Abstract
Background:
To evaluate the clinical efficacy and toxicity of single pemetrexed treatment compared with platinum-based pemetrexed doublet pemetrexed-based as first-line treatment for advanced nonsquamous nonsmall cell lung cancer (NS-NSCLC) in elderly Chinese patients.
Methods:
The study retrospectively reviewed 175 elderly Chinese patients with NS-NSCLC from June 2010 to September 2013: 90 patients received single pemetrexed treatment, 45 received pemetrexed plus oxaliplatin, and 40 received pemetrexed plus carboplatin. Clinical efficacy was assessed using disease control rate (DCR), overall survival (OS), and progression-free survival (PFS).
Results:
DCR, OS, and PFS did not significantly differ between single pemetrexed treatment (OS: 14.9 months; DCR: 62.2%; PFS: 3.3 months), pemetrexed plus oxaliplatin (OS: 16.5 months; DCR: 71.1%; PFS: 4.5 months), and pemetrexed plus carboplatin (OS: 15.5 months; DCR: 70.0%; PFS: 4.6 months) groups. Pemetrexed treatment caused significantly lower incidences of adverse events, such as hepatotoxicity and peripheral nerve injury. Performance status (PS), TNM stage, and Thymidylate synthase (TS) expression were predictive factors of DCR. Pemetrexed chemotherapy cycles, PS, and TNM stage were independent prognostic factors.
Conclusions:
Single pemetrexed was noninferior to platinum-based pemetrexed doublet for clinical efficacy and safety in elderly Chinese patients with advanced NS-NSCLC. Chemotherapy cycles, performance status, and TNM stage were independent prognostic factors.
Keywords: first-line treatment, nonsquamous nonsmall cell lung cancer, pemetrexed, platinum
1. Introduction
Lung cancer has become the most common cancer in China, and its incidence has doubled in the past decade.[1,2] In the United States, about 85% of lung cancers are nonsmall cell lung cancer (NSCLC), which is classified into three main subtypes: squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Standard treatment options for NSCLC include pemetrexed plus platinum compounds, cisplatin, and docetaxel.[3,4]
Pemetrexed is an antifolate drug and targets several folate-dependent enzymes, such as thymidylate synthase (TS). Pemetrexed-based chemotherapy has been used as first-line treatment and yields promising outcome.[5,6] For instance, superior efficacy of pemetrexed in nonsquamous (NS)-NSCLC patients compared with other standard treatment options has been proved using histology analyses.[7] Moreover, the effectiveness and well-tolerated performance of pemetrexed as maintenance treatment has also been reported for NS-NSCLC patients.[8] A retrospective study has reported that platinum-based pemetrexed doublet leads to a significant improvement of disease control in comparison with platinum-based gemcitabine doublet in first-line setting for advanced NS-NSCLC patients.[9] However, it is not clear whether single pemetrexed treatment is comparable to platinum-based pemetrexed doublet in first-line setting for advanced NS-NSCLC patients.
Increasing evidence has demonstrated that poor response to pemetrexed is associated with a high expression of TS in malignant tumors, and TS expression is a promising predictive marker in response to pemetrexed-based chemotherapy in NSCLC patients.[10,11] However, the association of TS expression with the prognosis of NS-NSCLC patients has not been elucidated.
The retrospective study was conducted to evaluate the clinical efficacy and toxicity of single pemetrexed treatment as first-line treatment in comparison with that of platinum-based pemetrexed doublet. Moreover, the factors, which were significantly associated with disease control rate (DCR) and overall survival (OS), were also explored.
2. Materials and methods
2.1. Patients
The retrospective study was conducted on Chinese patients with advanced NS-NSCLC from oncology departments of 3 hospitals, including institute of geriatrics of Jiangsu province, the first affiliated hospital of Nanjing medical university, and the second affiliated hospital of Nanjing medical university, between June 2010 and September 2013. Elderly Chinese patients with advanced NS-NSCLC, who were treated by pemetrexed or pemetrexed plus platinum (carboplatin or oxaliplatin) as first-line setting, were studied.
