors) 33%; tight control (ACE inhibitor) 33%; tight control (beta‐blocker) 33%. Allocation concealment was done with opaque, sealed envelopes with a check maintained on numerical sequence, dates of opening and results. Blinding: participants, providers, and assessors not blinded Loss to follow‐up: 4% Median duration of follow‐up: 8.4 years Analyses: by intention‐to‐treat |
|
| Participants | Geographic region: England, Scotland, and Northern Ireland Study setting: primary care Number of participants: 758 (54% men) Age range: 25 to 65 years (mean: 56.4 years) Race: white 651 (86%); black 62 (8%); Asian‐Indian 39 (5%); other 6 (1%) Exclusion criteria: ketonuria > 3 mmol/L; history of MI in the previous year; current angina or heart failure; > 1 vascular episode; serum creatinine concentration > 175 μmol/L; retinopathy requiring laser treatment; malignant hypertension; uncorrected endocrine abnormality; occupation which would preclude insulin treatment (such as heavy goods vehicle driver); a severe concurrent illness likely to limit life or require extensive treatment; or inadequate understanding or unwillingness to enter the study Mean BP at entry: 159/93 mmHg BP entry criteria: SBP ≥ 160 mmHg or DBP ≥ 90 mmHg, or both; or SBP ≥ 150 mmHg or DBP ≥ 85 mmHg in participants receiving antihypertensive medication Comorbid conditions: smoking: 171 (23%) |
|
| Interventions |
Beta‐blocker group: Step 1: atenolol 50 mg/day, increasing to 100 mg/day ACE inhibitor group: Step 1: captopril 25 mg twice daily, increasing to 50 mg twice daily Additional treatment for both groups: Step 2: frusemide 20 mg/day (maximum 40 mg twice daily) Step 3: nifedipine slow release 10 mg (maximum 40 mg) twice daily Step 4: methyldopa 250 mg (maximum 500 mg) twice daily; prazosin 1 mg (maximum 5 mg) 3 times daily Participants remaining on assigned therapy at study end: beta‐blocker group: 65%, ACE inhibitor group: 78% |
|
| Outcomes | Total mortality: death from any cause CHD mortality ‐ fatal MI, sudden death CHD morbidity ‐ non‐fatal MI Cerebrovascular mortality ‐ fatal stroke Cerebrovascular morbidity ‐ non‐fatal stroke Cardiovascular mortality ‐ fatal stroke, MI, sudden death Cardiovascular morbidity ‐ non‐fatal stroke, MI, heart failure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation concealment with opaque, sealed envelopes with a check maintained on numerical sequence, until dates of opening and results |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the outcome assessed (i.e. death) is unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not indicated whether reasons for missing outcome data were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
VA COOP 1982.
| Methods | Multicentre study
Randomisation: described as randomised controlled trial, but method of allocating participants to treatment not described Blinding: participants, providers, and assessors blinded Loss to follow‐up: 8% Mean duration of follow‐up: 12 months Participants withdrawn from the study for uncontrolled BP not included in the analysis |
|
| Participants | Geographic region: USA Study setting: hospital Number of participants: 683 (all men) Age range: 21 to 65 years (mean: 49.6 years) Race: 43% white and 57% black BP at entry: DBP 95 to 104 mmHg Comorbid conditions: not described |
|
| Interventions |
Beta‐blocker group: Propranolol 40 mg twice daily, increasing to 640 mg/day Diuretic group: Hydrochlorothiazide 25 mg twice daily, increasing to 200 mg/day Participants still on assigned baseline therapy at study end: beta‐blocker group, 39%, diuretic group: 52% |
|
| Outcomes | Total mortality Cerebrovascular disease CHD |
|
| Notes | Participants were withdrawn from the study if, on any follow‐up visit, DBP ≥ 120 mmHg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and providers blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 73 (10.7%) of the patients were dropped from the study after randomization. Of these, 42 (57.5%) were in the propranolol group and 31 were taking hydrochlorothiazide. The difference was not significant" Analyses by intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol. |
| Other bias | Unclear risk | Other antihypertensive drugs added to randomly allocated treatment to control BP. The observed effects may equally have resulted from the different additional drugs. |
ACE: angiotensin‐converting enzyme; BP: blood pressure; CHD: coronary heart disease; CHF: congestive heart failure; CVD: cardiovascular disease; DBP: diastolic blood pressure; LVH: left ventricular hypertrophy; MI: myocardial infarction; SBP: systolic blood pressure; SD: standard deviation; SR: sustained release; TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACCORD 2010 | Study designed to test the effect of BP lowering in addition to glycaemic control in people with diabetes. Participants were assigned to 2 BP treatment goals ‐ intensive (SBP < 120 mmHg) or standard (SBP < 140 mmHg). Various classes of antihypertensive drugs used but recommended start with combination of diuretic and ACE inhibitor or beta‐blocker. Beta‐blockers not first‐line or monotherapy. |
| ADaPT 2008 | Observational study conducted in primary care compared ACE inhibitor‐based treatment (ramipril) with a treatment based on diuretics or beta‐blockers. Not randomised. |
| APSIS 2006 | Study compared verapamil or metoprolol in people with stable angina pectoris. Not all participants had hypertension (27%). Mean baseline BP not given. |
| CAPP 1999 | This study compared the effects of ACE inhibitors and conventional therapy (diuretics and beta‐blockers) on cardiovascular morbidity and mortality in people with hypertension. Findings were not reported separately for beta‐blockers. |
