Supplemental Digital Content is available in the text
Keywords: adverse events, double-blind, gout, randomized controlled trial, recurrence of joint swelling and pain, therapeutic effect, traditional Chinese medicine, uric acid
Abstract
Trial Design:
In the double-blind, randomized, controlled trial, we aimed to evaluate the effects of compound tufuling oral liquid (CoTOL) on serum uric acid (sUA) levels and recurrence of acute gouty arthritis in intercritical and chronic gout treatment.
Methods:
A total of 210 patients with gout were screened from 8 hospitals to observe the sUA and acute gouty arthritis recurrence rate-reducing effects of CoTOL in intercritical and chronic gout during a 12-week treatment. We treated 139 and 71 patients with CoTOL and the placebo, respectively, and evaluated their sUA levels, acute gouty arthritis recurrence rate, and adverse events at week 0, 6, and 12.
Results:
Twenty-five and 12 patients in the treatment and control groups, respectively, had interrupted treatments, whereas 114 and 59 cases, respectively, completed their treatments. At the end of the 12-week treatment, the average decrease in sUA was 74.26 (95% confidence interval [CI]: 56.74–91.77 μmol/L) and 28.81 μmol/L (95% CI: 4.91–52.71 μmol/L) in the treatment and control groups, respectively (P = 0.004). The average decrease rate of sUA was 12.76% (95% CI: 9.82%–15.70%) and 4.57% (95% CI: 0.42%–8.71%) in the treatment and control groups, respectively (P = 0.004), and the gouty arthritis recurrence rate of the treatment group was lower than that of the control group (from week 6 to 12, 21.93% and 50.88% in the treatment and control group, respectively, P < 0.001; from baseline to week 12, 38.5% and 63.16%, respectively, P = 0.003). Severe adverse events were not observed in either groups, and fewer leucopenia incidences were observed in the treatment group than those in the control group (3/139 vs. 7/71, respectively, P = 0.033).
Conclusion:
CoTOL reduced sUA levels and effectively prevented acute arthritis recurrence in intercritical and chronic gout without serious adverse events.
1. Introduction
Gout is a metabolic rheumatism characterized by hyperuricemia and recurrent acute gouty arthritis, which is difficult to cure and relapses easily. The etiopathogenesis of gout is related to genetic[1,2] and endocrine factors,[3] purine-rich food intake,[4] and environmental factors,[5] which cause abnormal metabolism, resulting in hyperuricemia. In affected individuals, urate crystals are deposited in the joints, leading to acute gouty arthritis. The prevalence of gout is increasing worldwide[6] and was 1.4% in the UK and Germany from 2000 to 2005,[7] and increased from 6.7 per 1000 inhabitants in 2005 to 9.1 per 1000 inhabitants in 2009 in Italy.[8] A similar trend of increase in gout prevalence has also been observed in China. In Taiwan, the prevalence of a history of gout was found to be 15.2% (25/165) and 4.8% (11/231) in the Aboriginal men and women, respectively, compared with a prevalence rate of 0.3% in non-Aborigines.[9] In 2002, the age-standardized prevalence was 25.3% for hyperuricemia and 0.36% for gout in adults aged 20 to 74 years in Qindao, China.[10]
Conventional western medicines used to treat acute gout include anti-inflammatory and analgesic agents (such as colchicine, nonsteroidal anti-inflammatory drugs [NSAIDs], and glucocorticoids),[11] whereas hypouricemic agents (including allopurinol, benzbromarone,[12] and febuxostat[13]) are used for chronic gout.[14] Unfortunately, although these drugs show some therapeutic effects, they also cause numerous side effects. For example, colchicine, which inhibits leukocyte migration and lactic acid production to decrease inflammatory responses and urate crystal deposition,[15] may also induce abdominal pain, nausea, vomiting, diarrhea, bone marrow depression with aplasticanemia, agranulocytosis, thrombocytopenia, peripheralneuritis, alopecia, reversible azoospermia, myoneuropathy and myopathy, and rhabdomyolysis.[16] NSAIDs may exacerbate epeptic ulcers,[17] renal failure,[18] hypertension, and cardiovascular disease.[19] Similarly, glucocorticoids may exacerbate peptic ulcers,[20] hypertension, hyperlipidemia, and diabetes,[21] which results in patients with gout discontinuing their treatment. Allopurinol is also limited by adverse events such as hypersensitivity reactions and can even cause death. Therefore, it is of great concern to find effective herb medicines[22] and nonpharmacological treatment methods.[23]
In China, numerous traditional Chinese medicines (TCMs) have been used to treat gout for thousands of years. Several studies comparing Chinese herbal decoctions and traditional Western medicines for gout treatment have been reported over the past decade.[24,25] However, the absence of large sample, double-blind, randomized controlled trials (RCTs) affects the reliability and replicability of their conclusions.[26] Therefore, designing and conducting a large-sample double-blind RCT to demonstrate the effectiveness of TCM for gout treatment is of great importance.
