Abstract
The agony that accompanies the incidence and symptoms of temporomandibular disorders (TMDs) is an important concern in the oral and maxillofacial region. The objective of this study was to explore the clinical findings after centric relation occlusal splint (CROS) treatment and intra-articular injection treatment with liquid phase concentrated growth factors (LPCGFs) in patients with disc displacement without reduction (DDWOR).
The group under investigation of this retrospective cohort study included patients with DDWOR who received treatment from April 2014 until March 2016. The predictor variable was the therapeutic method. The outcome variables included joint crepitus sound, visual analog scale (VAS) of temporomandibular joint (TMJ) arthralgia, TMD-associated headache, myofascial pain with referral, deviation of the mandible during opening (DoM), and maximal interincisal opening (MIO). At the stage of CROS treatment, evaluation of all variables adopted the individual as the unit; at the stage after LPCGF injection, the evaluation of joint sound adopted the joint as the unit, whereas the other variables adopted the individual as the unit.
Among the 29 patients, 6 (20.68%) were males and 23 (79.31%) were females. Distribution by age ranged from 15 to 84 years (mean age 39.55 ± 15.49 years). After CROS treatment, except for the joint crepitus sound, which failed to achieve significant improvement (P > 0.05), other symptoms, such as DOM, TMD-associated headache, myofascial pain with referral, TMJ arthralgia, and MIO, all achieved statistically significant improvements (P < 0.05). After 2 mL of LPCGF was injected once after CROS treatment, 26 joint crepitus sound symptoms were relieved (P < 0.001) after an average of 48.5 ± 64.1 days.
CROS alone can alleviate TMD clinical symptoms, except for the joint crepitus sound. Approximately 72.2% of joint crepitus sounds could be improved within 48 days, on average, once 2 mL of LPCGF was injected. Comparisons were still required in the future, with the effects of other therapeutic methods.
Keywords: centric relation occlusal splint, disc displacement without reduction, liquid phase concentrated growth factors, temporomandibular disorders
1. Introduction
Temporomandibular disorder (TMD) is a great influence over the oral and maxillofacial region. It is estimated that approximately 20% to 30% of adults have suffered from TMD at least once.[1] Clinical symptoms of TMD are usually accompanied by pain in the masticatory muscle and temporomandibular joint (TMJ), mouth open limitation, deviation of the mandible during mouth opening, click or crepitus, headache, and ache of the shoulder and neck.[2] According to the classification of diagnostic criteria for TMD (DC/TMD), TMD can be divided into pain-related TMD and intra-articular TMD.[3] Nonsurgical methods of treatment of intra-articular TMD include drug therapies, diet alterations, physical therapies, different types of centric relation occlusal splint (CROS),[4,5] and psychological support treatment.[6] Among them, CROS treatment is the most widely adopted, which can improve many of the clinical symptoms of TMD[4,5] and which is even helpful for anxiety and depression.[7] Among intra-articular TMD, disc displacement without reduction (DDWOR) is severe and more difficult to cure than disc displacement with reduction (DDWR). If nonsurgical methods of treatment cannot completely relieve patients’ symptoms, positively invasive surgical treatment will be needed. Surgical methods of treatment with intra-articular TMD include arthrocentesis.[8] Among invasive treatments, minimally invasive arthrocentesis and intra-articular injection are relatively accepted by patients and are typically used.
Although platelet-rich plasma (PRP) was developed as early as the 1970s, it was not until 1997 that Pihut et al started to apply it in the accelerated healing process.[9] It was progressively substantiated in the twentieth century that platelets contain multiple growth factors, such as platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, insulin-like growth factor, fibroblast growth factor, and epidermal growth factor.[10] PRP has been widely applied in maxillofacial[11] and cosmetic surgery.[12] Many publications have described the application of PRP via intra-articular injection for curing TMD over recent years.[9,13–17] The above studies employed traditional PRP supplemented with anticoagulant and reagents. Nevertheless, there remains a vast space for exploration regarding the dosage of injection, frequency, and time interval.
Liquid phase concentrated growth factor (LPCGF) is the latest generation of PRP, which was developed by Sacco in 2006.[18] Unlike PRP, LPCGF does not require an anticoagulant or the addition of any other agent. A centrifugal machine with a specific speed of revolution alone can separate growth factors from blood, which are stronger and purer than PRP. Moreover, such growth factors that are centrifuged can continuously and slowly discharge for at least 7 to 10 days.[18]
No study has explored the clinical effect of CROS treatment combined with the intra-articular injection treatment of LPCGF in TMD. The authors hypothesize that LPCGF can improve the clinical symptoms beyond treatment with CROS. The specific aim of this study was to substantiate that a 2-mL LPCGF injection can alleviate the clinical symptoms better than CROS treatment.