| CAPRICORN 2001 | Trial evaluated the effects of carvedilol with placebo on survival in post‐MI participants with left ventricular dysfunction with or without symptomatic heart failure. All participants given ACE inhibitors for at least 48 hours before randomisation. Not all participants had hypertension (54%) and beta‐blockers not first‐line or monotherapy. |
| CARDHIAC 2008 | Study examined effects of doxazosin GITS and atenolol on 3 measures of target organ damage in people with type 2 diabetes and hypertension. Participants received ACE inhibitors or ARB and diuretic initially before receiving doxazosin GITS and atenolol. Beta‐blockers not first‐line or monotherapy. |
| CHHIPS 2009 | This RCT, which was conducted in 6 centres in the UK, evaluated the effects of active treatment with the ACE inhibitor, lisinopril, or beta‐blocker, labetalol, compared to placebo in people aged > 18 years with a clinical diagnosis of suspected stroke (with symptom onset < 36 hours) and hypertension (defined as SBP > 160 mmHg). After 2 weeks of treatment, study participants were routinely started on an ACE inhibitor with or without a diuretic irrespective of whether they had normal BP or hypertension, unless they were deemed to be unsuitable for such therapy. Decisions with regard to future antihypertensive therapy were delayed until the end of the trial intervention (2 weeks). The proportion of participants on assigned treatment at the end of the study was 71% in the beta‐blocker group, 68% in the ACE inhibitor group, and 80% in the placebo group. 172 participants, with mean age 74 years, were enrolled and the study reported mortality data at 3 months. We excluded this study because of the short duration (i.e. only 2 weeks) of relevant interventions. |
| CIBIS‐II 1999 | Trial compared bisoprolol and placebo in people with heart failure receiving standard therapy with an ACE inhibitor and diuretic. Not all participants had hypertension (mean baseline BP 139/80 mmHg) and beta‐blocker not first‐line or monotherapy. |
| COMET 2003 | Trial compared carvedilol and metoprolol in people with chronic heart failure. Not all had hypertension (36%). Mean baseline BP 126/77 mmHg. |
| CONVINCE 1998 | The Controlled ONset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE) Trial is a randomised, prospective, double‐blind, parallel‐group, 2‐arm, multicentre, international trial. The study recruited 15,000 people with hypertension, aged > 55 years, with an established second risk factor for cardiovascular disease and followed them for 5 years to compare the effects of controlled onset‐extended release verapamil 180 mg/day and hydrochlorothiazide 12.5 mg/day or atenolol 50 mg/day. Data has not been reported separately for hydrochlorothiazide and atenolol. |
| COPE 2005 | Study compared a combination of ARB, beta‐blocker, or thiazide diuretic in addition to a calcium‐channel blocker, benidipine hydrochloride, in Japanese people with hypertension. Beta‐blockers not first‐line treatment or monotherapy. |
| COPERNICUS 2004 | Study compared carvedilol vs placebo in people with chronic heart failure and receiving spironolactone or not at baseline. Not all participants had hypertension (mean baseline BP 123/76 mmHg). |
| COSMOS 2010 | People with stage 1 or 2 hypertension were randomised evenly to 1 of 15 groups for 6 weeks: extended‐release carvedilol (carvedilol CR) monotherapy 20 mg/day, 40 mg/day, or 80 mg/day; lisinopril monotherapy 10 mg/day, 20 mg/day, or 40 mg/day; or 1 of 9 combinations of carvedilol CR + lisinopril initiated simultaneously. The study has not reported effects on mortality or cardiovascular endpoints. |
| Dietz 2008 | This RCT was conducted in 85 centres in China, Germany, India, South Africa, Spain, and Turkey. People with hypertension (defined as mean sitting DBP 95 mmHg to 110 mmHg) were randomised to once‐daily aliskiren 150 mg (231 participants), atenolol 50 mg (231 participants), or the combination (150/50 mg; 232 participants) for 6 weeks, followed by a further 6 weeks on double the initial doses of aliskiren and atenolol. Aliskiren is the first direct renin inhibitor to be approved for the treatment of hypertension. The proportion of participants on assigned treatment at the end of the study was 92.2% in the beta‐blocker group, 91.3% in the direct renin inhibitor group, and 88.4% in the combination group. The trial followed up 694 participants (mean age 55.2 years, 23% aged ≥ 65 years) for 12 weeks. We excluded this study because of the short duration (i.e. only 12 weeks) of relevant interventions. |
| Dutch TIA 1993 | The trial evaluated the effects of a beta‐blocker (atenolol) in people after a transient ischaemic attack or non‐disabling ischaemic stroke in 56 collaborative centres in the Netherlands. Participants were randomised to atenolol or a matching placebo. The proportion of participants on assigned treatment in the beta‐blocker group was 71% at 2 years (and 64% at 3 years) and in the placebo group was 75% at 2 years (and 68% at 3 years). The trial followed up 1473 participants (52% aged > 65 years) for a mean duration of 2.7 years. We excluded the trial because only 29% of participants had hypertension at baseline. |
| GEMINI 2008 | Trial compared effects of carvedilol with metoprolol on glycaemic control in people with hypertension and type‐2 diabetes. BP was stabilised using ACE inhibitors or ARB antihypertensive regimens (or both) prior to randomisation. Beta‐blockers not first‐line or monotherapy. |
| IMPACT‐HF 2004 | Study assessed the use of carvedilol therapy initiated before discharge in people hospitalised with heart failure compared with 'usual care'. Not all participants had hypertension (64%). Baseline mean BP 124/69.5 mmHg) |
| MAPHY 1988 | This multicentre study was a subset of the HAPPHY trial. Analysis take into consideration only 1 of the 2 beta‐blockers (metoprolol). Including this trial alongside the HAPPHY trial would count those participants twice. |