The inclusion criteria for eligible patients were: histologically or cytologically confirmed NS-NSCLC according to the World Health Organization criteria[12]; stage III or IV[13]; wild-type epidermal growth factor receptor (EGFR); age ≥70 year; at least 1 measurable lesion; Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 to 2[14]; adequate organ function according to the results of routine blood test,[15] and normal electrocardiogram; expected survival time ≥3 months; pemetrexed chemotherapy cycles ≥2; adequate cancer tissue could be obtained for assessing the expression level of TS. Exclusion criteria: brain metastases; spinal metastasis and pancoast which needed radiotherapy first. At last, 175 eligible patients were enrolled onto the study. The protocol of the study was approved by the Ethic committee of the first affiliated hospital of Nanjing Medical University and the second affiliated hospital of Nanjing Medical University, and each enrolled patient provided written informed consent.
2.2. Therapy protocol
Among these patients, 90 patients received single pemetrexed (pemetrexed group), 45 patients received pemetrexed plus oxaliplatin (pemetrexed plus oxaliplatin group), and 40 patients received pemetrexed plus carboplatin (pemetrexed plus carboplatin group). Pemetrexed treatment: patients received intravenous (I.V.) pemetrexed 500 mg/m2 for 15 minutes every 21 days. Folic acid 500 μg/d was taken orally from 1 week before the pemetrexed treatment to 5 days after the treatment. Oral intake of dexamethasone (4.5 mg, 2 times per day) started from 1 day before the pemetrexed treatment, and maintained for 3 days. Meanwhile, intramuscular administration of vitamin B12 1000 μg was received every 9 weeks until 3 weeks after the pemetrexed treatment. Pemetrexed plus carboplatin treatment: pemetrexed was administrated as stated above. Patients received IV carboplatin (AUC = 5) on day 1 of a 21-day cycle. Pemetrexed plus oxaliplatin treatment: pemetrexed was administrated as stated above. Patients received IV oxaliplatin 100 mg/m2 on day 1 of a 21-day cycle. Routine hematology and biochemistry measurements, and electrocardiography were conducted weekly to monitor the vital signs, liver function, and kidney function of patients.
2.3. Outcome measures
The tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) evaluation criteria.[16] Disease control was categorized as complete response (CR), partial response (PR), stable disease (SD), and treatment failure that included progressive disease (PD) or early treatment discontinuation. DCR was defined as “CR+PR+SD,” and evaluated after 2 treatment cycles. DCR after 2 pemetrexed-based treatment cycles was the major outcome measure for evaluation of short-term curative effect of treatment. Adverse events (AEs) were graded according to World Health Organization Common Terminology Criteria. Clinicians determined the tumor response and progression status.
The platinum or oxaliplatin treatment continued for 4 cycles at most. If the patients still had a CR, PR, or SD after 2 to 4 treatment cycles, single pemetrexed would be given to the patients as maintenance therapy. Patients with a PD or intolerable toxicity were given supportive treatment or additional treatment at their physician's discretion.
2.4. Follow-up
Prognosis of patients was evaluated by telephone review or case review until death or dropping out. OS was defined from the date of chemotherapy administration until the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of chemotherapy administration until the date of disease progression or death.
2.5. Assessment of TS expression in cancer tissue using western blot
Cancer tissue samples were obtained from the patients for detection of TS. Two chief pathologists confirmed that more than 85% of the tissue samples were cancer cells. Briefly, total protein was extracted from the tissue samples, and used for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred onto immobilon-P transfer membranes. The membranes were blocked in TBS containing 5% Bovine Serum Albumin and incubated with primary antibody according to manufacturer's instruction (Rockland, Gilbertsville, PA). After washing, the membranes were incubated with secondary antibodies (Sigma, St. Louis, MO; diluted 1:10,000), and further detected using 3,3′-diaminobenzidine (DAB). The target protein was quantified by gray scanning with the help of Bandscan software (PROZYME, San Leandro, CA). Beta-actin (Sigma) was used as an internal reference marker.
2.6. Statistical analysis
All statistical analyses were performed by SPSS software, version 18 (IBM, Armonk, NY). Scheffe test or χ2 tests were used to examine the differences in baseline characteristics and outcomes of patients between the 3 treatment groups. OS was estimated using Kaplan–Meier analysis, and compared using Log-rank test. Logistic and Cox regression analyses were used to identify predictors of DCR and overall survival. P value <0.05 suggested significant difference.