Our previous studies showed that CoTOL (also named Quzuo Tongbi recipe), which we developed after years of clinical and experimental research, reduces serum uric acid (sUA) levels in intercritical and chronic gout and alleviates acute arthritis without severe adverse reactions even after long-term use.[27–30] In the present study, we sought to further explore the effect of CoTOL on the sUA levels of patients in the intercritical and chronic gout phase, and the recurrence rate of acute gouty arthritis by conducting a double-blind, multicenter, placebo-controlled, RCT (S1 File).
2. Methods
2.1. Study design
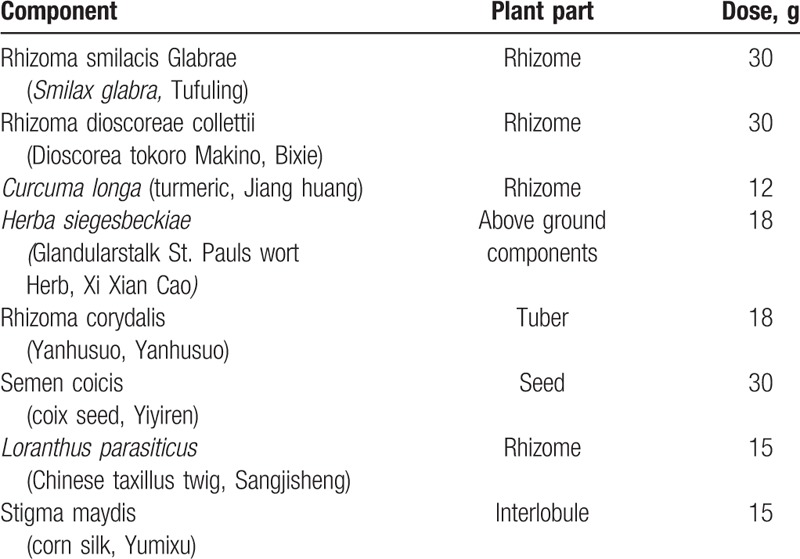
The trial was designed as a prospective, multicenter, randomized, placebo-controlled, and double-blind trial of CoTOL (Table 1) in patients with intercritical and chronic gout, conducted in China. The trial was approved by the Ethics Committee of Zhejiang Chinese Medical University, and the procedures were conducted in accordance with the Declaration of Helsinki 1975, as revised in 2008.[31] Informed consent was given to every participant, and all participants had provided the written informed consent.
Table 1.
Composition of compound tufuling oral-liquid recipe.
2.2. Participants
The inclusion criteria included: male patients, aged 18 to 60 years, with hyperuricemia (blood uric acid [UA] >480 μmol/L), meeting the American College of Rheumatology Classification standard for primary gout revised in 1977 [32] based on the patient's medical history, without gouty arthritis attack at the time of enrolment, and willing to participate in the trial and sign informed consent certificates. The exclusion criteria included patients: with acute gouty arthritis at the baseline, administered a uricosuric medicine within 2 weeks, with secondary gout, with serum creatinine (Scr) >133 μmol/L or urinary calculi, with serious organ dysfunction, mental illness, or cancer, and body mass index >50 kg/m2 or alcoholism. The suspension criteria included: occurrence of serious adverse drug reactions (ADRs) leading to the study team deciding to suspend the patient from the study after adequate evaluation; patients whose sickness worsened after treatment or whose syndrome was influenced by unexpected factors and should be treated as invalid cases; treatment with other gout therapies during the study period, which rendered the participant as an invalid case; and an accumulated treatment interruption of 28 or 21 days at a stretch was regarded as an invalid case. The withdrawal criteria included patients who did not complete the whole treatment course because of serious ADRs or other possible reasons. The cases that were considered withdrawals were managed as follows:
-
(1)
After patients had withdrawn from the study, the researcher coordinators contacted them using every possible method such as personal visits, telephone, or mail to identify their reasons for withdrawing. In addition, the time of their last medication was recorded, and all the possible evaluations were completed.
