Abstract
Background:
The comprehensive meta-analysis aimed to explore the reductive effect of aerobic exercise on blood pressure of hypertensive patients.
Methods:
The related researches were selected from PubMed and Embase databases up to June 2016. Based on specific inclusive criteria, the eligible studies were selected, and the heterogeneities in their results were estimated by χ2-based Q-test and I2 statistics. Quantitative meta-analysis was assessed by R 3.12 software, and results were presented by standardized mean difference (SMD) and their 95% confidence intervals (CIs). Outcome indicators were systolic blood pressure (SBP) and diastolic blood pressure (DBP). The publication biases were estimated by Egger test. Besides, the “leave one out” method was used for sensitivity evaluations.
Results:
As a result, a total of 13 papers with 802 samples were included. Based on the meta-analysis results, there were no significant differences in SBP and DBP between aerobic and control groups before exercise (SMD = 0.15, 95%CI: −0.16–0.46; SMD = 0.16, 95% CI: −0.23–0.55). However, significant reductions were obviously in aerobic group after aerobics, compared with control (SMD = −0.79, 95% CI: −1.29 to −0.28; SMD = −0.63, 95% CI: −1.14 to −0.12). A significant publication bias was detected in SBP (t = −2.2314, P = 0.04549) but not in DBP (t = −1.4962, P = 0.1604). Additionally, the DBP result would be altered after the exclusion of 2 individual papers.
Conclusion:
Aerobic exercise may be a potential nonpharmacological treatment for blood pressure improvement in essential hypertensive patients.
Keywords: aerobic exercise, diastolic blood pressure, essential hypertension, meta-analysis, systolic blood pressure
1. Introduction
Physical activity is an important adjunct to medical management in the therapy of cardiovascular diseases (CVD).[1] Previous studies reported that moderate-intensive exercise was closely related with a low incidence of adverse CVD events and improved health outcomes,[2] and a higher-intensity activity may provide a better benefit.[3] Meanwhile, large-scale population-based trials have documented that 15 minutes of daily exercise in leisure-time will confer about 15% mortality reduction in cardiovascular mortality.[4] Physical activity has variety physiologic effects on the cardiovascular system, and the most notably influences are improving vascular endothelial function via enhancing flow-mediated vasodilation, reducing the resting heart rate by increasing parasympathetic tone, increasing vasculogenesis via endothelial progenitor cells, and strengthening tolerance for ischemia and reperfusion injury.[5] In China, the incidence of CVD mortality has increased more than 50% from 1990 to 2009, and most of them are attributed to lack of physical activity.[6] All of these evidences may indicate that physical activity plays a crucial role in the administration of CVD. Therefore, it is very useful to perform a comprehensive evaluation on the clinical outcome of physical activity on CVD.
Aerobic exercise is one of nonpharmacological treatment methods and is recommended by European and American hypertension guidelines to reduce blood pressure.[7,8] It is reported that moderate-intensity aerobic exercise is able to reduce both systolic blood pressure (SBP) and diastolic blood pressure (DBP) in both male and female patients with essential hypertension in pre- or stage 1.[9] Meanwhile, previous research also reported that exercise training altered the balance between vasodilatation- and vasoconstriction-related cytokines, such as nitric oxide,[10] prostacyclin, and thromboxane.[11] However, other studies support the opposite opinions. Fagard[12] and Whelton et al[13] have reported that exercise training has not caused a reduction on blood pressure. Moreover, a trimester exercise training also shows no reduction on blood pressure, except in a few resistance hypertension.[14] In spite of this difference, these results are mainly obtained from small sample researches, thus, the effect of aerobic exercise on hypertensive blood pressure may have some deviations. Therefore, the aim of this comprehensive meta-analysis was to exposit the real effect of aerobic exercise on blood pressure in hypertensive patients, so that it can provide us with some reliable and useful views for essential hypertensive treatment.
2. Methods and materials
2.1. Data sources
The systematic search strategies were conducted according to the Meta-analysis of Observation Studies in Epidemiology (MOOSE) guidelines.[15] Based on the searching strategies, the English papers were selected from Pubmed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase (http://www.embase.com) databases, as well as by literature tracing method from their inception to June 2015. Key search terms were essential hypertension (OR “Primary hypertension” OR “Essential hypertensive”) AND aerobics (OR “aerobic” OR “cardio”).
