Abstract
Introduction:
Cyclin D1-positive tumor cells are commonly found in mantle cell lymphoma but they are very rare in diffuse large B-cell lymphoma.
Clinical findings/Patient concerns:
Here we present a rare case of cyclin D1-positive diffuse large B-cell lymphoma in the right tonsil of a 50-year-old man. Computed tomographic imaging detected a mass, about 2.5 cm × 1.8 cm in size, in the left side of the oropharynx.
Diagnoses:
Microscopically, the tumor cells were located under the pharyngeal mucosa and diffusely arranged. The tumor cells were large, with marked nuclear atypia. On performing immunohistochemistry, the tumor cells showed diffuse positive staining for CD10, CD20, cyclin D1, and Pax-5, and negative staining for CD3, CD15, CD30, CD56, and CK. Bcl-6 and Mum-1 expression were observed in 60% and 80% of tumor cells, respectively. The tumor Ki67 index was about 60%. Based on these findings, The tumor was diagnosed as a rare cyclin D1-positive diffuse large B-cell lymphoma rather than a mantle cell lymphoma.
Conclusion:
Cyclin D1-positive large B-cell lymphoma is rare, but as large B-cell lymphoma is a common type of lymphoma, cyclin D1-positive large B-cell lymphoma should be considered a major possibility during differential diagnosis, including in the tonsils.
Keywords: case report, cyclin D1, large B-cell lymphoma, lymphoma, mantle cell lymphoma
1. Introduction
Cyclin D1 is a useful marker for distinguishing mantle cell lymphoma from other types of small B-cell lymphoma.[1] Overexpression of cyclin D1 in mantle cell lymphoma is due to the t(11;14)(q13;q32)/CCND1-IGH translocation/fusion.[1] However, it is also found to be overexpressed in other hematopoietic and lymphoid tumors. Ehinger et al[2] reported that the positive rate of cyclin D1 expression in 231 diffuse large B-cell lymphomas (DLBCLs) was about 4%. The main differentiation of cyclin D1-positive DLBCL is the pleomorphic blastoid subtype of mantle cell lymphoma, which has large tumor cells with marked atypia.[1] Here, we report a cyclin D1-positive DLBCL in the right tonsil in a 50-year-old man. The tonsils belong to Waldeyer ring, which is the most common site of lymphoma involvement in the head and neck. The most common histological type of non-Hodgkin lymphoma in the tonsils is DLBCL.[3] However, cyclin D1-positive DLBCLs in the tonsils are rare.
2. Case presentation
2.1. Clinical history
A 50-year-old man was referred to our hospital for a 1-month history of discomfort during swallowing. The patient had experienced weight loss of about 5 kg, but no fever or pruritus during this period. He had no previous history of severe disease or tumors. The patient had no family history of tumors of the lymphatic and hematopoietic systems. A left tonsil tumor was suspected following fiberoptic laryngoscopic examination and computed tomographic imaging. A clear diagnosis was not made until the pathological examination was performed. The patient had not received any prior therapy for lymphoma. The International Prognosis Index of the patient was 1 (low risk). He received radiotherapy and chemotherapy (6 courses of CHOP) after positive diagnosis and the tumor in the right tonsil shrank by more than 50%.
3. Materials and methods
The resected tumor samples were embedded in paraffin blocks and sectioned. Immunohistochemistry was performed using an SP kit (Maixin Biotechnology, Fuzhou, Fujian, China) according to the manufacturer's instructions. The sections were incubated overnight at 4 °C with the following primary antibodies: Bcl-2 (1:200, Dako, Carpinteria, CA), Bcl-6 (1:100, Dako), CD10 (1:100, Dako), CD15 (1:100, Dako), CD3 (1:100, Dako), CD20 (1:100, Dako), CD21 (1:100, Dako), CD30 (1:100, Dako), CD56 (1:200, Dako), CK (Pan) (1:200, Dako), Cyclin D1 (1:200, Dako), Ki-67 (1:200, Dako), Mum-1 (1:100, Dako), and Pax-5 (1:100, Dako). Immunostaining showing a granular brown substance in the appropriate subcellular locations was considered positive. This study was prospectively performed and approved by the institutional Ethics Committees of China Medical University and conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
4. Results
4.1. Imaging and gross features
Figure 1 shows the computed tomographic imaging. A mass, about 2.5 cm × 1.8 cm in size, was observed in the left side of the oropharynx (Fig. 1A, B, white arrow). The boundary of the mass was not clear. The parapharyngeal space had disappeared. Two enlarged lymph nodes were detected in the right side of the neck, of which the largest one was about 2.7 cm × 2.3 cm (Fig. 1B, black arrow). No enlarged lymph nodes were detected in the left side of the neck. The fiberoptic laryngoscope revealed that the right tonsil was bulging. A biopsy specimen was obtained from the right tonsil. The specimen was about 0.5 cm in size, and the cut surface was gray-white.
Figure 1.