3. Results
3.1. Baseline characteristics of patients
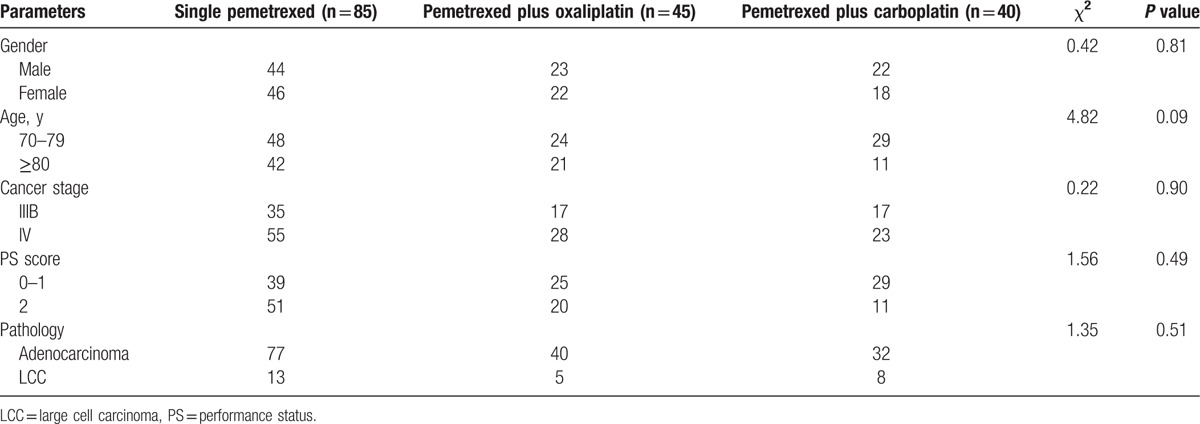
Of the 175 included patients, 89 were male and 86 were female with the median age of 77 years (range, 70–92 years). As shown in Table 1, single pemetrexed group (n = 85), pemetrexed plus oxaliplatin group (n = 45), and pemetrexed plus carboplatin group (n = 40) did not significantly differ in age, gender, clinical stage, PS, and pathological type. Expression of TS in cancer tissue of the patients varied. TS equal to or above the median value was defined as high TS expression; TS below the median value was defined as low TS expression. Thirty patients were still alive at the last follow-up, and 14 patients were dropped out of the follow-up.
Table 1.
Baseline characteristics of patients with advanced nonsquamous nonsmall cell lung cancer.
3.2. Disease control
A total of 582 treatment cycles were completed in the 175 patients, and the mean number of treatment cycles for all patients was 3.3. After 4 weeks of first-line treatment, 77 cases were treated with pemetrexed alone as maintenance treatment, and completed 212 cycles of maintenance treatment. The mean number of maintenance treatment cycles for these patients was 2.8.
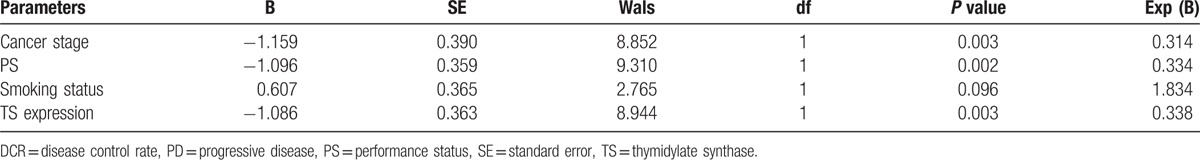
After 2 treatment cycles, DCR of all patients was 67.3%. DCR of the pemetrexed group, pemetrexed plus oxaliplatin group, and pemetrexed plus carboplatin group was 62.2%, 71.1%, and 70.0%, separately. The difference in the DCR did not achieve significance among the 3 groups (P > 0.05). χ2 test (Table 2) revealed that DCR after 2 pemetrexed-based treatment cycles was significantly associated with smoking status (current or previous history of smoking cigarettes), PS score, TNM stage, and TS expression levels. Logistic regression analysis (Table 3) further found that PS score, TNM stage, and TS expression were significantly associated with DCR, suggesting that these parameters might be predictive factors of short-term curative effect of pemetrexed-based treatments.