2.2. Inclusive criteria
Studies were included in this meta-analysis if they met the following criteria: the study[1] must be an English paper about the effect of aerobics effect on primary hypertension reduction [2]; must have a definitely diagnostic criterion for primary hypertension: SBP ≥140 mm Hg and DBP ≥90 mm Hg, or being medicated for hypertension[3]; and can provide or calculate the SBP and DBP for hypertensive patients. Studies were excluded if they were reviews, reports, letters, or comments.
2.3. Data extraction
Two authors independently abstracted all related data from the enrolled papers, including: 1st author, publication year, study country, the participant numbers in aerobic group and control group, general demographic characteristics (eg, gender ratio, age composition, and BMI), and SBPs and DBPs before and after aerobic exercise. A 3rd author would be involved to solve the disagreements and differences during data extraction.
2.4. Statistical analysis
In the current study, meta-analysis was conducted by R 3.12 software (R Foundation for statistical Computing, Beijing, China, Meta-package), the effect indicators of quantitative data were showed by standardized mean difference (SMD) and their 95% confidence intervals (CIs). Heterogeneity was assessed by χ2-based Q-test[16] and I2 statistics, by which P < 0.05 or I2 > 50% was recognized heterogeneous and the random-effects model was selected to calculate the pooled effective size; or else, fixed-effects model was selected to calculate the pooled effective size.[17] Publication bias was evaluated by Egger test and the threshold was set as P < 0.05.[18] Moreover, the sensitivity analysis was evaluated by “leave one out” method with I2 > 50% as the criterion.[18]
3. Results
3.1. Characteristics of included studies
The detailed screening flow chart is presented in Fig. 1. Based on the aforementioned selection strategies, a total of 348 researches were included. After repeated studies were excluded, 204 researches were put through title scanning and abstract reading. As a result, 162 of them were not met the selected criteria. After reading full texts of the remaining 42 researches, 29 of them were excluded, including reviews (n = 3), case reports (n = 2), and studies that were unable to obtain related data (n = 11). Finally, a total of 13 researches were included in the meta-analysis.[19–31]
Figure 1.
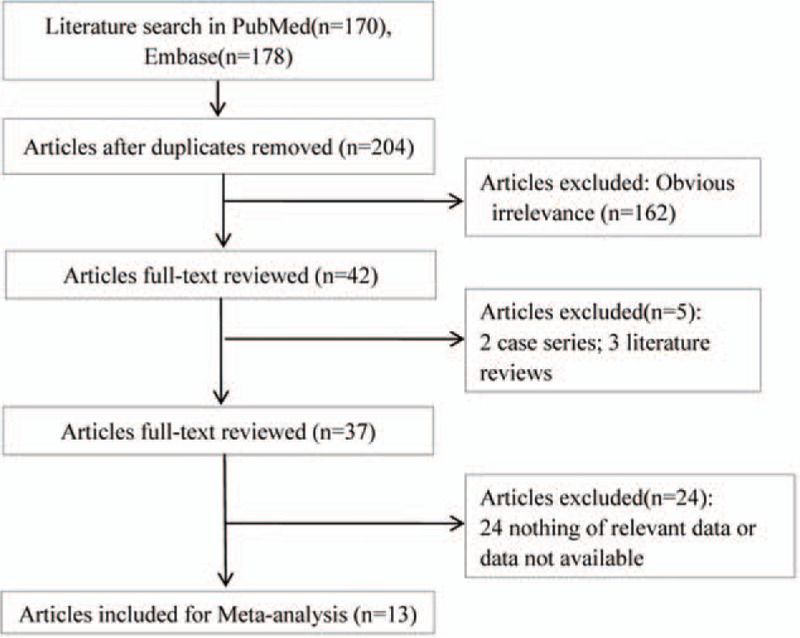
Flow chart of literature search and study selection.