| Marazzi 2011 | This trial compared the effects of long‐term treatment with nebivolol vs carvedilol on left ventricular ejection fraction in people with hypertensive chronic heart failure. We excluded this study because the majority of participants were already taking other antihypertensives at baseline, mainly ACE inhibitors. |
| MERIT‐HF 2002 | Trial evaluated metoprolol compared to placebo added to standard therapy in people with heart failure. Not all participants had hypertension (44%). Mean baseline BP not given. |
| Nilsson 2007 | This trial compared 2 first‐line antihypertensive therapies for initiating treatment in hypertension, i.e. the ACE inhibitor zofenopril and the beta‐blocker atenolol. The study has not reported effects on mortality or cardiovascular endpoints. |
| NORDIL 2000 | The Nordic Diltiazem (NORDIL) study enrolled 10,881 people with hypertension aged 50 to 74 years at health centres in Norway and Sweden and randomly assigned them to either diltiazem, or diuretics with/without beta‐blockers. Morbidity and mortality were not reported separately for participants assigned to beta‐blocker therapy. |
| REASON 2009 | Trial compared the effects of atenolol and perindopril/indapamide on BP and carotid‐femoral pulse wave velocity, which is a marker for aortic stiffness and arterial wall alterations. No morbidity or mortality data reported. |
| RESOLVD 2000 | Trial compared metoprolol or placebo in people with heart failure who had received treatment with either an ACE inhibitor (enalapril) or ARB (candesartan) or both for 5 months prior to trial commencement (+ a diuretic in 84% of participants). Beta‐blocker not first‐line or monotherapy. |
| SENIORS 2005 | Study compared the effects of nebivolol with placebo, in addition to standard therapy, in elderly people with chronic heart failure. Not all participants had hypertension (62%). Mean baseline BP 139/81 mmHg. |
| STOP 1991 | This study compared the effects of active hypertensive treatment (1 of 3 beta‐blockers or a diuretic) and placebo in elderly people with hypertension. Morbidity and mortality were not reported separately for participants assigned to beta‐blocker therapy. |
| STOP‐2 1999 | Conventional antihypertensive drugs (1 of 3 beta‐blockers or a diuretic) were compared with newer agents, ACE inhibitors and calcium‐channel blockers. Findings were not reported separately for participants taking beta‐blockers. |
| TEST 1995 | The trial was conducted in 21 centres in Sweden between July 1988 and June 1992. The study evaluated the effects of a beta‐blocker (atenolol) in people aged > 40 years enrolled within 3 weeks of a stroke or transient ischaemic attack. Participants were randomised to atenolol or a matching placebo. The proportion of participants on assigned treatment at the end of the study not stated. The trial followed up 720 participants (mean age 70.4 years) for a mean duration of 2.5 years. We excluded this study because not all participants had hypertension at baseline. |
ACE: angiotensin‐converting enzyme; ARB: angiotensin receptor blocker; BP: blood pressure; DBP: diastolic blood pressure; MI: myocardial infarction; RCT: randomised controlled trial; SBP: systolic blood pressure.
Differences between protocol and review
We have decided to have clearly defined strict eligibility criteria regarding duration of treatment, which we have now set at one year or more on trial medications. In the protocol and initial version of the review published in 2007, duration of treatment was not included as a criterion for eligibility. We have now used the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool was not yet developed when the protocol was written.
Contributions of authors
CSW and HB screened the search output, selected studies, assessed the risk of bias, and extracted data. At each stage, the two review authors resolved differences by discussion and consensus; with arbitration by JV.
CW conducted the analyses.
All review authors read and approved the final version before submission.
CSW and HB contributed equally to this review and share first authorship.
Sources of support
Internal sources
South African Medical Research Council (CSW), South Africa.
Stellenbosch University (CSW, JV), South Africa.
University of the Western Cape (HB), South Africa.
University of Cape Town (BMM, LHO), South Africa.
External sources
No sources of support supplied
Declarations of interest
We have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of this systematic review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AASK 2002 {published data only}
- Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001;285:2719‐28. [DOI] [PubMed] [Google Scholar]
- Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, et al. AASK Study Group. Comparison of measured GFR, serum creatinine, cystatin C, and beta‐trace protein to predict ESRD in African Americans with hypertensive CKD. American Journal of Kidney Disease 2011;58:886‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EM, Appel LJ, Wang X, Greene T, Astor BC, Rahman M, et al. African American Study of Kidney Disease and Hypertension Research Collaborative Group. Limitations of analyses based on achieved blood pressure: lessons from the African American study of kidney disease and hypertension trial. Hypertension 2011;57:1061‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. American Journal of Kidney Disease 2006;48:739‐51. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421‐31. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, et al. Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Controlled Clinical Trials 1996;17(4):3S‐16S. [DOI] [PubMed] [Google Scholar]
ASCOT 2005 {published and unpublished data}
- ASCOT Study Investigators. Anglo Scandinavian Cardiac Outcomes Trial. ASCOT‐BPLA preliminary results. www.ascotstudy.org/home.htm (accessed 30 March 2005).