2. Materials and methods
2.1. Participants
The group under investigation in this retrospective cohort study was screened out among 450 patients who received treatment with TMD at Tainan Sin Lau Hospital from April 2014 until March 2016. The inclusion criteria were patients 15 years or older, patients who have been diagnosed with DDWOR in accordance with the DC/TMD classification and magnetic resonance imaging (MRI) findings, people who had received 3-mm thickness CROS treatment for 1 year on average but who remained with clinical symptoms, and patients willing to be rediagnosed for 4 months after LPCGF injection. The exclusion criteria were uncontrolled systemic disease, hematologic or neurologic disorders, head and neck cancer, inflammatory disease or connective tissue disease, anticoagulant drug use, oral submucosal fibrosis, edentulous patients, previous TMJ surgery, and incomplete data. Ultimately, 29 patients were recruited.
2.2. Treatment protocols
The same physician (J-WY) performed all treatments. Landmarks in the surgical intervention corresponded with the method of Murakami.[19] A tragus-lateral canthus line from the tragus to the lateral canthus was drawn. A line was made along the crease at the point of the skin crease ahead of the tragus, and then a point (landmark A) was made at the midpoint between this crease line and the tragal tip. Landmark B was 1 cm from point A, and landmark C was 1 cm from point B. Next, landmark D was made 2 mm from point B, and then landmark E was made 1 cm from point C perpendicular to the tragus-lateral canthus line (Fig. 1A). Then, LPCGF was prepared according to Sacco protocol.[18] Ten milliliters of blood were drawn from the patient's vein, and the blood was transferred into plastic white cap tubes (Greiner Bio-One, GmbH, Kremsmunster, Austria) without anticoagulants and reagents. These tubes were then immediately centrifuged at 2400 to 3000 rpm in a special machine (Medifuge; Silfradent srl, Sofia, Italy) using a program with the following characteristics: 30′′ acceleration, 2′ 2700 rpm, 4′ 2400 rpm, 4′ 2700 rpm, 3′ 3000 rpm, and 36′′ deceleration and stop.[18] At the end of the process, there were 3 blood fractions: the upper layer was represented by platelet-poor plasma; the middle layer was represented by a very large and dense LPCGF; and the lower layer was red blood cells. Then, the middle layer (LPCGF) was separated. Using two 22-gauge needles at points D and E, it was injected into the superior joint space before LPCGF injection. Then, 2 mL of physiological saline was injected, and the smoothness of inflow and outflow was observed to ensure that the position of the injection was within the superior joint space (Fig. 1B). Next, the no. 22 void needle was removed from point E before injecting the extracted 2 mL of LPCGF at point D (Fig. 1C). All patients orally took 500 mg of acetaminophen once every 8 h for 3 days as necessary, with soft food for 1 week. All patients were advised to continue wearing the CROS at night after the injection, with follow-ups in the first week and every month after the injection for 6 consecutive months. The minimum follow-up was 16 months, and the longest was 24 months.
Figure 1.
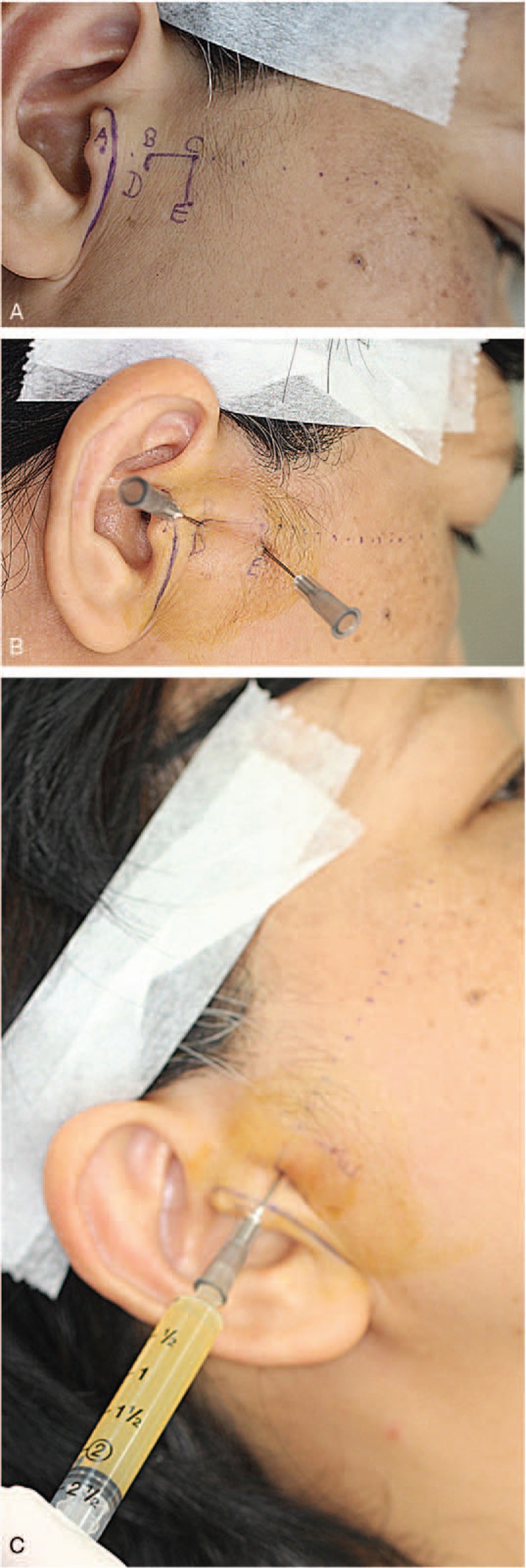
(A) Landmarks in surgical intervention approaches. (B) Double needle technique. The position where the needle was injected was within the superior joint space. (C) Injected 2 mL of liquid phase concentrated growth factors along the No. 22 void needle at point D.