Table 2.
χ2 test to evaluate factors associated with short-term curative effect.
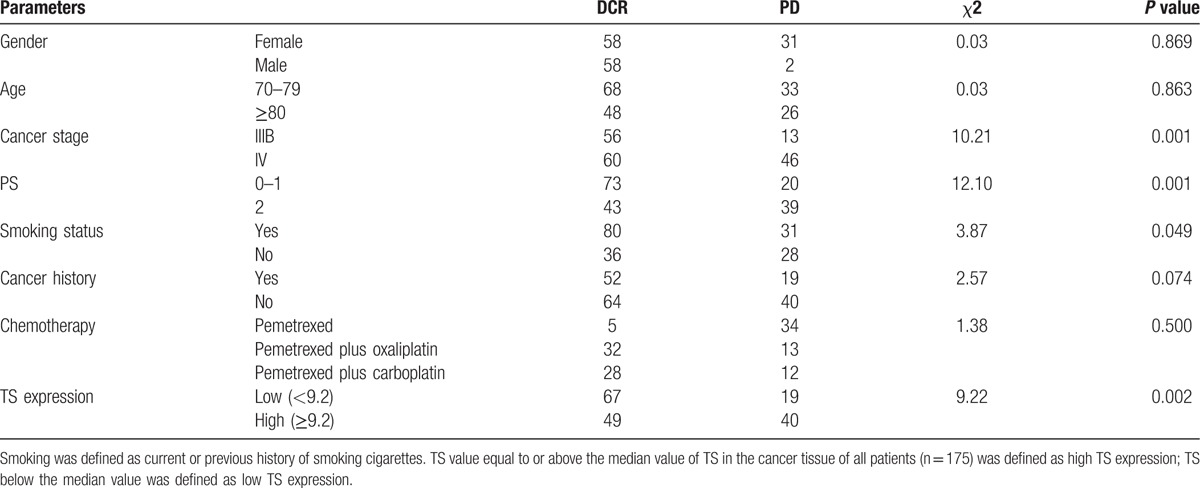
Table 3.
Logistic regression analysis to evaluate factors associated with DCR.
3.3. OS and PFS
Median OS of all patients was 15.6 months (95% confidence interval (95% CI): 14.1–17.1). As shown in Fig. 1A, OS was not significantly different among the 3 treatment groups (χ2 = 2.79, P = 0.25; single pemetrexed: 14.9 (95% CI: 12.9–17.1); pemetrexed combined with oxaliplatin: 16.5 (95% CI: 14.1–17.8); pemetrexed combined with carboplatin: 15.5 (95% CI: 13.8–17.4)). Median PFS of all patients was 4.5 months (95% CI: 4.3–4.7). Median PFS was 3.3 months (95% CI: 2.2–4.4) in the single pemetrexed group, 4.5 months (95% CI: 4.3–4.7) in the pemetrexed combined plus oxaliplatin group, and 4.6 months (95% CI: 2.7–6.5) in the pemetrexed plus carboplatin group. Figure 1B shows no significant difference in PFS among the 3 treatment groups (χ2 = 5.22, P = 0.07).
Figure 1.
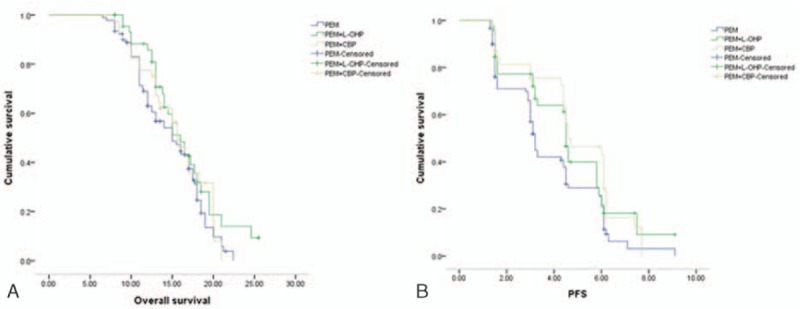
Overall survival and progression-free survival curves for chemotherapy administrations, including pemetrexed, pemetrexed plus oxaliplatin, and pemetrexed plus carboplatin. A, Overall survival (OS) curve. B, Progression-free survival (PFS) curves.