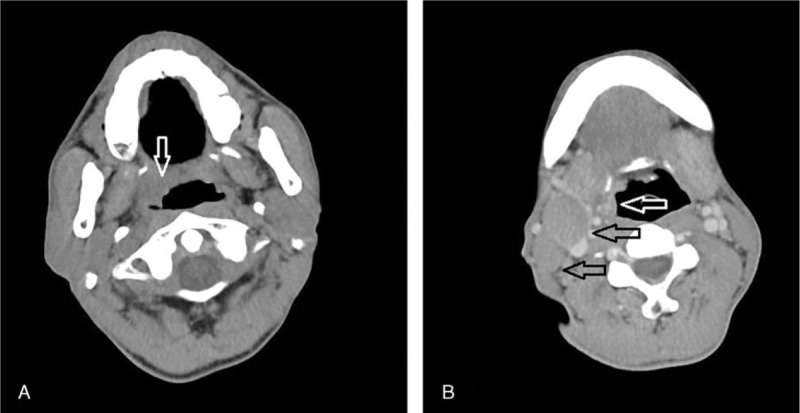
Computed tomographic (CT) imaging of the tumor. A mass about 2.5 cm × 1.8 cm was detected in the left side of the oropharynx (A, B, white arrow). The boundary of the mass was not clear. There were 2 enlarged lymph nodes in the left side of the neck (B, black arrow).
4.2. Microscopic features
Figure 2 shows the histological findings of the tumor. The surface of the tissue was covered with stratified squamous cells (A). The tumor lymphoid cells proliferated under the mucosa and were diffusely arranged (A, B). The tumor cells were dense and showed no clear nodular structure. There were scattered eosinophilic cells and tumor cell debris in the tumor tissues (C). The tumor cells were large, and the cell boundaries were not clear. The nuclei of the tumor cells were large and round or irregular, with sparse chromatin and single or several prominent nucleoli. Mitoses were >10/10 high-power fields (D).
Figure 2.

Morphological features of the tumor. The tumor was located under the mucosa (A). The tumor cells were diffusely arranged and showed no clear nodular structure (A, B). Scattered eosinophilic cells and tumor cell debris were found in the tumor tissues (C). The tumor cells were large and showed notable atypia (D).
4.3. Immunophenotype
Figure 3 shows the immunostaining features of the tumor. The tumor cells were diffusely positive for Bcl-2, CD10, CD20, cyclin D1, and Pax-5. The tumor cells were negative for CD3, CD5, CD21, CD30, CD56, and CK. The percentages of tumor cells positive for Bcl-6 and Mum-1 were 60% and 80%, respectively. The Ki67 index was about 60%.
Figure 3.

Immunostaining features of the tumor. Bcl-2, CD10, CD20 Cyclin D1, and Pax-5 were diffusely positive in the tumor cells. The positive rates of Bcl-6 and Mum-1 were 60% and 80%, respectively. CD3, CD15, CD21, CD30, CD56, and CK were negative in the tumor cells. The Ki67 index was about 60%.
5. Discussion
Lymphoma is a very common malignant disease in the head and neck region.[4] DLBCL is the most common histological type of non-Hodgkin lymphoma in the tonsils.[1] However, cyclin D1-positive DLBCL is rare. The rate of expression of cyclin D1 in DLBCL was about 4% according to the findings of Ehinger et al.[2] In the present study, we report a DLBCL with cyclin D1 expression in the right tonsil in a 50-year-old man.
As cyclin D1 expression is generally positive in mantle cell lymphoma, it is a very critical marker for the diagnosis of mantle cell lymphoma. However, about 2% of these tumors are cyclin D1 negative.[5] In addition, cyclin D1 has also been found to be positive in other histological types of hematopoietic and lymphoid tumors. For example, cyclin D1 was found to be overexpressed in about half the plasma cell myelomas.[6] Overexpression of cyclin D1 was also detected in a case of chronic lymphocytic leukemia.[6] The blastoid subtype of mantle cell lymphoma is the main differentiation for cyclin D1-positive DBCLC. Expression of CD10 is commonly negative in both the common mantle cell lymphoma and the blastoid subtype.[7] Expression of Bcl-6 is also commonly negative in mantle cell lymphoma.[8] In this case, the tumor cells were positive for both CD10 and Bcl-6, findings that were not consistent with a diagnosis of mantle cell lymphoma. The subtype of DLBCL in the present case was the germinal center type based on diffuse CD10 expression in the tumor cells. In the study from Han, CD10 expression was positive in all 7 reported cases.[9] The reports from Ok did not show an association between cyclin D1 expression and the subtype of DLBCL.[10]
Cyclin D1 plays critical roles in accelerating cell cycle progression.[12,13] Cyclin D1 is generally overexpressed in mantle cell lymphoma and has been considered a critical oncogene in this tumor owing to its function in promoting the G1/S transition of the cell cycle.[13] Cyclin D1 has also been found to be rarely overexpressed in a few other tumors of the lymphoid hematopoietic system, including DLBCL.[1,2,6,13] Al-Kawaaz et al[13] reported a case of DLBCL that demonstrated overexpression of cyclin D1 in the relapsed tumor but not the original tumor, which indicates that cyclin D1 may also be involved in tumor progression. According to the reports from Han et al,[9] patients with DLBCLs in the tonsils tend to have relatively good prognosis. The study by Wang and coworkers[11] indicates that expression of cyclin D1 suggests poor prognosis in patients with DLBCL. However, Ok's study did not find a significant link between cyclin D1 expression and clinical outcome.[10] Qin's et al report[3] showed no significant association between the histological type of non-Hodgkin lymphoma and patients’ clinical outcome. The study by Ok indicated that cyclin D1-positive DLBCL was more often seen in younger males, and there were no significant differences in other clinical features including patient survival in comparison to cyclin D1-negative tumors.[10] The study by Coiffier et al[14] showed that it was more effective to treat patients with DLBCL by adding rituximab to the CHOP regimen than using CHOP alone. Ok's et al study indicated that the patients of the Rituximab-CHOP Consortium Program with cyclin D1-positive DLBCLs had the same overall survival as those with cyclin D1-negative tumors.[10] The factors affecting the clinical outcome of patients with cyclin D1-positive DBCLC still need to be investigated.