2.3. Variables and assessments
The evaluated variables included a joint crepitus sound, TMJ arthralgia, TMD-associated headache, myofascial pain with referral, deviation of the mandible during opening (DoM), and maximal interincisal opening (MIO). At the stage of CROS treatment, evaluation of all variables adopted the individual as the unit; at the stage after LPCGF injection, the evaluation of the joint crepitus sound adopted the joint as the unit, while the other variables adopted the individual as the unit. The joint crepitus sound was evaluated by combining 3 means: palpation by the physician in the TMJ zone, auscultation with a stethoscope, and the patient's self-report regarding any joint noise heard during mouth opening movements, lateral excursive movements, and protrusive movements. If any of the above 3 means was detected, the patient would be defined as having a joint crepitus sound; on the contrary, if none of the above 3 means was detected, the patient was defined as not having a joint crepitus sound. TMJ arthralgia was evaluated by the visual analog scale (VAS) questionnaire after palpating the TMJ region and inquiring about patient's pain when asking them to make maximal mouth opening and mandible movements. A VAS score of 0 represents no pain, whereas a score of 10 represents pain beyond toleration. We evaluated TMD-associated headache and myofascial pain with referral as follows: the physician inquired about any pain after palpating the temporalis, shoulder-neck, and masseter regions. DoM was recorded when the patients are asked to make mouth opening movements with a soft caliper placed in the middle of the central incisor. MIO is to measure a patient's maximum mouth opening (in mm) and make a record using a hard caliper. At the stage of CROS treatment, all records were made during a follow-up visit for patients every month (return visits were shifted to an earlier time if there were changes in symptoms); at the stage of LPCGF injection, records were made in the first week, and then once per month until the 6th month after injection. The same physician completed all evaluations, and the same assistant completed all of the above recordings.
2.4. Data analysis
The authors entered data in an Excel worksheet and analyzed it with SPSS statistical software (version 20 for Windows; IBM, New York, NY). Comparing the disparity before and after CROS treatment, the McNemar chi-squared test was adopted in the presence of categorical variables. The study applied the Kolmogorov–Smirnov test to examine whether continuous variables had a normal distribution. Then, TMJ arthralgia VAS and MIO did not present a normal distribution in the Kolmogorov–Smirnov test. Pain VAS and MIO were analyzed with the Wilcoxon signed-rank test for non-normal distributions; multiple regression was used for analysis in comparing the variables between different time trends, and the proportional test was used for analysis in comparing the incidences of joint crepitus sounds. The level of significance was set at 0.05 for all statistical tests.
2.5. Ethics
To protect patients, this clinical study explored the physical medical research standards that complied with the Declaration of Helsinki[20] and that underwent verification by Tainan Sin Lau Hospital's committee of medical ethics were issued with a license. The license number was (protocol number SLH919–105–007). All the patients signed an informed consent form. All the authors are accountable for the integrity and authenticity of this study material.