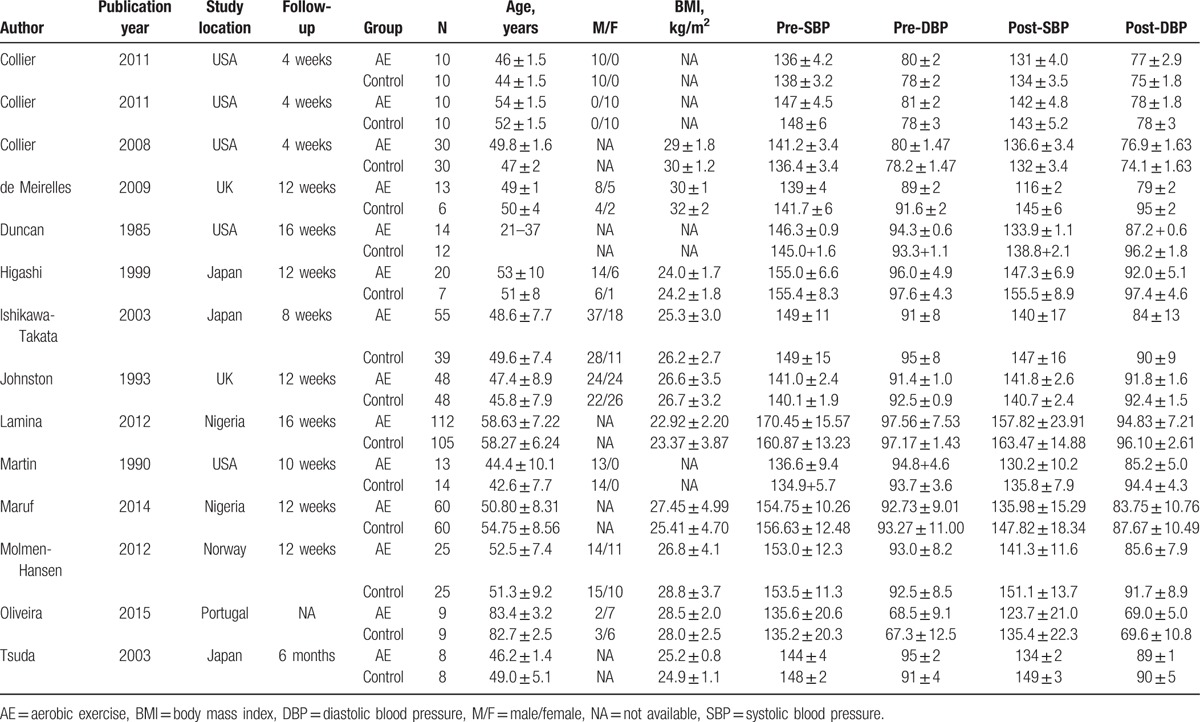
By integrating the 13 included studies, a total of 802 primary hypertensive patients were enrolled, including 424 cases in aerobics group and 378 cases in control group. The general characteristics of the included researches were tabulated (Table 1). It was clearly showed that although the included papers were published from 1985 to 2015, most of them were performed after 2003; their follow-up time ranged from 4 weeks to 6 months. For the included patients, their nationalities were mainly concentrated in America, the United Kingdom, Japan, Norway, Nigeria, and so on; meanwhile, the overall patients were unbalanced in gender composition with more males than females; however, the distribution in 2 groups was balanced. Furthermore, there were no significant differences in ages and BMIs between 2 groups in each study.
Table 1.
Characteristics of included researches.
3.2. Meta-analysis for pooled quantitative data
Based on the thresholds of P < 0.05 and or I2 > 50%, the significant heterogeneous results on SBP and DBP evaluations were identified (P < 0.05, I2 > 50%), so the random-effects model was selected. According to the pooled results, no significant differences were identified in both SBP and DBP between aerobic and control groups before aerobic exercise (SBP: SMD = 0.15, 95%CI: −0.16–0.46, Fig. 2A; DBP: SMD = 0.16, 95% CI: −0.23–0.55, Fig. 2B). However, significant reductions on SBP and DBP were observed in aerobic group, compared with their respective control groups after aerobic exercise (SBP: SMD = −0.79, 95% CI: −1.29 to −0.28, Fig. 3A; DBP: SMD = −0.63, 95% CI: −1.14 to −0.12, Fig. 3B).
Figure 2.