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895‐906. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Nasothimiou EG, Chang CL, Sever PS, Dahlöf B, Poulter NR, ASCOT investigators. Baseline predictors of resistant hypertension in the Anglo‐Scandinavian Cardiac Outcome Trial (ASCOT): a risk score to identify those at high‐risk. Journal of Hypertension 2011;29:2004‐13. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Prieto‐Merino D, Dahlöf B, Sever PS, Poulter NR, ASCOT Investigators. Metabolic syndrome, impaired fasting glucose and obesity, as predictors of incident diabetes in 14 120 hypertensive patients of ASCOT‐BPLA: comparison of their relative predictability using a novel approach. Diabetic Medicine 2011;28:941‐7. [DOI] [PubMed] [Google Scholar]
- Server PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac outcomes Trial ‐ Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149‐58. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Rationale, design, methods and baseline demography of participants of the Anglo‐Scandinavian Cardiac Outcomes Trial. Journal of Hypertension 2001;19(6):1139‐47. [DOI] [PubMed] [Google Scholar]
Berglund 1981 {published data only}
- Berglund G, Andersson O. Beta‐blockers or diuretics in hypertension? A six year follow‐up of blood pressure and metabolic side effects. Lancet 1981;1:744‐7. [DOI] [PubMed] [Google Scholar]
- Berglund G, Andersson O, Widgren B. Low‐dose antihypertensive treatment with a thiazide diuretic is not diabetogenic. A 10‐year controlled trial with bendroflumethiazide. Acta Medica Scandinavica 1986;220:419‐24. [DOI] [PubMed] [Google Scholar]
Coope 1986 {published data only}
- Coope JR, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. British Medical Journal 1986;293:1145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
ELSA 2002 {published data only}
- Zanchett A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, et al. Calcium antagonist Lacidipine slows down progression of asymptomatic carotid atherosclerosis. Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double‐blind, long‐term trial. Circulation 2002;106:2422‐7. [DOI] [PubMed] [Google Scholar]
- Zanchetti A. Prevalence of carotid atherosclerosis in hypertension: preliminary baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). Blood Pressure Supplement 1996;4:30‐5. [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Henning M, Neiss A, Mancia G, Dal Palù C, et al. Risk factors associated with alterations in carotid intima‐media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. Journal of Hypertension 1998;16:949‐61. [DOI] [PubMed] [Google Scholar]
HAPPHY 1987 {published data only}
- Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, et al. Beta‐blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. Journal of Hypertension 1987;5(5):561‐72. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L, Berglund G, Elmfeldt D, Wedel H. Beta‐blockers versus saluretics in hypertension. Comparison of total mortality, myocardial infarction, and sudden death: study design and early results on blood pressure reduction. Preventive Medicine 1981;10(1):38‐49. [DOI] [PubMed] [Google Scholar]
INVEST 2003 {published data only}
- Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper‐DeHoff R, et al. INVEST Investigators. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR‐Trandolapril study. Hypertension 2004;44(5):637‐42. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Handberg EM, Cooper‐DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805‐16. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Handberg‐Thurmond E, Marks RG, Conlon M, Cooper‐DeHoff R, Volkers P, et al. Rationale and design of the International Verapamil SR/Trandolapril (INVEST). Journal of the American College of Cardiology 1998;32:1228‐37. [DOI] [PubMed] [Google Scholar]
IPPPSH 1985 {published data only}
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). Journal of Hypertension 1985;3(4):379‐92. [DOI] [PubMed] [Google Scholar]
- The IPPPSH Collaborative Group. The International Prospective Primary Prevention Study in Hypertension (IPPPSH): objectives and methods. European Journal of Clinical Pharmacology 1984;27:379‐91. [DOI] [PubMed] [Google Scholar]
LIFE 2002 {published data only}
- Dahlof B, Devereux R, Faire U, Fyhrquist F, Hedner T, Ibsen H, et al. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE Study Group. American Journal of Hypertension 1997;10(7 Pt 1):705‐13. [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Julius S, Kjeldsen SE, Beevers G, Faire U, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint Reduction in Hypertension. Hypertension 1998;32(6):989‐97. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Deveureux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995‐1003. [DOI] [PubMed] [Google Scholar]
- Lindhom LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004‐10. [DOI] [PubMed] [Google Scholar]
MRC 1985 {published data only}
- Dollery C, Brennan PJ. The Medical Research Council Hypertension Trial: the smoking patient. American Heart Journal 1988;115(No 1 Pt 2):276‐81. [DOI] [PubMed] [Google Scholar]
- Dollery CT. An update on the Medical Research Council Trial. Journal of Hypertension 1987;5(Suppl 3):S75‐8. [PubMed] [Google Scholar]
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. British Medical Journal 1985;291:97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Coronary heart disease in the Medical Research Council trial of treatment of mild hypertension. British Heart Journal 1988;59(3):364‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild to Moderate Hypertension. Adverse reactions to bendrofluazide and propranolol for the treatment of mild hypertension. Lancet 1981;ii:539‐43. [PubMed] [Google Scholar]