-
(2)
Patients who withdrew from the study because of allergies or other ADRs were properly treated with other therapies.
-
(3)
All the test data of the withdrawn cases were properly kept as records for use in the total analysis and calculation.
The participants were recruited from June 9, 2012 to May 31, 2013 in 9 hospitals, which were the Rheumatism Institute, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China; Department of Rheumatism, the Second Affiliated Hospital of Zhejiang University Medical College, Hangzhou, Zhejiang, China; Department of Rheumatism, Zhejiang Hospital, Hangzhou, Zhejiang, China; Department of Rheumatism, Chinese Medical Academy Gate Hospital, Beijing, Beijing, China; Department of Rheumatism, Zhejiang People's Hospital, Hangzhou, Zhejiang, China; Department of Rheumatism, Sir Run Run Shaw Hospital of Zhejiang University Medical College, Hangzhou, Zhejiang, China; Department of Rheumatism, the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China; Department of Public Healthy, Medical College, Zhejiang University, Hangzhou, Zhejiang, China; and Department of Rheumatism, the Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China.
This trial was registered at the Chinese Clinical Trial Registry on June 9, 2012 before participant recruitment and is available on the http://www.chictr.org/ChiCTR-TRC-12002245 website. The authors confirm that all ongoing and related trials for this drug/intervention are registered.
3. Randomization and masking
3.1. Sample size
The sample size of the measurement data was calculated by using the superiority test:
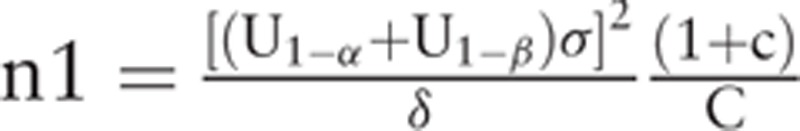 |
(where, σ is calculated by pooled standard deviation Sc, δ is equivalent standard, and c is the ratio of 2 sample size); n2 = c n1; c = 2; α = 0.05, β = 0.1, U1-α = 1.64, and U1-β = 1.28. Previous clinical observations showed that the decreased rate of blood UA level after TCM and placebo treatments was 19.37% ± 17.74% and 2.12% ± 4.23%, respectively and, therefore, σ = 14.74 and δ = 6.8. Based on the above formula, the sample size was set at 210 with an expected 20% withdrawal rate based on data from our previous trials.[27] A moderate treatment duration of 12 weeks was chosen out of consideration for patients receiving placebo and for ethical reasons.
3.2. Stochastic methods
This study adopted the envelope random method. With each center as a block, the Quality Control Department of the Affiliated Hospitals of Nanjing University of Chinese Medicine used the Statistical Analysis Software (SAS) version 9.2 (SAS Institute, Cary, NC) to generate randomized numbers for each group and patient to determine whether they should be assigned to the treatment or control group.
3.3. Masking design
The double-blind method was used to mask the treatments. The researcher coordinators adjusted the basic recipe based on each patient's temporary symptoms and subsequently informed the specially assigned drug management personnel to adjust the procedures afterward. The drug management personnel first obtain each patient's random and group numbers and then informed the pharmacist to decoct their specific treatment recipe or placebo based on the patient's group information (Figs. 1 and Supplementary Fig. 1).
Figure 1.