3. Results
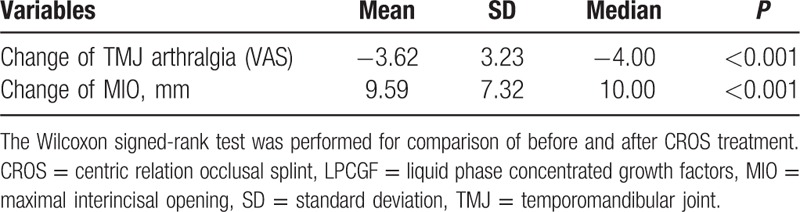
Among the 29 patients, there were 6 males (20.68%) and 23 females (79.31%). Distribution by age ranged from 15 to 84 years (mean age 39.55 ± 15.49 years). The mean time affected by TMD was 856.5 ± 1635.8 days (min: 7.0, max: 7200.0, median: 120.0). The mean time of CROS treatment was 345.0 ± 161.3 days (min: 8.0, max: 722.0, median: 309.0). DoM, TMD-associated headache, and myofascial pain with referral all improved significantly after CROS treatment; namely, 16 had deviation on the first visit; of these, the symptoms of 11 patients were relieved after CROS treatment (P < 0.05). There were 13 with TMD-associated headache on the first visit; of these, the symptoms of 10 patients were relieved after CROS treatment (P < 0.05). There were 27 patients with myofascial pain with referral on the first visit; of these, the symptoms of 19 patients were relieved after CROS treatment (P < 0.05). Nevertheless, the symptoms of joint sound did not improve significantly (P > 0.05) (Table 1). TMJ arthralgia VAS scored 4.72 ± 2.58 in average on the first visit and scored 1.10 ± 1.72 after CROS treatment, with the mean degree of pain declining by 3.62 ± 3.23 (P < 0.001). Mean MIO was 37.48 ± 6.66 mm on the first visit, and MIO was 47.07 ± 6.08 mm after CROS treatment, increasing on average by 9.59 ± 7.32 mm (P < 0.001) (Table 2).
Table 1.
Comparison of categorical indicators before and after CROS treatment (n = 29).
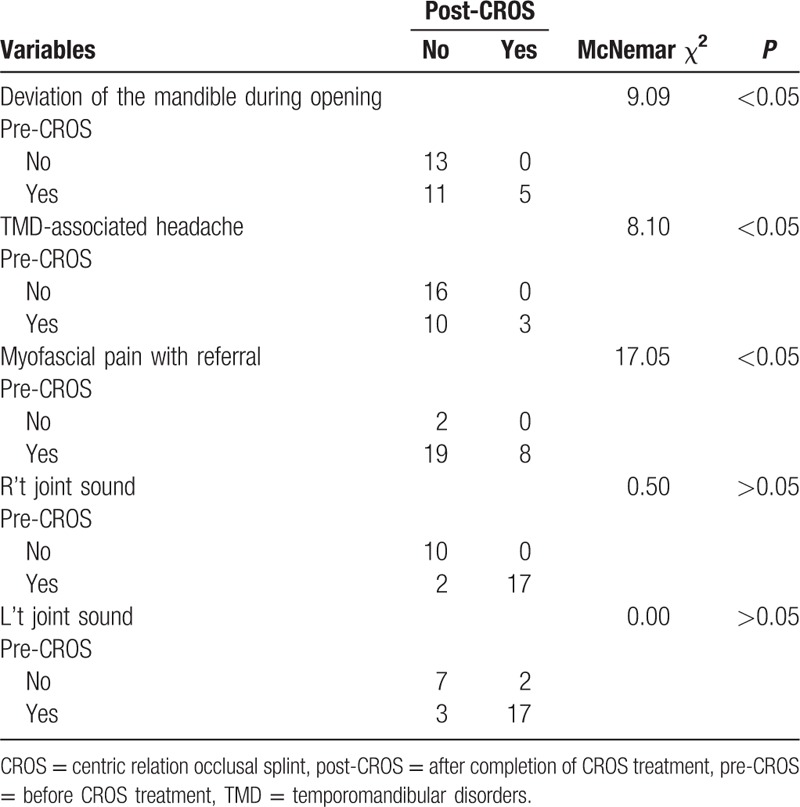
Table 2.
Comparison of changes in the continuous indicators before and after CROS treatment (n = 29).
No severe complications occurred after the 36 joints in total among the 29 patients who received the LPCGF injection. The only slight sensation of swelling and aching was felt over the TMJ region during the first 3 days. No significant differences in DoM, TMD-associated headache, myofascial pain with referral, TMJ arthralgia, and MIO were observed before and after LPCGF injection (P > 0.05). Prior to the injection, the joint crepitus sound was observed in 100% of the patients (36 joints). In the first postinjection week, the number of patients with symptoms dropped by 42% compared with preinjection (P < 0.001). In the first postinjection month, the number of patients still with symptoms dropped by 50% compared with preinjection (P < 0.001). In the fourth postinjection month, the number of patients still with symptoms dropped by 50% compared with preinjection (P < 0.001) (Fig. 2). Among the 36 joints, 26 exhibited relief of the joint crepitus sound after 2 mL of LPCGF was injected. The mean time of symptom relief was 48.5 ± 64.1 days (min: 7.0, max: 258.0, median: 21.5).
Figure 2.