- The Treatment of Mild Hypertension Study Group. A randomized, placebo‐ controlled trial of a nutritional‐hygienic regimen along with various drug monotherapies.. Archives of Internal Medicine 1991;151(7):1413‐23. [DOI] [PubMed] [Google Scholar]
MRCOA 1992 {published data only}
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
UKPDS‐39‐1998 {published data only}
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. British Medical Journal 1998;317:713‐20. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPRDS 38. British Medical Journal 1998;317:703‐13. [PMC free article] [PubMed] [Google Scholar]
VA COOP 1982 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. I. Results of short‐term titration with emphasis on racial difference in response. JAMA 1982;248:1996‐2003. [PubMed] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long‐term therapy. JAMA 1982;248(16):2004‐11. [PubMed] [Google Scholar]
References to studies excluded from this review
ACCORD 2010 {published data only}
- The ACCORD Study Group. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. New England Journal of Medicine 2010;362:1575‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADaPT 2008 {published data only}
- Zidek W, Schrader J, Lüders S, Matthaei S, Hasslacher C, Hoyer J, et al. First‐line antihypertensive treatment in patients with pre‐diabetes: rationale, design and baseline results of the ADaPT investigation. Cardiovascular Diabetology 2008;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
APSIS 2006 {published data only}
- Hjemdahl P, Eriksson SV, Held C, Forslund L, Nasman P, Rehnqvist N. Favourable long term prognosis in stable angina pectoris: an extended follow up of the angina prognosis study in Stockholm (APSIS). Heart 2006;92:177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
CAPP 1999 {published data only}
- Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. the Captopril prevention Project (CAPP) study group. Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPP) randomised trial. Lancet 1999;353:611‐6. [DOI] [PubMed] [Google Scholar]
- The CAPP group. The Captopril Prevention Project: a prospective intervention trial of angiotensin converting enzyme inhibition in the treatment of hypertension. Journal of Hypertension 1990;8(11):985‐90. [DOI] [PubMed] [Google Scholar]
CAPRICORN 2001 {published data only}
- CAPRICORN Investigators. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385‐90. [DOI] [PubMed] [Google Scholar]
CARDHIAC 2008 {published data only}
- Barrios V, Escobar C, Tomás JP, Calderon A, Echarri R. Comparison of the effects of doxazosin and atenololon target organ damage in adults with type 2 diabetes mellitus and hypertension in the CARDHIAC study: a 9‐month, prospective, randomized, open‐label, blinded‐evaluation trial. Clinical Therapeutics 2008;30:98‐107. [DOI] [PubMed] [Google Scholar]
CHHIPS 2009 {published data only}
- Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling Hypertension and Hypotension Immediately Post‐Stroke (CHHIPS): a randomised, placebo‐controlled, double‐blind pilot trial. Lancet Neurology 2009;8(1):48‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CIBIS‐II 1999 {published data only}
- CIBIS‐II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9‐13. [PubMed] [Google Scholar]
COMET 2003 {published data only}
- Poole‐Wilson PA, Swedberg K, Cleland JG, Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7‐13. [DOI] [PubMed] [Google Scholar]
CONVINCE 1998 {published data only}
- Black HR, Elliot WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003;289:2073‐82. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, et al. Rationale and design for the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial. Controlled Clinical Trials 1998;19(4):370‐90. [DOI] [PubMed] [Google Scholar]
COPE 2005 {published data only}
- Ogihara T, Matsuzaki M, Matsuoka H, Shimamoto K, Shimada K, Rakugi H, et al. COPE Trial Group. The combination therapy of hypertension to prevent cardiovascular events (COPE) trial: rationale and design. Hypertension Research 2005;28:331‐8. [DOI] [PubMed] [Google Scholar]
COPERNICUS 2004 {published data only}
- Rouleau JL, Roecker EB, Tendera M, Mohacsi P, Krum H, Katus HA, et al. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Journal of the American College of Cardiology 2004;43:1423‐9. [DOI] [PubMed] [Google Scholar]
COSMOS 2010 {published data only}
- Bakris GL, Iyengar M, Lukas MA, Ordronneau P, Weber MA. Effect of combining extended‐release carvedilol and lisinopril in hypertension: results of the COSMOS study. Journal of Clinical Hypertension (Greenwich) 2010;12:678‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietz 2008 {published data only}
- Dietz R, Dechend R, Yu CM, Bheda M, Ford J, Prescott MF, et al. Effects of the direct renin inhibitor aliskiren and atenolol alone or in combination in patients with hypertension. Journal of the Renin‐Angiotensin‐Aldosterone System 2008;9:163‐75. [DOI] [PubMed] [Google Scholar]
Dutch TIA 1993 {published data only}
- The Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993;24:543‐8. [DOI] [PubMed] [Google Scholar]
GEMINI 2008 {published data only}
- Phillips RA, Fonseca V, Katholi RE, McGill JB, Messerli FH, Bell DS, et al. GEMINI Investigators. Demographic analyses of the effects of carvedilol vs metoprolol on glycemic control and insulin sensitivity in patients with type 2 diabetes and hypertension in the Glycemic Effects in Diabetes Mellitus: Carvedilol‐Metoprolol Comparison in Hypertensives (GEMINI) study. Journal of Cardiometabolic Syndrome 2008;3:211‐7. [DOI] [PubMed] [Google Scholar]