3.4. Adverse events
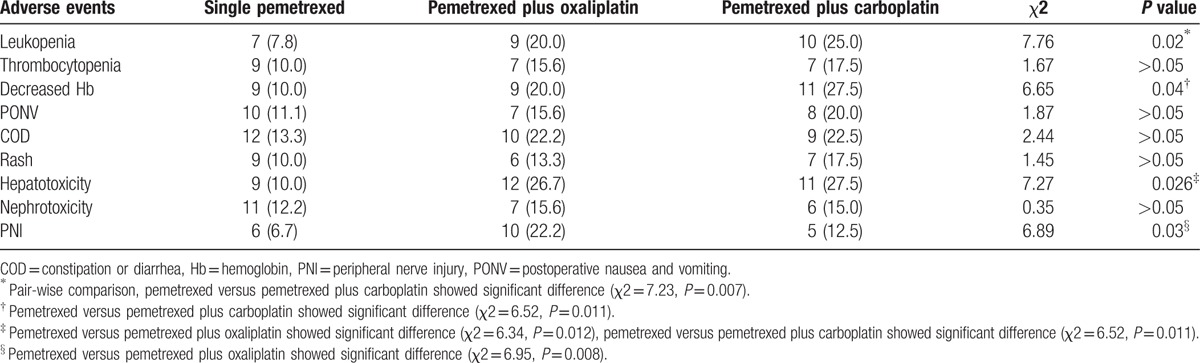
Myelosuppression (12 cases, grades 1–2), gastrointestinal reaction (12 cases, grades 1–2), and fatigue (25 cases, grades 1–2) were 3 main adverse events, which occurred 1 to 3 days after chemotherapy administration. Symptoms relieved lasting for 2 to 5 days. Among patients with myelosuppression (grades 3–4), leukocyte count deceased in 18 cases, of which fever was induced by deceased leukocyte in 5 cases. They were relieved from symptoms after granulocyte colony-stimulating factor (G-CSF) treatment. A total of 23 cases experienced thrombocytopenia (grades 3–4) which was relieved after thrombopoietin (TPO) treatment for 1 week. In addition, adverse events such as hepatotoxicity, nephrotoxicity, and loss of appetite were also observed. Seven cases stopped the treatment because of grade 4 leukopenia, and no treatment-related deaths occurred.
Grade 3–4 adverse events of patients in the 3 groups are summarized in Table 4. Pemetrexed group had significantly lower incidences of leukopenia, hemoglobin decreased, hepatotoxicity, and peripheral nerve injury (PNI) than the pemetrexed plus carboplatin group. Pemetrexed group had significantly lower incidences of hepatotoxicity and PNI than the pemetrexed plus oxaliplatin group.
Table 4.
Adverse events (grades 3–4) induced by chemotherapy administration [case (%)].
3.5. Univariate and multivariate analysis of prognostic factors
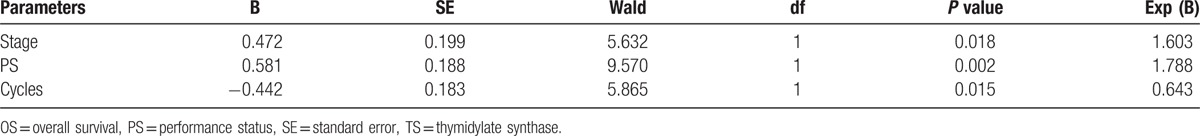
Univariate analysis unveiled that OS was significantly correlated with PS scores, TNM stage, and pemetrexed-based chemotherapy cycle (Table 5). Moreover, the correlations between PS scores, TNM stage, pemetrexed-based chemotherapy cycle, and OS are shown in Fig. 2. Furthermore, Cox-type regression analysis revealed that PS score, TNM stage, and pemetrexed-based chemotherapy cycle were all independent prognostic factors (Table 6). Moreover, pemetrexed-based chemotherapy cycle played as a protective factor, and OS of patients treated with more than 4 cycles of chemotherapy was better than patients treated by less than 4 cycles of chemotherapy (RR = 0.621, 95% CI: 0.415–0.930). It suggested that pemetrexed maintenance therapy was important for prognosis of patients. OS of patients with PS scored 0 to 1 points was better than patients with PS scored 2 points (RR = 1.546, 95% CI: 1.039–2.300).