6. Conclusion
Cyclin D1-positive large B-cell lymphomas are rare. The main differentiation is pleomorphic mantle cell lymphoma. Cyclin D-positive large B-cell lymphoma is rare, but as large B-cell lymphoma is a common type of lymphoma, cyclin D1-positive large B-cell lymphoma should be considered a major possibility in the differential diagnosis, even in cases in which tumor cells are positive for cyclin D1, including in the tonsils. The gene profiles and the immunostaining features may be useful in differentiating these tumors. The prognosis still requires further investigation.
Footnotes
Abbreviations: CT = computed tomographic, DLBCL = diffuse large B-cell lymphoma, HPF = high-power field, IPI = International Prognosis Index.
Funding/support: This work was supported by the National Natural Science Foundation of China (no. 81472599 to CF).
The authors have no conflicts of interest to disclose.
References
- [1].Rodriguez-Justo M, Huang Y, Ye H, et al. Cyclin D1-positive diffuse large B-cell lymphoma. Histopathology 2008;52:900–3. [DOI] [PubMed] [Google Scholar]
- [2].Ehinger M, Linderoth J, Christensson B, et al. A subset of CD5- diffuse large B-cell lymphomas expresses nuclear cyclin D1 with aberrations at the CCND1 locus. Am J Clin Pathol 2008;129:630–8. [DOI] [PubMed] [Google Scholar]
- [3].Qin Y, Shi YK, He XH, et al. Clinical features of 89 patients with primary non-Hodgkin's lymphoma of the tonsil. Ai Zheng 2006;25:481–5. [PubMed] [Google Scholar]
- [4].Kaur P, Nazeer T. B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma presenting in the tonsil: a case report and review of literature. Am J Otolaryngol 2004;25:121–5. [DOI] [PubMed] [Google Scholar]
- [5].Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol 2015;90:739–45. [DOI] [PubMed] [Google Scholar]
- [6].Vela-Chávez T1, Adam P, Kremer M, et al. Cyclin D1 positive diffuse large B-cell lymphoma is a post-germinal center-type lymphoma without alterations in the CCND1 gene locus. Leuk Lymphoma 2011;52:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parrens M, Belaud-Rotureau MA, Fitoussi O, et al. Blastoid and common variants of mantle cell lymphoma exhibit distinct immunophenotypic and interphase FISH features. Histopathology 2006;48:353–62. [DOI] [PubMed] [Google Scholar]
- [8].Jaffe ES, Harris NE, Stein H, et al. WHO Classification of Tumors of Heamatopoitic and Lymphoid Tissues. Lyon: IRAC; 2003. [Google Scholar]
- [9].Han TT, Wang L, Fan L, et al. Clinical pathological analysis of 7 cases of primary tonsil diffuse large B cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014;22:104–7. [DOI] [PubMed] [Google Scholar]
- [10].Ok CY, Xu-Monette ZY, Tzankov A, et al. Prevalence and clinical implications of cyclin D1 expression in diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Cancer 2014;120:1818–29. [DOI] [PubMed] [Google Scholar]
- [11].Liang X, Wang J, Bai W, et al. Expression of CD68, cyclin D1 and rearrangement of bcl-6 gene are adverse prognostic factors in diffuse large B-cell lymphoma. Zhonghua Bing Li Xue Za Zhi 2015;44:559–64. [PubMed] [Google Scholar]
- [12].Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 2003;15:158–63. [DOI] [PubMed] [Google Scholar]
- [13].Al-Kawaaz M, Mathew S, Liu Y, et al. Cyclin D1-positive diffuse large B-cell lymphoma with IGH-CCND1 translocation and BCL6 rearrangement: a report of two cases. Am J Clin Pathol 2015;143:288–99. [DOI] [PubMed] [Google Scholar]
- [14].Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010;116:2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