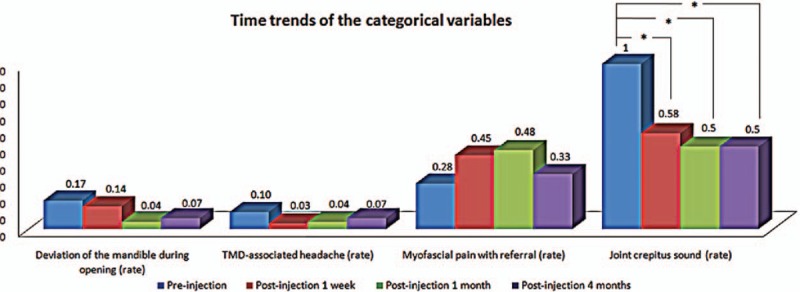
Time trends of the categorical variables. ∗P < 0.001.
4. Discussion
The objective of this study was to present all the clinical discoveries after CROS treatment and treatment with DDWOR using LPCGF injection. The authors hypothesized that LPCGF could improve the clinical symptoms beyond treatment with CROS. This hypothesis was substantiated after a single injection of 2 mL of LPCGF at the joint on the affected side of TMD.
The study by Ohnuki et al used arthroscopic surgery to treat closed lock of the jaw, and 1 year later, none of the disc positions improved, as shown by MRI; instead, the disc was displaced forward to a higher degree and caused a high deformity rate.[21] In the present study, patients with improvement in the joint crepitus sound underwent MRI examination, and the results were consistent with the study of Ohnuki et al; that is, disc position was not improved. Additionally, the authors checked cone-beam computed tomography before CROS treatment for these patients to rule out degenerative joint disease (DJD). However, the authors could not find evidence of erosion, osteophytes, subchondral cyst, and generalized sclerosis. The authors speculated that these patients were at the stage between DDWOR and initial DJD because the time affected by TMD was >2 years. After CROS treatment, the authors still had no evidence to prove DJD. Therefore, these patients were still categorized to DDWOR. The authors discovered that even for the relatively severe DDWOR in TMD taxonomy, DoM, TMD-associated headache, myofascial pain with referral, TMJ arthralgia, and MIO could attain a statistically significant improvement after 3-mm CROS treatment. Among the clinical symptoms, only the joint crepitus sound did not exhibit significant improvement beyond that of CROS (7.69%, P > 0.05). This was consistent with the findings of Wassell et al. According to their comparison of the effects of stabilization splint, other symptoms of TMD relative to the TMJ clicking sound all retained a degree of progress in the post-treatment year, and 81% of the patients unanimously believed that their symptoms entirely or partially improved.[5] CROS treatment relieves the symptoms of disc displacement not by improving the disc position but is related to the basic decoupling of neuromuscular reflex mechanism and the reduction of TMJ stress.[22] Therefore, the authors speculated that the improvement might be attributed to reducing abnormal muscle activity, producing neuromuscular balance, widening the joint space, lowering disc adhesion, and shifting the range of disc mobility.
In recent years, it has been clearly demonstrated that TMD enables extracellular matrix, collagen, and other excessive metabolic reactions to change the microenvironment around the TMJ so that the cartilage degenerates and the subchondral bone is damaged.[23] Aside from the displacement of the disc position, pain and TMJ dysfunction are also related to the pressure in joint and the cytokine level in synovial fluid.[24] All these factors are beyond the solution of CROS. By injecting PRP into the joint, not only does the volume of the joint cavity expand so that the internal pressure in the joint can be modulated, but growth factors are synthesized through the alpha granule in degranulation plaques.[10] The growth factors can restore the disc, capsule, and retro-discal pad[25] and achieve efficacy in inhibiting pro-inflammatory cytokines by inhibiting interleukin-1 released by the activated macrophages.[26] Kutuk et al performed animal experiments on rabbits and showed that PRP can help regenerate new bone, fibrocartilage, hyaline cartilage, and better ultrastructural architecture of the collagen fibrils.[27] In the present study, among the 36 joints of the 29 patients involved, 26 patients with joint crepitus sound were relieved of symptoms for an average of 48.5 ± 64.1 days after 2 mL of LPCGF was injected, and no one progressed to DJD. The authors deduced that LPCGF might inhibit more pro-inflammatory cytokines, produce more collagen fibrils, modulate more joint pressure, widen the joint space, and shift the disc mobility range because of stronger and purer growth factors relative to PRP.