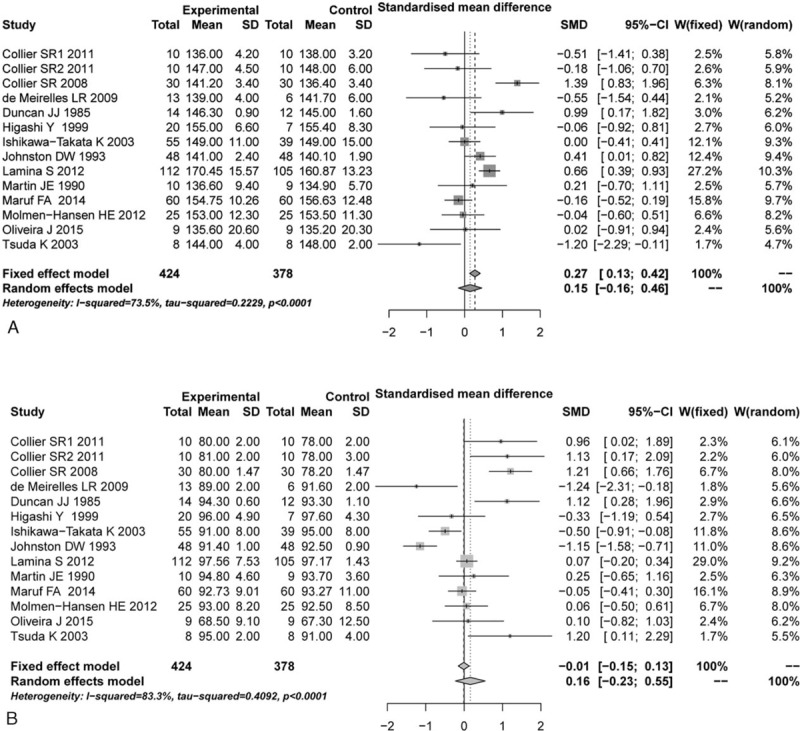
Comparison of blood pressure between aerobic group and control group before aerobic exercise. (A) SBP, (B) DBP. CI = confidence interval, DBP = diastolic blood pressure, SBP = systolic blood pressure, SD = standard deviation, SMD = standardized mean difference.
Figure 3.
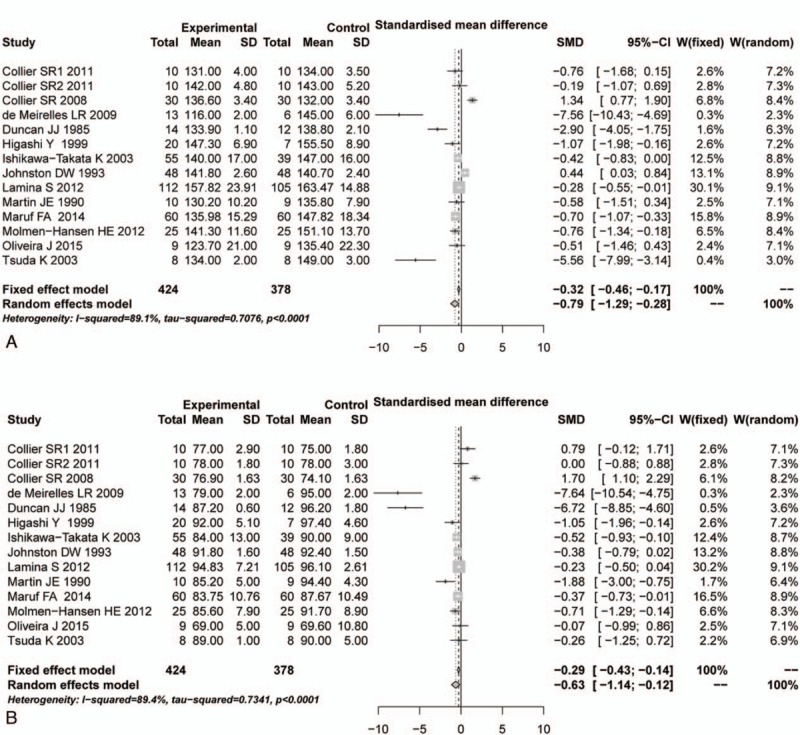
Comparison of blood pressure between aerobic group and control group after aerobic exercise. (A) SBP, (B) DBP. CI = confidence interval, DBP = diastolic blood pressure, SBP = systolic blood pressure, SD = standard deviation, SMD = standardized mean difference.