Table 5.
Univariate analysis to evaluate factors associated with overall survival.
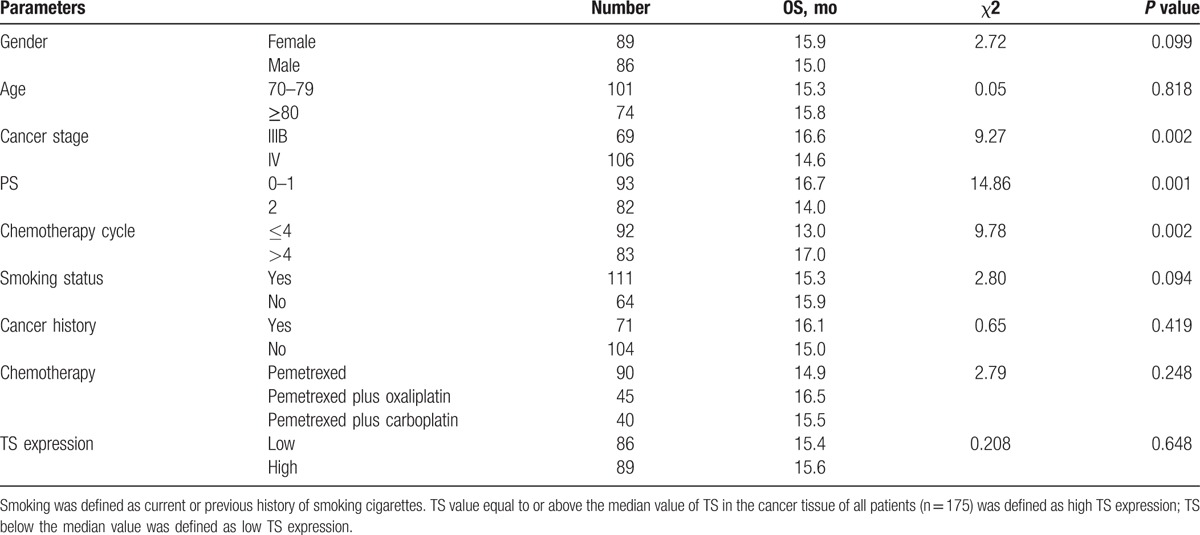
Figure 2.
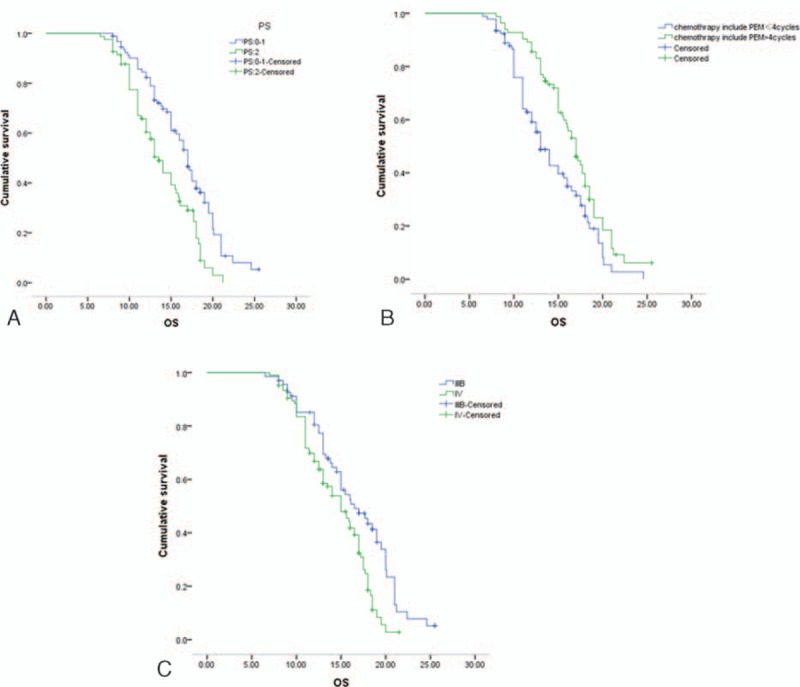
Overall survival for performace status (PS), tumor node metastasis (TNM) stage, and pemetrexed chemotherapy cycle. A, Overall survival for PS graded 0–1 versus PS graded 2. B, Overall survival for chemotherapy cycle ≤4 versus chemotherapy cycle > 4. C, Overall survival for IIIB TNM stage versus IV TNM stage.