According to Hanci et al, their experimental group included 10 DDWR patients for whom conservative treatments were ineffective. Six months after 0.6 mL of PRP was injected once into 17 joints in total, the clinical symptoms all exhibited statistically significant improvement. Among them, the VAS pain score dropped from 6.69 ± 2.21 to 0.07 ± 0.27 (P < 0.05), MIO ascended from preinjection 32 ± 8.53 mm to 39.7 ± 10.39 mm (P = 0.01), and only 2/12 of the joint sounds remained unrelieved (P < 0.05).[16] In the present study, the unrelieved rate of the joint crepitus sound was 27.78% (10/36) on the 48th day, whereas the unrelieved rate after 6 months was 16.67% (2/12) in the study by Hanci et al. The proportional test was utilized to compare the present study with that by Hanci et al. The result of unrelieved rate under investigation was z = 0.77 (P > 0.05). In other words, the patients with severe DDWOR could achieve the same result as the study by Hanci et al regarding the relieved rate of joint crepitus sound for 48 days after injection treatment with 2 mL of LPCGF. This result suggested that 2 mL of LPCGF was more applicable to TMD than 0.6 mL of PRP, especially for severe DDWOR. The authors assumed that 2 mL might be the most efficient volume because it is the highest volume of joint space. Kim et al also demonstrated that the average volume of the joint space was 1490.5 ± 512.2 mm3 (between 1 and 2 mL).[28] Targeted at 10 DDWR-equivalent patients at RDC/TMD Stage IIa without effect over 3 months after the occlusal splint treatment, the study by Pihut et al found that the intensity of pain significantly improved 6 weeks after the injection of 0.5 mL of PRP, with a mean decrease in VAS score from 6.5 to 0.6 (P = 0.00005).[9] The study by Machon et al focused on 10 patients with osteoarthritis (OA) of the experimental group. These 10 patients received an occlusal splint, arthrocentesis, hyaluronic acid injection, and arthroscopic lavage without any improvement. After 1 mL of PRP was injected once every other 2 weeks and twice in total over 3 months of observation, it was discovered that the average VAS pain score dropped from 7.3 ± 0.55 to 4.1 ± 0.77 (P = 0.005) by 3.2 ± 0.81 in total and that MIO improved by 1.6 ± 1.0 mm over the 3 months from preinjection to postinjection.[17] The study by Giacomello et al targeted 13 patients in whom articular pain was continuously present after a mandibular repositioning splint. After 1.5 to 2 mL PRP was injected once every other month and twice in total over 6 months of observation, it was discovered that the VAS pain score dropped from 7.69 ± 1.9 to 0.23 ± 0.65 (P < 0.0001), and MIO increased from preinjection 30.15 ± 4.44 to 39.54 ± 4.55 mm (P < 0.0001) at the 6th postinjection month, which improved by 9.38 ± 2.21 mm in total.[15] The above 3 studies by Pihut et al, Machon et al, and Giacomello et al have all been mentioned in their inclusion conditions. Their groups under investigation have all undergone treatment by occlusal splint without any improvement. It was only after PRP injection was received that the intensity of pain and MIO were improved. However, the present study found that MIO, TMJ arthralgia, myofascial pain with referral, and other clinical symptoms had very good reactions to CROS treatment. In the study by Machon et al, the patients had no reaction to other treatments such as arthrocentesis, hyaluronic acid injection, and arthroscopic lavage, except for the occlusal splint, which was ineffective; it was only effective for PRP. As far as the authors’ clinical experience, groups like this are clinically rare. The study by Hegab et al targeted 50 patients with TMJ osteoarthritis (TMJOA) who had never received any treatment for TMD. The 25 patients in the experimental group received a 1-mL PRP injection once every other week and thrice in total, and all received arthrocentesis with 50 mL of Ringers lactate solution prior to PRP injection. Over 12 months of observation, it was discovered that VAS dropped from 7.36 ± 1.11 to 0.4 ± 0.76 (power = 97%), whereas MIO increased from 33.88 ± 3.09 to 41.56 ± 2.31 mm. The joint sound significantly improved in the first 3 postinjection months, without a significant difference from 3 to 6 months; after 12 months, the incidence of joint sounds decreased dramatically compared with the previous 3 months, but without a significant difference compared with the later 6 months.[14] Comparing the inclusion conditions of this study with the previous 3, the authentic effect of PRP could be noticed in terms of VAS and MIO because the patients had not received any treatment prior to PRP injection. However, because they had received arthrocentesis with 50 mL of Ringers lactate solution, the findings of Hegab et al cannot completely exclude the effect of the treatment of arthrocentesis. Additionally, although they were injected with PRP once every other week and 3 times in total, the presentation of the improvement in joint sounds was not clear enough. The study by Kilic et al targeted 30 patients who had never undergone treatment protocols for TMJOA without improvement. Arthrocentesis applied with 100 mL of Ringers lactate solution to the 32 joints in a total of 18 patients in the experimental group before a 1-mL PRP injection once per month and 4 times in total. Over 1 year of observation, it was discovered that VAS pain complaints dropped from preinjection 5.70 ± 1.35 to 1.02 ± 1.88 (P < 0.001), MIO dropped from preinjection 38.72 ± 7.84 mm to postinjection 38.39 ± 8.02 mm (P > 0.05), and the joint sound VAS score dropped from preinjection 5.48 ± 3.46 to 0.70 ± 0.85 (P < 0.001). The joint sound assessment in this study adopted self-perceived pathologic noise intensity during joint movement. The joint sound was evaluated with a VAS score of patients’ self-perceived pathologic noise intensity. However, as far as this research experience is concerned, evaluation, with or without joint sound, must be concurrently subjective and objective. Thus, it might be inappropriate to evaluate joint sounds using the VAS score to evaluate any reaction to treatments except PRP, but provided no data about the lack of reaction; except for the study by Hanci et al, other studies failed to discuss the observation of joint sound clearly.