3.3. Publication bias estimation
According to the Egger test results, there were no significant publication biases identified between aerobic and control groups in SBP and DBP before aerobic exercise (t = −1.4047, P = 0.1855; t = 1.0943, P = 0.2953). These results meant that these combined results were reliable and credible. Meanwhile, there was also no publication bias in DBP between 2 groups after aerobic exercise (t = −1.4962, P = 0.1604). Nonetheless, an unexpected publication bias was identified in SBP between 2 groups after aerobic exercise (t = −2.2314, P = 0.04549).
3.4. Sensitivity estimation
After sensitivity analysis using the “leave one out” method, the pooled DBP was altered after leaving 2 included articles out,[21,22] but the SBP was not. This result meant that SBP had a relatively stable analysis result in this research.
4. Discussion
Based on the selected criteria, a total of 13 papers with 802 essential hypertensive patients were included in this study to illustrate the effect of aerobic exercise on hypertensive control. The pooled data of meta-analysis showed that there were no significant differences of SBP and DBP between aerobic group and control group before aerobic exercise training, and there were also no publication biases identified for them. However, significant reductions of SBP and DBP were obviously identified in aerobic group after aerobic exercise training while compare with control group, and a significant publication bias was identified in SBP due to aberrant age, BMI, and training time, while DBP was not. Besides, the sensitivity estimation showed a relatively stable result for SBP, but the result of DBP was altered after excluding 2 individual studies.
As many researches and guidelines present previously, aerobic exercise training is an important complementary treatment strategy for hypertensive control. A research of Cornelissen and Fagard[32] has showed that aerobic exercise is able to reduce SBP and DBP by about 7 and 5 mm Hg, respectively. Meanwhile, long-term results (12 and 16 weeks) also show that aerobic exercise plays a beneficial effect on SBP and DBP level controls.[33,34] Besides, Molmen-Hansen et al[30] have also reported that the reducing effects of aerobic exercise on SBP and DBP are intensity-dependent. Therefore, according to the previous researches and our findings in this study, it might be acknowledged that aerobic exercise might play a crucial role in the reductions of SBP and DBP in essential hypertensive patients. Devan et al[35] consider that one explanation for this reduction effect was the regular aerobic exercise could prevent the age-associated vascular endothelial dysfunction. An experiment in rats shows that aerobic exercise training can reduce blood pressure via improving vascular stiffness and endothelial function.[36] However, a previous meta-analysis showed that aerobic exercise training was not able to induce arterial stiffness in hypertensive patients, unless having a prolonged duration and/or an obvious reduction in SBP,[37] but how does it affect essential hypertension is still elusive. Hence, a comparison of different training time is required. Nyberg et al[10] have reported that blood pressure can be regulated by nitric oxide and prostanoid systems, which are substances for blood vessel dilation and can be influenced by aerobic exercise training. Psychosocial stress is an important factor in the pathogenesis of essential hypertension, while appropriate intensive aerobic exercise can alleviate the mental stress to decrease the sympathetic nervous system which is related to the origin of essential hypertension.[38–40] Besides, improving insulin sensitivity may be another potential mechanism of aerobic training on blood pressure reduction.[41] Furthermore, it was worth noting that the pooled SBP after aerobic exercise had a significant publication bias, and the DBP effect was altered after excluding 2 of included papers. In the research of Meirelles, a relatively high BMI with a long-term aerobic training might induce a more significant reduction on SBP, which was too different to other results, so that the result was obviously altered after the removal of this study. A plausible explanation for the research of Duncan et al was age-bracket. Essential hypertension is an age-dependent chronic disease.[42] However, the patients’ ages of Duncan et al were narrowed to a range of 21 to 37 years, which might be a causative factor for the reverse result when it was removed.