IMPACT‐HF 2004 {published data only}
- Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial. Journal of the American College of Cardiology 2004;43:1534‐41. [DOI] [PubMed] [Google Scholar]
MAPHY 1988 {published data only}
- Olsson G, Tuomilehto J, Berglund G, Elmfeldt D, Warnold I, Barber H, et al. Primary prevention of sudden cardiovascular death in hypertensive patients. Mortality results from the MAPHY Study. American Journal of Hypertension 1991;4(2 Pt 1):151‐8. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Wikstrand J, Olsson G, Elmfeldt D, Warnold I, Barber H, et al. Decreased coronary heart disease in hypertensive smokers. Mortality results from the MAPHY study. Hypertension 1989;13(6 Pt 2):773‐80. [DOI] [PubMed] [Google Scholar]
- Wikstrand J, Warnold I, Olsson G, Tuomilehto J, Elmfeldt D, Berglund G. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA 1988;259(13):1976‐82. [PubMed] [Google Scholar]
- Wikstrand J, Warnold I, Tuomilehto J, Olsson G, Barber HJ, Eliasson K, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHY Study. Hypertension 1991;17(4):579‐88. [DOI] [PubMed] [Google Scholar]
Marazzi 2011 {published data only}
- Marazzi G, Volterrani M, Caminiti G, Iaia L, Massaro R, Vitale C, et al. Comparative long term effects of nebivolol and carvedilol in hypertensive heart failure patients. Journal of Cardiac Failure 2011;17:703‐9. [DOI] [PubMed] [Google Scholar]
MERIT‐HF 2002 {published data only}
- Gottlieb SS, Fisher ML, Kjekshus J, Deedwania P, Gullestad L, Vitovec J, et al. Tolerability of beta blocker initiation and titration in the MetoprololCR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT‐HF). Circulation 2002;105:1182‐8. [DOI] [PubMed] [Google Scholar]
Nilsson 2007 {published data only}
- Nilsson P. Antihypertensive efficacy of zofenopril compared with atenolol in patients with mild to moderate hypertension. Blood Pressure Supplement 2007;2:25‐30. [DOI] [PubMed] [Google Scholar]
NORDIL 2000 {published data only}
- Hansson L, Hedner T, Lund‐Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of the effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359‐65. [DOI] [PubMed] [Google Scholar]
- The Nordic Diltiazem Study (NORDIL). A prospective intervention trial of calcium antagonist therapy in hypertension. Blood Pressure Supplement 1993;2(4):312‐21. [DOI] [PubMed] [Google Scholar]
REASON 2009 {published data only}
- Protogerou A, Blacher J, Stergiou GS, Achimastos A, Safar ME. Blood pressure response under chronic antihypertensive drug therapy: the role of aortic stiffness in the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double‐Blind) study. Journal of the American College of Cardiology 2009;53:445‐51. [DOI] [PubMed] [Google Scholar]
RESOLVD 2000 {published data only}
- RESOLVD Investigators. Effects of metoprolol CR inpatients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation 2000;101:378‐84. [DOI] [PubMed] [Google Scholar]
SENIORS 2005 {published data only}
- Flather MD, Shibata MC, Coats AJ, Veldhuisen DJ, Parkhomenko A, Borbola J, et al. SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European Heart Journal 2005;26:215‐25. [DOI] [PubMed] [Google Scholar]
STOP 1991 {published data only}
- Dahlof B, Hansson L, Lindholm L, Rastam L, Schersten B, Wester P‐O. STOP‐Hypertension: Swedish Trial in Old Patients with Hypertension. Journal of Hypertension 1986;4:511‐3. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Hansson L, Lindholm L, Schersten B, Wester P‐O. STOP‐Hypertension ‐ preliminary communication from the pilot study of the Swedish Trial in Old Patients with Hypertension. Journal of Hypertension 1987;5(Suppl 5):S607‐10. [PubMed] [Google Scholar]
- Dahlof B, Lindholm L, Hansson L, Schersten B, Ekbom T, Wester P‐O. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP‐Hypertension). Lancet 1991;338:1281‐5. [DOI] [PubMed] [Google Scholar]
- Ekbom T, Dahlof B, Hansson L, Lindholm L, Schersten B, Wester P‐O. Antihypertensive efficacy and side effects of three beta‐blockers and a diuretic in elderly hypertensives: a report from the STOP‐Hypertension study. Journal of Hypertension 1993;11(Suppl 2):S19‐24. [DOI] [PubMed] [Google Scholar]
- Ekbom T, Dahlof B, Hansson L, Lindholm L, Schersten B, Wester P‐O. Antihypertensive efficacy and side‐effects of three beta‐blockers and a diuretic in elderly hypertensives: a report from the STOP‐Hypertension study. Journal of Hypertension 1992;10:1525‐30. [DOI] [PubMed] [Google Scholar]
STOP‐2 1999 {published data only}
- Hansson L, Lindholm L, Ekbom T, Dahlof B, Lanke J, et al. the STOP‐Hypertension‐2 Study Group. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999;354:1751‐6. [DOI] [PubMed] [Google Scholar]
TEST 1995 {published data only}
- Eriksson S, Olofsson BO, Wester PO. Atenolol in the secondary prevention after stroke. Cerebrovascular Diseases 1995;5:21‐5. [Google Scholar]
Additional references
Angeli 2004
- Angeli F, Verdecchia P, Reboldi GP, Gattobigio R, Bentivoglio M, Staessen JA, et al. Calcium channel blockade to prevent stroke in hypertension: a meta‐analysis of 13 studies with 103,793 subjects. American Journal of Hypertension 2004;17:817‐22. [DOI] [PubMed] [Google Scholar]
Balamuthusamy 2009
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta‐blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta‐analysis. American Journal of Therapeutics 2009;16:133‐42. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem B, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Broze J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Bangalore 2007