It remains inconclusive whether CROS treatment or invasive surgery is superior. The present study adopted treatment with CROS and an intra-articular joint LPCGF injection to relieve clinical symptoms. The authors suggest that CROS be worn continuously after injection. This order of treatment is accepted by patients. This study presented complete situations that would likely be encountered in clinical treatment. Although the efficacy of CROS treatment remains disputed, it is undeniably the most widely used conservative therapeutic method. The present study presented the effectiveness of CROS in alleviating TMJ arthralgia, MIO, myofascial pain with referral, and TMD-associated headache and deviation, as well as the extraordinary effectiveness of LPCGF in joint crepitus sounds. Through this course, a second injection can be considered if symptoms of joint sound remain unrelieved until the 48th postinjection day. Because no symptoms significantly relapsed during a maximum follow-up period of 258 days, we suggest that 2 mL of LPCGF is highly stable and effective when used to assuage TMD.
The limitations of this study include the following points. First, the study design was a retrospective cohort design, for which the bias produced was unavoidable. Second, due to the small sample size, external validity remains inadequate to be expanded and extended. Third, because CROS is continuously worn after injection, there is no way to entirely preclude the realization that the improvement in symptoms has benefited from the delayed efficacy of CROS. This remains possible even though experience and other studies suggest that the joint crepitus sound of TMD cannot completely rely on CROS for improvement. Lastly, the operation requires physicians to be highly skillful. Because the test tubes contain no anticoagulant, the injection must be completed within 5 min; otherwise, the blood will become coagulated and LPCGF cannot be been extracted.
In conclusion, CROS alone can relieve the clinical symptoms of TMD except for joint sounds. Approximately 72.2% of joint sounds can be improved on 48 days once 2 mL of LPCGF is injected. A second injection can be considered if symptoms remain unrelieved 48 days after injection. Comparisons are still required in the future with the effects of other therapeutic methods.
Acknowledgments
The authors would like to thank Professor Wei-Fan Chiang, a long-term advisor and instructor who provided training in surgical skills.
Footnotes
Abbreviations: CROS = centric relation occlusal splint, DC/TMD = diagnostic criteria for TMD, DDWOR = disc displacement without reduction, DDWR = disc displacement with reduction, DJD = degenerative joint disease, DoM = deviation of the mandible during opening, LPCGF = liquid phase concentrated growth factor, MRI = magnetic resonance imaging, MIO = maximal interincisal opening, PRP = platelet-rich plasma, TMD = temporomandibular disorders, TMJ = temporomandibular joint, TMJOA = temporomandibular joint osteoarthritis, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Guo C, Shi Z, Revington P. Arthrocentesis and lavage for treating temporomandibular joint disorders. Cochrane Database Syst Rev 2009;7:CD004973. [DOI] [PubMed] [Google Scholar]
- [2].Gencer ZK, Ozkiris M, Okur A, et al. A comparative study on the impact of intra-articular injections of hyaluronic acid, tenoxicam and betametazon on the relief of temporomandibular joint disorder complaints. J Craniomaxillofac Surg 2014;42:1117–21. [DOI] [PubMed] [Google Scholar]
- [3].Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Ani Z, Gray RJ, Davies SJ, et al. Stabilization splint therapy for the treatment of temporomandibular myofascial pain: a systematic review. J Dent Educ 2005;69:1242–50. [PubMed] [Google Scholar]
- [5].Wassell RW, Adams N, Kelly PJ. The treatment of temporomandibular disorders with stabilizing splints in general dental practice: one-year follow-up. J Am Dent Assoc 2006;137:1089–98. [DOI] [PubMed] [Google Scholar]
- [6].Manfredini D, Marini M, Pavan C, et al. Psychosocial profiles of painful TMD patients. J Oral Rehabil 2009;36:193–8. [DOI] [PubMed] [Google Scholar]