In the present study, a comprehensive meta-analysis was conducted to estimate the reduction effect of aerobic exercise on blood pressure control for essential hypertensive patients. Therefore, our results might be of great value to provide guidelines for the control of blood pressure via aerobic exercise. However, there were still some limitations in this study. First, there lacked a comprehensive demographic data, such as populations, exercise way and intensity, job category, and other related diseases, which was unable to conduct subgroup analyses stratified by these factors. For example, Collier et al[20] have reported that moderate intensive resistance exercise plays an important benefit for females with a greater reduction in blood pressure control and a significant increase in flow-mediated dilation. Second, due to a significant publication bias identified in pooled SBP after aerobic exercise, the analysis result of SBP might be affected. Third, because the pooled DBP result was altered by excluding 2 papers, the final reduction effect of aerobic exercise on DBP might be misestimated. Hence, age, aerobic training time, obesity, and even diabetes might have some inevitable influences on the benefits of aerobic exercise in essential hypertension, and these influences might be attenuated by including more related research with larger samples. Additionally, the wider range of follow-up times after aerobic exercise in the included studies might be another risk factor for the heterogeneity. Thus, subgroup analysis with different follow-up times should be considered in future investigations. Last but not the least, due to different types of included papers, the qualitative estimation was not allowed.
In conclusion, it could be inferred that aerobic exercise training might cause a mild but significant reduction on blood pressure in essential hypertensive patients. However, more large and long-term clinical trials would be still required in the future to drive more precise conclusion.
Footnotes
Abbreviations: CI = confidence interval, CVD = cardiovascular diseases, DBP = diastolic blood pressure, SBP = systolic blood pressure, SMD = standardized mean difference.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Pescatello LS, Arena R, Riebe D, et al. Acsm's Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- [2].Myers J. Exercise and cardiovascular health. Circulation 2003;107:2e–e5. [DOI] [PubMed] [Google Scholar]
- [3].Ismail H, Mcfarlane JR, Nojoumian AH, et al. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC Heart Fail 2013;1:514–22. [DOI] [PubMed] [Google Scholar]
- [4].Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–53. [DOI] [PubMed] [Google Scholar]
- [5].Gielen S, Schuler GV. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 2010;122:1221. [DOI] [PubMed] [Google Scholar]
- [6].Lan C, Chen SY, Wong MK, et al. Tai chi chuan exercise for patients with cardiovascular disease. Evid Based Complement Alternat Med 2013;2013:983208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mansia G, De BG, Dominiczak A, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;34:2159–219. [DOI] [PubMed] [Google Scholar]
- [8].Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014;32:162–70. [DOI] [PubMed] [Google Scholar]
- [9].Collier SR, Kanaley JA, Frechette R, Jr, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 2008;22:678–86. [DOI] [PubMed] [Google Scholar]
- [10].Nyberg M, Jensen LG, Thaning P, et al. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 2012;590(Pt 6):1481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hansen AH, Nyberg M, Bangsbo J, et al. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension 2011;58:943–9. [DOI] [PubMed] [Google Scholar]
- [12].Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001;33:493–4. [DOI] [PubMed] [Google Scholar]
- [13].Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288:1882–8. [DOI] [PubMed] [Google Scholar]
- [14].Braith RW, Pollock ML, Lowenthal DT, et al. Moderate- and high-intensity exercise lowers blood pressure in normotensive subjects 60 to 79 years of age. Am J Cardiol 1994;73:1124–8. [DOI] [PubMed] [Google Scholar]
- [15].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [16].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [17].Feng RN, Zhao C, Sun CH, et al. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. Plos One 2011;6:e18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luo AJ, Wang FZ, Luo D, et al. Consumption of vegetables may reduce the risk of liver cancer: results from a meta-analysis of case-control and cohort studies. Gastroentérol Clin Biol 2015;39:45–51. [DOI] [PubMed] [Google Scholar]