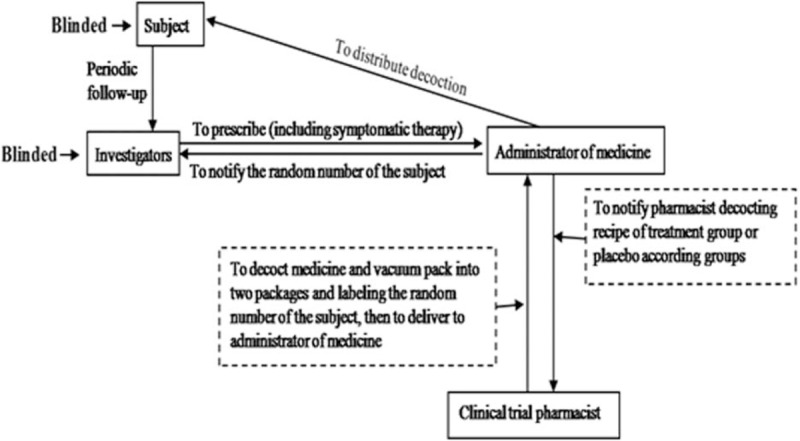
Flowchart of distribution of decoction to implement double-blind trial.
4. Interventions
At baseline, every patient was instructed to reduce their purine-rich food intake and to drink >2 L of water a day. The patients randomly assigned to the treatment and placebo groups were administered CoTOL or the placebo (each 2 packs a day), respectively. The age, course of gout, frequency of acute arthritis over 1 year after treatment, joint function, and comorbidities were recorded for each patient.
The treatment recipe was decocted twice by refluxing with water (1:8, w/v) for 1 hour, and then it was vacuum packed into two 250-mL packages. A rapid-performance liquid chromatography identification method was used to authenticate the compound recipe. The control group patients were treated with a placebo consisting of 20 g each of charred millet sprout (Millet sprout, JiaoGuya) and fructus hordei germinatus (Barley sprout, Maiya), 12 g of charred fructus crataegi (Chinese Hawthorn fruit, JiaoShanzha), and 3 g of edible bitter principle, which each have no therapeutic effects on gout. The placebo solution was prepared using the same procedure used for the study drug and vacuum packed into two 250-mL packages, and the color, taste, smell, and physical form of the placebo were the same as those of the treatment recipe.
5. Measurements
5.1. Efficacy assessments
The primary outcomes were the average decrease and decrease rate of sUA at the end of the 12-week treatment. The average decrease was calculated by using the following formula: C = a − b (where, a and b are the sUAs at baseline, and the end of the 12-week treatment, respectively and C is the decreased sUA value). The decreased rate was calculated by using the formula: R(%) = 100 × (a − b)/a (where, a and b are the sUAs at baseline, and the end of the 12-week treatment, respectively and R is the decreased rate of sUA). The secondary outcome was the decrease in the frequency of recurrent joint swelling or pain. During the 12-week study, fasting venous blood samples were collected in the morning to monitor the biochemical indexes (including sUA). A complete blood count measurement was performed at the baseline as well as at the end of 6 and 12 weeks. The frequency of recurrent joint swelling and pain was recorded at the 6- and 12-week treatment points.
5.2. Safety assessments
The adverse events (AEs) including drug-induced liver or renal injury (alanine transaminase [ALT], aspartate transaminase [AST], Scr, urea nitrogen) and hematological parameters (leukocyte, erythrocyte, hemoglobin, glucose (GLU), total cholesterol (TC), high-density lipoprotein cholesterol [HDL-c], and triglyceride [TG]), and vital signs (systolic blood pressure and diastolic blood pressure) were recorded at all scheduled clinic visits.
5.3. Quality control
Each patient's clinical information was recorded in a case report form (CRF). Then, 2 data entry staff members entered the data into the electronic CRF on the e-clinical study website (http://www.njecdm.com) administered by the Quality Control Department of the China State Clinical Trial Center of TCM, which was assigned by the State Administration of TCM of the People's Republic of China. The data management was performed by using the web-based clinical trial management platform, ResMan. The data were blindly managed and analyzed by the clinical and statistical staff of the Department of Public Health, Medical College, Zhejiang University.