- Bangalore S, Parkar S, Grossman E, Messerli FH. A meta‐analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new‐onset diabetes mellitus. American Journal of Cardiology 2007;100:1254‐62. [DOI] [PubMed] [Google Scholar]
Bangalore 2008
- Bangalore S, Wild D, Parkar S, Kukin M, Messerli FH. Beta‐blockers for primary prevention of heart failure in patients with hypertension insights from a meta‐analysis. Journal of the American College of Cardiology 2008;52:1062‐72. [DOI] [PubMed] [Google Scholar]
Bath 2014
- Bath PMW, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD000039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Breslow 1980
- Breslow NE, Day NE. Combination of results from a series of 2 X 2 tables; control of confounding. Statistical Methods in Cancer Research, Vol 1: the Analysis of Case‐control Data. Lyon: International Agency for Health Research on Cancer, 1980. [Google Scholar]
Broeders 2000
- Broeders MA, Doevendans PA, Bekkers BC, Bronsaer R, Gorsel E, Heemskerk JW, et al. Nebivolol: a third‐generation beta‐blocker that augments vascular nitric oxide release: endothelial beta(2)‐adrenergic receptor‐mediated nitric oxide production. Circulation 2000;102:677‐84. [DOI] [PubMed] [Google Scholar]
Carlberg 2004
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice?. Lancet 2004;364:1684‐9. [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003654.pub4] [DOI] [PubMed] [Google Scholar]
Collins 1990
- Collins R, Peto R, MacMahon SW, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2. Lancet 1990;325:827‐38. [DOI] [PubMed] [Google Scholar]
Dahlöf 2007
- Dahlöf B, Devereux RB, Kjeldsen SE, Lyle PA, Zhang Z, Edelman JM. Atenolol as a comparator in outcome trials in hypertension: a correct choice in the past, but not for the future?. Blood Pressure Supplement 2007;16:6‐12. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2013
- ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. Journal of Hypertension 2013;31:1925‐38. [DOI] [PubMed] [Google Scholar]
Fergus 2015
- Fergus IV, Connell KL, Ferdinand KC. A comparison of vasodilating and non‐vasodilating beta‐blockers and their effects on cardiometabolic risk. Current Cardiology Reports 2015;17:38. [DOI] [PubMed] [Google Scholar]
GBD 2015
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(10010):2287‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gradman 2010
- Gradman AH, Weir MR, Wright M, Bush CA, Keefe DL. Efficacy, safety and tolerability of aliskiren, a direct renin inhibitor, in women with hypertension: a pooled analysis of eight studies. Journal of Human Hypertension 2010;24:721‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howlett 2014
- Howlett JG. Nebivolol: vasodilator properties and evidence for relevance in treatment of cardiovascular disease. Canadian Journal of Cardiology 2014;30:S29‐37. [DOI] [PubMed] [Google Scholar]
James 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507‐20. [DOI] [PubMed] [Google Scholar]
Jennings 2013
- Jennings GL. Recent clinical trials of hypertension management. Hypertension 2013;62:3‐7. [DOI] [PubMed] [Google Scholar]
JNC‐6 1997
- JNC‐6. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Archives of Internal Medicine 1997;157(24):2413‐46. [DOI] [PubMed] [Google Scholar]
Kalinowski 2003
- Kalinowski L, Dobrucki LW, Szczepanska‐Konkel M, Jankowski M, Martyniec L, Angielski S, et al. Third‐generation beta‐blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003;107:2747‐52. [DOI] [PubMed] [Google Scholar]
Kamp 2010
- Kamp O, Metra M, Bugatti S, Bettari L, Dei Cas A, Petrini N, et al. Nebivolol: haemodynamic effects and clinical significance of combined beta‐blockade and nitric oxide release. Drugs 2010;70:41‐56. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan N, McAlister FA. Re‐examining the efficacy of [beta]‐blockers for the treatment of hypertension: a meta‐analysis. CMAJ 2006;174:1737‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuyper 2014
- Kuyper LM, Khan NA. Atenolol vs non‐atenolol beta‐blockers for the treatment of hypertension: a meta‐analysis. Canadian Journal of Cardiology 2014;30:S47‐53. [DOI] [PubMed] [Google Scholar]
Larochelle 2014
- Larochelle P, Tobe SW, Lacourciere Y. Beta‐blockers in hypertension: studies and meta‐analyses over the years. Canadian Journal of Cardiology 2014;30:S16‐22. [DOI] [PubMed] [Google Scholar]
Lindhom 2005
- Lindholm LH, Carlberg B, Samuelsson O. Should [beta] blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet 2005;366:1545‐53. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
Materson 1993
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single‐drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. New England Journal of Medicine 1993;328(13):914‐21. [DOI] [PubMed] [Google Scholar]
Messerli 1998
- Messerli FH, Grossman E, Goldbourt U. Are beta‐blockers efficacious as first‐line therapy for hypertension in the elderly? A systematic review. Journal of the American Medical Association 1998;279(23):1903‐7. [DOI] [PubMed] [Google Scholar]
Messerli 2003
- Messerli FH, Beevers DG, Franklin SS, Pickering TG. [Beta]‐blockers in hypertension ‐ the emperor has no clothes: an open letter to present and prospective drafters of new guidelines for the treatment of hypertension. American Journal of Hypertension 2003;16:870‐3. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2004
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis andmanagement. Clinical guideline. Published: 24 August 2011 nice.org.uk/guidance/cg127.. Available from https://www.nice.org.uk/guidance/cg127/resources/hypertension‐in‐adults‐diagnosis‐and‐management‐35109454941637.