- [19].Collier SR. Sex differences in the effects of aerobic and anaerobic exercise on blood pressure and arterial stiffness. Gend Med 2008;5:115–23. [DOI] [PubMed] [Google Scholar]
- [20].Collier SR, Frechette V, Sandberg K, et al. Sex differences in resting hemodynamics and arterial stiffness following 4 weeks of resistance versus aerobic exercise training in individuals with pre-hypertension to stage 1 hypertension. Biol Sex Differ 2011;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Meirelles LR, Mendes-Ribeiro AC, Mendes MA, et al. Chronic exercise reduces platelet activation in hypertension: upregulation of the l-arginine-nitric oxide pathway. Scand J Med Sci Sports 2009;19:67–74. [DOI] [PubMed] [Google Scholar]
- [22].Duncan JJ, Farr JE, Upton SJ, et al. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA J Am Med Assoc 1985;254:2609–13. [PubMed] [Google Scholar]
- [23].Higashi Y, Sasaki S, Sasaki N, et al. Daily aerobic exercise improves reactive hyperemia in patients with essential hypertension. Hypertension 1999;33:591–7. [DOI] [PubMed] [Google Scholar]
- [24].Ishikawa-Takata K, Ohta T, Tanaka H. How much exercise is required to reduce blood pressure in essential hypertensives: a dose–response study. Am J Hypertens 2003;16:629–33. [DOI] [PubMed] [Google Scholar]
- [25].Johnston DW, Gold A, Kentish J, et al. Effect of stress management on blood pressure in mild primary hypertension. BMJ Clin Res 1993;306:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kazushi T, Akiyoshi Y, Keizo K, et al. Effects of mild aerobic physical exercise on membrane fluidity of erythrocytes in essential hypertension. Clin Exp Pharmacol Physiol 2003;30:382–6. [DOI] [PubMed] [Google Scholar]
- [27].Lamina S, Okoye GC. Effects of aerobic exercise training on psychosocial status and serum uric acid in men with essential hypertension: a randomized controlled trial. Ann Med Health Sci Res 2012;2:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martin JE, Dubbert PM, Cushman WC. Controlled trial of aerobic exercise in hypertension. Circulation 1991;81:1560–7. [DOI] [PubMed] [Google Scholar]
- [29].Maruf FA, Akinpelu AO, Salako BL. A randomized controlled trial of the effects of aerobic dance training on blood lipids among individuals with hypertension on a thiazide. High Blood Press Cardiovasc Prev 2014;21:275–83. [DOI] [PubMed] [Google Scholar]
- [30].Molmen-Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol 2012;19:151–60. [DOI] [PubMed] [Google Scholar]
- [31].Oliveira J, Mesquita-Bastos J, Argel de Melo C, et al. Postaerobic exercise blood pressure reduction in very old persons with hypertension. J Geriatr Phys Ther 2015;39:8–13. [DOI] [PubMed] [Google Scholar]
- [32].Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005;46:667–75. [DOI] [PubMed] [Google Scholar]
- [33].Tsai JC, Chang WY, Kao CC, et al. Beneficial effect on blood pressure and lipid profile by programmed exercise training in Taiwanese patients with mild hypertension. Clin Exp Hypertens 2002;24:315–24. [DOI] [PubMed] [Google Scholar]
- [34].Pitsavos C, Chrysohoou C, Koutroumbi M, et al. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hellenic J Cardiol 2011;52:6–14. [PubMed] [Google Scholar]
- [35].Devan AE, Eskurza I, Pierce GL, et al. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci 2012;124:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roque FR, Briones AM, García-Redondo AB, et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol 2013;168:686–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 2014;173:361–8. [DOI] [PubMed] [Google Scholar]
- [38].Harshfield GA, Yan DB, Kapuku GK, et al. Stress-induced sodium retention and hypertension: a review and hypothesis. Curr Hypertens Rep 2009;11:29–34. [DOI] [PubMed] [Google Scholar]
- [39].Flaa A, Eide IK, Kjeldsen SE, et al. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension 2008;52:336–41. [DOI] [PubMed] [Google Scholar]
- [40].Mayorov DN. Brain angiotensin AT 1 receptors as specific regulators of cardiovascular reactivity to acute psychoemotional stress. Clin Exp Pharmacol Physiol 2011;38:126–35. [DOI] [PubMed] [Google Scholar]
- [41].Pescatello LS, Blanchard BE, Heest JLV, et al. The metabolic syndrome and the immediate antihypertensive effects of aerobic exercise: a randomized control design. BMC Cardiovasc Disord 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xiong XJ, Li SJ, Zhang YQ. Massage therapy for essential hypertension: a systematic review. J Hum Hypertens 2014;29:143–51. [DOI] [PubMed] [Google Scholar]