- [7].Costa YM, Porporatti AL, Stuginski-Barbosa J, et al. Additional effect of occlusal splints on the improvement of psychological aspects in temporomandibular disorder subjects: a randomized controlled trial. Arch Oral Biol 2015;60:738–44. [DOI] [PubMed] [Google Scholar]
- [8].Rigon M, Pereira LM, Bortoluzzi MC, et al. Arthroscopy for temporomandibular disorders. Cochrane Database Syst Rev 2011;5:CD006385. [DOI] [PubMed] [Google Scholar]
- [9].Pihut M, Szuta M, Ferendiuk E, et al. Evaluation of pain regression in patients with temporomandibular dysfunction treated by intra-articular platelet-rich plasma injections: a preliminary report. BioMed Res Int 2014;2014:132369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004;62:489–96. [DOI] [PubMed] [Google Scholar]
- [11].Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol 2012;13:1231–56. [DOI] [PubMed] [Google Scholar]
- [12].Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg 2001;107:229–37. [DOI] [PubMed] [Google Scholar]
- [13].Kiliç SC, Güngörmüş M, Sümbüllü MA. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis alone in the treatment of temporomandibular joint osteoarthritis? A randomized clinical trial. J Oral Maxillofac Surg 2015;73:1473–83. [DOI] [PubMed] [Google Scholar]
- [14].Hegab AF, Ali HE, Elmasry M, et al. Platelet-rich plasma injection as an effective treatment for temporomandibular joint osteoarthritis. J Oral Maxillofac Surg 2015;73:1706–13. [DOI] [PubMed] [Google Scholar]
- [15].Giacomello M, Giacomello A, Mortellaro C, et al. Temporomandibular joint disorders treated with articular injection: the effectiveness of plasma rich in growth factors—Endoret. J Craniofac Surg 2015;26:709–13. [DOI] [PubMed] [Google Scholar]
- [16].Hanci M, Karamese M, Tosun Z, et al. Intra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesis. J Craniomaxillofac Surg 2015;43:162–6. [DOI] [PubMed] [Google Scholar]
- [17].Machon V, Rehorová M, Šedý J, et al. Platelet-rich plasma in temporomandibular joint osteoarthritis therapy: a 3-month follow-up pilot study. Arthritis 2013;2:1–4. [Google Scholar]
- [18].Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 2011;74:772–7. [DOI] [PubMed] [Google Scholar]
- [19].Murakami K, Hosaka H, Moriya Y, et al. Short-term treatment outcome study for the management of temporomandibular joint closed lock: a comparison of arthrocentesis to nonsurgical therapy and arthroscopic lysis and lavage. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 1995;80:253–7. [DOI] [PubMed] [Google Scholar]
- [20].World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- [21].Ohnuki T, Fukuda M, Nakata A, et al. Evaluation of the position, mobility, and morphology of the disc by MRI before and after four different treatments for temporomandibular joint disorders. Dentomaxillofac Radiol 2006;35:103–9. [DOI] [PubMed] [Google Scholar]
- [22].Miernik M, Więckiewicz W. The basic conservative treatment of temporomandibular joint anterior disc displacement without reduction—review. Adv Clin Exp Med 2015;24:731–5. [DOI] [PubMed] [Google Scholar]
- [23].De Leeuw R, Klasser GD. Orofacial pain: guidelines for assessment, diagnosis, and management. Am J Orthod Dentofacial Orthop 2008;134:171. [Google Scholar]
- [24].Nishimura M, Segami N, Kaneyama K, et al. Comparison of cytokine level in synovial fluid between successful and unsuccessful cases in arthrocentesis of the temporomandibular joint. J Oral Maxillofac Surg 2004;62:284–7. [DOI] [PubMed] [Google Scholar]
- [25].Springer, Woodell-May JE, Pietrzak WS. Platelet-rich plasma in orthopedics. Musculoskeletal Tissue Regeneration 2008;547–68. [Google Scholar]
- [26].Woodall J, Jr, Tucci M, Mishra A, et al. Cellular effects of platelet rich plasmainterleukin1 release from PRP treated macrophages. Biomed Sci Instrum 2007;44:489–94. [PubMed] [Google Scholar]
- [27].Kutuk N, Bas B, Soylu E, et al. Effect of platelet-rich plasma on fibrocartilage, cartilage, and bone repair in temporomandibular joint. J Oral Maxillofac Surg 2014;72:277–84. [DOI] [PubMed] [Google Scholar]
- [28].Kim JY, Kim BJ, Park KH, et al. Comparison of volume and position of the temporomandibular joint structures in patients with mandibular asymmetry. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:772–80. [DOI] [PubMed] [Google Scholar]