5.4. Statistical analysis
The dataset was first exported from the electronic CRF on the e-clinical study website. The intention-to-treat (ITT) or safety set (SS) analysis included patients who responded to at least 1 follow-up survey, and the per-protocol population set (PPS) analysis included patients who adhered to the treatment protocol. The values are presented as the median (interquartile range) for continuous variable and proportion or ratio for categorical variables. The differences in the decreased value or decreased rate of the sUA between the groups were analyzed using the Wilcoxon signed-rank test. The χ2 test was used to analyze the difference in the proportion or ratio of categorical variables between the groups. The difference in the recurrence of joint swelling and pain between groups during the treatment course was analyzed by using the Wilcoxon signed-rank test. A 2-tailed P value <0.05 was considered statistically significant, and the statistical analyses were performed using the statistical package for the social sciences (SPSS) version 18.0 for Windows (SPSS Inc, Chicago, IL) and the SAS software version 9.2.
6. Results
6.1. Subjects
The flow of the participants through the trial is shown in Fig. 2 and Supplementary Fig. 2. Of the 332 male patients with gout who were screened for eligibility, 114 did not meet the inclusion criteria and 8 did not choose to enroll. Of the 210 patients assessed for eligibility at baseline, 139 and 71 were assigned to the treatment and control groups, and received CoTOL and the placebo, respectively. During the 12-week follow-up, a total of 25 and 12 patients in the treatment and control groups had interrupted treatment. Thus, 114 patients who underwent the complete course of treatment and had endpoint assessment were included in the PPS analysis and 139 were included in the ITT and safety analyses in the treatment group. Furthermore, 59 patients were included in PPS analysis and 71 were included in ITT and safety analyses in the control group.
Figure 2.

Flow diagram of participant enrollment and treatment. Of the 332 male patients with gout screened for eligibility, 114 did not meet the inclusion criteria while eight did not enroll. Of the 210 patients assessed for eligibility at baseline, 139 and 71 were assigned to the treatment and control groups, and received compound tufuling oral-liquid and the placebo, respectively. During the 12-week follow-up, a total of 25 and 12 patients in the treatment and control groups had interrupted treatment. Thus, 114 patients