Table 6.
Cox regression analysis to evaluate factors associated with overall survival.
4. Discussion
The retrospective cohort study demonstrated that single pemetrexed treatment for elderly Chinese patients with advanced NS-NSCLC was noninferior to pemetrexed plus oxaliplatin and pemetrexed plus carboplatin in terms of short-term DCR, OS, and PFS for treatment of NS-NSCLC. Single pemetrexed treatment could significantly lower the incidences of adverse events, such as hepatotoxicity and PNI. Adverse reactions of most patients were mainly evaluated as grade 1 or 2, and no treatment-related death was reported. Moreover, PS score, TNM stage, and TS expression might be predictive factors of short-term DCR of pemetrexed-based treatments. Number of pemetrexed chemotherapy cycles, PS scores, and TNM stage were demonstrated as independent factors for prognosis.
Recently, varied treatment outcomes of pemetrexed chemotherapy administration in elderly patients have been reported. Gridelli et al[17] put forward that the median PFS and OS for elderly patients with stage IIIB/IV NSCLC are 4.5 months and 3.3 months, respectively. Kim et al[18] have found that median PFS and OS of elderly NS-NSCLC patients who received pemetrexed as first-line treatment are 3.3 and 17.5 months, respectively. In the present study, OS, PFS, and DCR of all patients receiving pemetrexed-based treatment in first-line setting was 15.6 months, 4.5 months, and 67.3%, separately, supporting favorable antitumor activity of pemetrexed-based treatment in elderly patients. The inconsistent data of these studies might due to different individual backgrounds, therapeutic schedule, and nursing care.
The present study suggested that TS expression, PS score, TNM stage were predictive factors of DCR after 2 cycles of pemetrexed-based treatments, which was in concordance with a previous finding that a high TS expression is related to a reduced sensitivity of pemetrexed treatment.[19] TS expression, PS score, TNM stage might be suggestive of the short-term curative effect of pemetrexed-based treatment. However, TS was not significantly associated with OS in the present study, indicating that it is a predictive factor of the short-term curative effect of pemetrexed-based treatment, but not an independent prognostic factor. Furthermore, emerging evidence has proved that patient's PS, chemotherapy cycle, and second-line therapy might be prognostic factors in elderly patients with advanced NSCLC, who were treated with chemotherapy.[20,21] Moreover, a recent study on older NSCLC patients has found that survival rate is significantly associated with PS, disease stage, and chemotherapy using multivariate Cox regression analysis.[22] Similarly, the study found that pemetrexed chemotherapy cycles, PS score, and TNM stage were independent prognostic factors for advanced NS-NSCLC patients receiving pemetrexed or pemetrexed in combination with platinum chemotherapy. Therefore, we infer that advanced NS-NSCLC patients with PS 0–1, early stage cancer (IIIB vs IV), and pemetrexed chemotherapy as maintenance treatment (<4 weeks versus ≥4 weeks) might be more likely to have longer overall survival.
In conclusion, this study suggests that single pemetrexed treatment was noninferior to pemetrexed plus oxaliplatin or carboplatin in terms of clinical efficacy and safety for older Chinese patients with advanced NS-NSCLC. PS score, TNM stage, and TS expression might be predictive factors of short-term curative effect of pemetrexed-based treatments. Number of pemetrexed chemotherapy cycles, PS scores, and TNM stage might be independent prognostic factors. An open-label randomized trial was needed to verify the findings of this study.