NICE 2006
- National Collaborating Centre for Chronic Conditions. Hypertension: Management in Adults in Primary Care: Pharmacological Update. London: Royal College of Physicians, 2006. [PubMed] [Google Scholar]
Opie 1997
- Opie LH. Evidence is needed that beta blockade alone reduces mortality in hypertension. BMJ 1997;315:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Opie 2002
- Opie LH, Schall R. Evidence‐based evaluation of calcium channel blockers for hypertension: equality of mortality and cardiovascular risk relative to conventional therapy. Journal of the American College of Cardiology 2002;39:315‐22. [DOI] [PubMed] [Google Scholar]
Opie 2004
- Opie LH, Schall R. Old antihypertensives and new diabetes. Journal of Hypertension 2004;22:1453‐58. [DOI] [PubMed] [Google Scholar]
Opie 2014
- Opie LH, Wiysonge CS. β‐Blocker therapy for patients with hypertension. JAMA 2014;311:862‐3. [DOI] [PubMed] [Google Scholar]
Park 2007
- Park IU, Taylor AL. Race and ethnicity in trials of antihypertensive therapy to prevent cardiovascular outcomes: a systematic review. Annals of Family Medicine 2007;5:444‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 2007
- Pedersen ME, Cockcroft JR. The vasodilatory beta‐blockers. Current Hypertension Reports 2007;9:269‐77. [DOI] [PubMed] [Google Scholar]
Philipp 1997
- Philipp T, Anlauf M, Distler A, Holzgreve M, Michaelis J, Welleck S. Randomised, double blind, multicentre comparison of hydrochlorothiazide, atenolol, nifedipine and enalapril in antihypertensive treatment: results of the HANE study. BMJ 1997;315:154‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poirier 2014
- Poirier L, Tobe SW. Contemporary use of beta‐blockers: clinical relevance of sub‐classification. Canadian Journal of Cardiology 2014;30:S9‐15. [DOI] [PubMed] [Google Scholar]
Polónia 2010
- Polónia J, Barbosa L, Silva JA, Bertoquini S. Different patterns of peripheral versus central blood pressure in hypertensive patients treated with β‐blockers either with or without vasodilator properties or with angiotensin receptor blockers. Blood Pressure Monitoring 2010;15:235‐9. [DOI] [PubMed] [Google Scholar]
Psaty 1997
- Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, et al. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA 1997;277:739‐45. [PubMed] [Google Scholar]
Pucci 2016
- Pucci G, Ranalli MG, Battista F, Schillaci G. Effects of β‐blockers with and without vasodilating properties on central blood pressure: systematic review and meta‐analysis of randomized trials in hypertension. Hypertension 2016;67(2):316‐24. [DOI] [PubMed] [Google Scholar]
Ramsay 1999
- Ramsay LE, Williams B, Johnson GD, MacGregor GA, Poston L, Potter JF, et al. British Hypertension Society guidelines for hypertension management 1999; summary. BMJ 1999;319:630‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ripley 2014
- Ripley TL, Saseen JJ. Beta‐blockers: a review of their pharmacological and physiological diversity in hypertension. Annals of Pharmacotherapy 2014;48:723‐33. [DOI] [PubMed] [Google Scholar]
Sander 2011
- Sander GE, Giles TD. Thiazide diuretics and β‐blockers in the treatment of hypertension in diabetes mellitus. Journal of Clinical Hypertension (Greenwich) 2011;13:296‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sciarretta 2011
- Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta‐analysis of studies in patients with hypertension and high cardiovascular risk. Archives of Internal Medicine 2011;171:384‐94. [DOI] [PubMed] [Google Scholar]
Smithwick 1949
- Smithwick RH. An evaluation of the surgical treatment of hypertension. Bulletin of the New York Academy of Medicine 1949;25:698‐716. [PMC free article] [PubMed] [Google Scholar]
Staessen 2003
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. Journal of Hypertension 2003;21:1055‐76. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2015
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs overview and meta‐analyses. Journal of Hypertension 2015;33:195‐211. [DOI] [PubMed] [Google Scholar]
Verdecchia 2004
- Verdecchia P, Reboldi G, Angeli F, Borgioni C, Gattobigio R, Filippucci L, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension 2004;43:963‐9. [DOI] [PubMed] [Google Scholar]
Verdecchia 2005
- Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, et al. Angiotensin‐converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension 2005;46:386‐92. [DOI] [PubMed] [Google Scholar]
Weber 2005
- Weber MA. The role of the new beta‐blockers in treating cardiovascular disease. American Journal of Hypertension 2005;18:169‐76. [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006;113:1213‐25. [DOI] [PubMed] [Google Scholar]
Wiysonge 2007a
- Wiysonge CS, Volmink J, Opie LH. Beta‐blockers and the treatment of hypertension: it is time to move on. Cardiovascular Journal of Africa 2007;18:351‐2. [PMC free article] [PubMed] [Google Scholar]
Wong 2014a
- Wong GWK, Boyda HN, Wright JM. Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD007450.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2014b
- Wong GWK, Wright JM. Blood pressure lowering efficacy of nonselective beta‐blockers for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007452.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1999
- Wright JM, Cheng‐Han L, Chalmers GK. Systematic review of anti‐hypertensive therapies: does the evidence assist in choosing a first‐line drug?. CMAJ 1999;161:25‐32. [PMC free article] [PubMed] [Google Scholar]
Wright 2000
- Wright JM. Choosing a first‐line drug in the management of elevated blood pressure: what is the evidence? 2: Beta‐blockers. CMAJ 2000;163:188‐92. [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]
Zanchetti 2005
- Zanchetti A. Evidence‐based medicine in hypertension: what type of evidence?. Journal of Hypertension 2005;23:1113‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bradley 2006
- Bradley HA, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence for use of beta‐blockers as first‐line therapy for hypertension? Systematic review and meta‐analysis. Journal of Hypertension 2006;24:2131‐41. [DOI] [PubMed] [Google Scholar]
Wiysonge 2007b
- Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, et al. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PubMed] [Google Scholar]
Wiysonge 2012
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002003.pub4] [DOI] [PubMed] [Google Scholar]
Wiysonge 2013
- Wiysonge CS, Opie LH. β‐Blockers as initial therapy for hypertension. JAMA 2013;310:1851‐2. [DOI] [PubMed] [Google Scholar]