who underwent the complete course of treatment and had endpoint assessment were included in per-protocol population set analysis and 139 were included in ITT and safety analyses in the treatment group. In addition, 59 patients were included in the per-protocol population set analysis and 71 were included in the ITT and safety analyses in the control group. ITT = intention-to-treat, TCMs = traditional Chinese medicines.
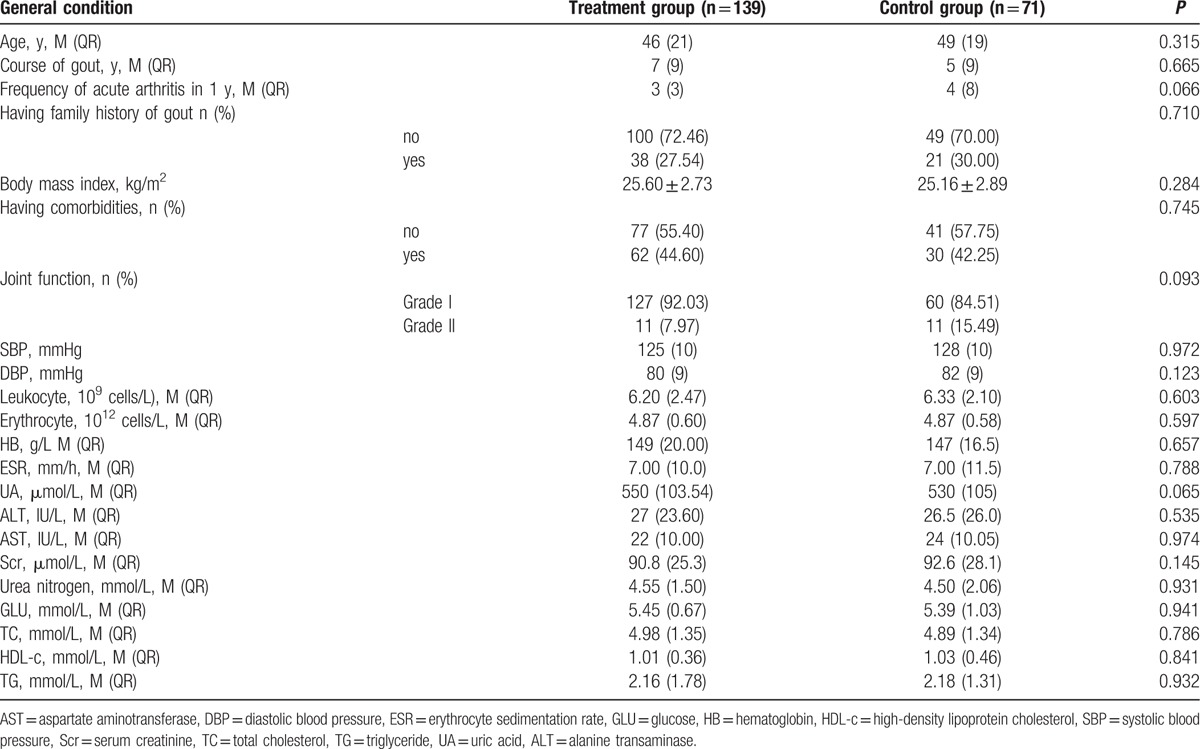
6.2. Baseline characteristics
Table 2 shows the baseline characteristics of the included patients. The demographical and clinical characteristics were well balanced between both groups. The median age was 46 and 49 years in the treatment and control groups, respectively. The median course of disease was 7 and 5 years in the treatment and control groups, respectively. The median frequency of acute arthritis in 1 year was 3 and 4 in the treatment and control groups, respectively. The median sUA levels were 550 and 530 μmol/L in the treatment and control groups, respectively.
Table 2.
Patient baseline characteristics.
6.3. Efficacy
Using an ITT analysis, we found that at the end of week 12, the average decrease in the sUA was 74.26 μmol/L (95% confidence interval [CI]: 56.74–91.77 μmol/L) in treatment group versus 28.81 μmol/L (95% CI: 4.91–52.71 μmol/L) in the control group (z = −2.87, P = 0.004, Wilcoxon rank sum test). Moreover, the average decreased rate of the sUA was 12.76% (95% CI: 9.82%–15.70%) in the treatment group versus 4.57% (95% CI: 0.42%–8.71%) in the control group (z = −2.86, P = 0.004, Wilcoxon rank sum test, Fig. 3 A and B and Supplementary Fig. 3A and B).
Figure 3.
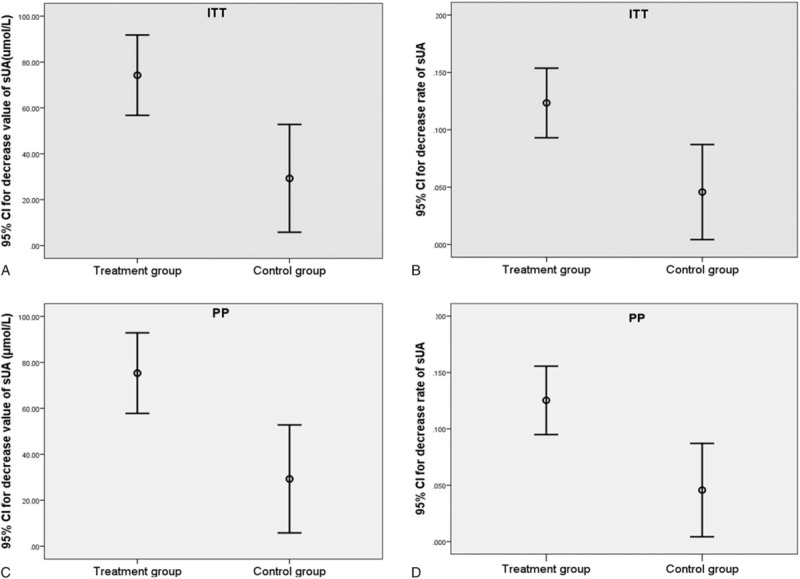
sUA reduction by compound tufuling oral-liquid. (A) ITT analysis of decreased sUA after 12-week treatment. The difference between both groups was statistically significant (z = −2.87, P = 0.004, Wilcoxon rank sum test). (B) Intention-to-treat analysis of decreased rate of sUA after 12-week treatment. The difference between the 2 groups was statistically significant (z = −2.86, P = 0.004, Wilcoxon rank sum test). (C) PPS analysis of decrease sUA after 12-week treatment. The difference between the 2 groups was statistically significant (z = −2.956, P = 0.003, Wilcoxon rank sum test). (D) PPS analysis of decrease rate of sUA after 12-week treatment. The difference between the 2 groups was statistically significant (z = −2.954, P = 0.003, Wilcoxon rank sum test). CI = confidence interval, PPS = per-protocol population set, sUA = serum uric acid.