Acknowledgment
No one else or the organization needs to be acknowledged.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, AEs = adverse events, CR = complete response, DAB = 3,3′-diaminobenzidine, DCR = disease control rate, ECOG = Eastern Cooperative Oncology Group, EGFR = wild-type epidermal growth factor receptor, G-CSF = granulocyte colony-stimulating factor, NSCLC = nonsmall cell lung cancer, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PNI = peripheral nerve injury, PR = partial response, PS = performance status, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease, SDS-PAGE = sodium dodecyl sulfate polyacrylamide gel electrophoresis, TPO = thrombopoietin, TS = thymidylate synthase.
The authors have no conflicts of interest to disclose.
References
- [1].Chen WQ, Zheng RS, Zhang SW, et al. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer 2014;33:402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhao P, Dai M, Chen W, et al. Cancer trends in China. Japan J Clin Oncol 2010;40:281–5. [DOI] [PubMed] [Google Scholar]
- [3].Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. [DOI] [PubMed] [Google Scholar]
- [4].Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- [5].Esteban E, Casillas M, Cassinello A. Pemetrexed in first-line treatment of non-small cell lung cancer. Cancer Treat Rev 2009;35:364–73. [DOI] [PubMed] [Google Scholar]
- [6].Wu YL, Lu S, Cheng Y, et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 2014;85:401–7. [DOI] [PubMed] [Google Scholar]
- [7].Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncolo 2011;6:64–70. [DOI] [PubMed] [Google Scholar]
- [8].Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–55. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Chen J, Wu S, et al. Clinical effectiveness and clinical toxicity associated with platinum-based doublets in the first-line setting for advanced non-squamous non-small cell lung cancer in Chinese patients: a retrospective cohort study. BMC Cancer 2014;14:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takezawa K, Okamoto I, Okamoto W, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer 2011;104:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chung-Yu C, Yih-Leong C, Jin-Yuan S, et al. Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer 2011;74:132–8. [DOI] [PubMed] [Google Scholar]
- [12].Gibbs A, Thunnissen F. Histological typing of lung and pleural tumours. J Clin Pathol 2001;54:498–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nair A, Klusmann MJ, Jogeesvaran KH, et al. Revisions to the TNM staging of non-small cell lung cancer: rationale, clinicoradiologic implications, and persistent limitations 1. Radiographics 2011;31:215–38. [DOI] [PubMed] [Google Scholar]
- [14].Bhosle J, Hall G. Eastern cooperative oncology group performance status. Surgery (Oxford) 2009;4:173–7. [Google Scholar]
- [15].Yu S, Zhang B, Xiang C, et al. Prospective assessment of pemetrexed or pemetrexed plus platinum in combination with gefitinib or erlotinib in patients with acquired resistance to gefitinib or erlotinib: a phase II exploratory and preliminary study. Clinical Lung Cancer 2015;16:121–7. [DOI] [PubMed] [Google Scholar]
- [16].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [17].Gridelli C, Kaukel E, Gregorc V, et al. Single-agent pemetrexed or sequential pemetrexed/gemcitabine as front-line treatment of advanced non-small cell lung cancer in elderly patients or patients ineligible for platinum-based chemotherapy: a multicenter, randomized, phase II trial. J Thorac Oncol 2007;2:221–9. [DOI] [PubMed] [Google Scholar]
- [18].Kim YH, Hirabayashi M, Kosaka S, et al. Phase II study of pemetrexed as first-line treatment in elderly (>/=75) non-squamous non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0901. Cancer Chemother Pharmacol 2013;71:1445–51. [DOI] [PubMed] [Google Scholar]
- [19].Ozasa H, Oguri T, Uemura T, et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci 2010;101:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pirker R, Pereira JR, Szczesna A, et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer 2012;77:376–82. [DOI] [PubMed] [Google Scholar]
- [21].Inal A, Kaplan MA, Kucukoner M, et al. Prognostic factors in elderly patients with advanced non-small cell lung cancer treated with first-line cisplatin-based chemotherapy: a retrospective analysis of single institution. J BUON 2012;17:533–6. [PubMed] [Google Scholar]
- [22].Su Q, Sun YP, Liu YH, et al. Prognostic factors in older patients with advanced non-small cell lung cancer in China. Tumori 2014;100:69–74. [DOI] [PubMed] [Google Scholar]