The PPS analysis also showed similar results and the average decrease in the sUA at the end of week 12 was 75.34 μmol/L (95% CI: 57.80–92.88 μmol/L) in treatment group versus 28.81 μmol/L (95% CI: 4.91–52.71 μmol/L) in the control group (z = −2.956, P = 0.003, Wilcoxon rank sum test). Furthermore, the average decrease rate of sUA was 12.96% (95% CI: 10.01%–15.90%) in the treatment group versus 4.57% (95% CI: 0.42%–8.71%) in the control group (z = −2.954, P = 0.003, Wilcoxon rank sum test, Fig. 3C and D and Supplementary Fig. 3C and D).
The PPS analysis (Pearson χ2 test) revealed that the recurrence rate of gouty arthritis (joint swelling or pain) in the treatment group was lower than that in the control group. The specific values were: from baseline to the end of week 6, 32.14% and 48.28% in the treatment and control groups, respectively, P = 0.04; from the end of week 6 to the end of week 12, 22.02% and 50.88% in the treatment and control groups, respectively, P < 0.001; and from baseline to the end of week 12, 38.53% and 63.16% in the treatment and control groups, respectively, P = 0.003, Fig. 4 A and Supplementary Fig. 4A. The ITT analysis (Pearson χ2 test) revealed that the recurrence rate of gouty arthritis in the treatment group was lower than that in the control group. The specific values were: from baseline to the end of week 6, 30.77% and 44.44% in the treatment and control groups, respectively, P = 0.062; from the end of 6 to the end of week 12, 21.93% in the treatment and control groups, respectively; P = 0.000; and from baseline to the end of week 12, 39.50% and 63.16% in the treatment and control groups, respectively, P = 0.003, Fig. 4B and Supplementary Fig. 4B.
Figure 4.
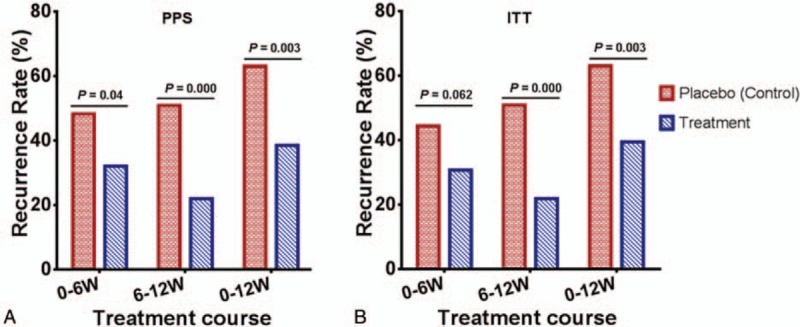
Prevention of gouty arthritis recurrence by compound tufuling oral-liquid. (A) PPS analysis (Pearson χ2 test): from baseline to end of week 6, 32.14% and 48.28% in treatment and control group, respectively, P = 0.04; from end of week 6 to end of week 12, 22.02% and 50.88% in treatment and control group, respectively, P < 0.001; and from baseline to end of week 12, 38.53% and 63.16%in treatment and control group, P = 0.003. (B) ITT analysis (Pearson χ2 test): from baseline to end of week 6, 30.77% and 44.44% in treatment and control group, respectively, P = 0.062; from end of week 6 to end of week 12, 21.93% and 50.88% in treatment and control group, respectively, P < 0.001; and from baseline to end of week 12, 39.50% and 63.16% in treatment and control group, respectively, P = 0.003. ITT = intention-to-treat, PPS = per-protocol population set.
6.4. Safety
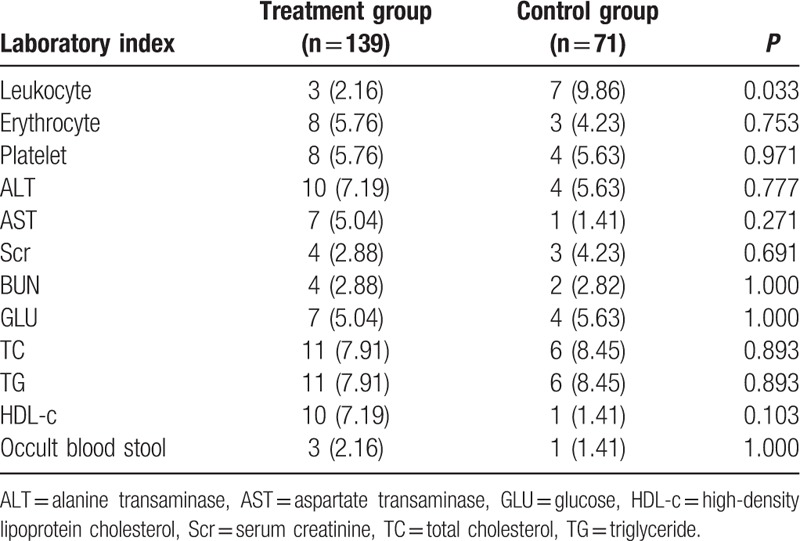
At week 0 and the end of week 12 after treatment, several laboratory indices including the blood cell count, alanine transaminase, aspartate transaminase, GLU), Scr, TC, TG, HDL-c, and occult blood stool were measured. Except for the leukocyte count, no obvious abnormalities or indications of worsening symptoms were observed in the 2 groups during the treatment. Fewer cases of leukopenia were observed in the treatment group than in the control group (3/139 and 7/71 in treatment and control group, respectively, P = 0.033, Table 3).
Table 3.
Incidence rates of abnormal laboratory indices (M [QR]).
7. Discussion
Gout is a metabolic rheumatic disease caused by long-term abnormal purine metabolism. The developmental process of primary gout can be divided roughly into the hyperuricemia (without gouty symptoms), acute gout, and chronic gout (including the development of chronic gouty arthritis and gouty stones) phases. The prevalence of gout is increasing worldwide [6] and conventional medicines including colchicine, NSAIDS, allopurinol, and benzbromarone have some therapeutic effects on gout,[33] but they also have numerous side effects. For example, allopurinol is limited by an AE profile that includes hepatic, gastrointestinal, renal, hematological, and skin toxicities that occur in approximately 20% of patients. In addition, this drug may cause the death of 2% to 4% of patients with hypersensitivity reactions. Notably, patients tend to show low compliance and tolerance to these drugs, thereby limiting their long-term effectiveness. Consequently, there is an urgent need for the identification of new strategies to control acute gouty arthritis and the development of new drugs to lower sUA levels.[34,35]
Based on previous studies,[27–30] we adopted a double-blind, placebo-controlled, multicenter, randomized trial design to determine the effect of CoTOL. Our results indicate that the decrease in the sUA level of the treatment group was significantly higher than that of the control group. Furthermore, the frequency of recurrent joint swelling or pain in the treatment group was obviously lower than that in the control group, whereas the number of patients with an exacerbated white blood cell count was lower in the treatment group than it was in the control group. In addition, severe AEs did not occur in either group. Two and 8 patients in the treatment and control groups, respectively, experienced mild AEs. Therefore, our results indicate that CoTOL reduced the sUA levels of patients in the intercritical and chronic gout phases and prevented the recurrence of acute gouty arthritis with a better efficiency than the placebo did.
CoTOL consists mainly of Rhizoma smilacis Glabrae (Smilax glabra, Tufuling), Rhizoma dioscoreae collettii (Dioscoreatokoro Makino, Bixie), Curcuma longa (turmeric, Jianghuang), Herba siegesbeckiae (Glandularstalk St.Paul's Wort herb, Xixiancao), and Rhizoma corydalis (Yanhusuo, Yanhusuo), Semen coicis (coix seed, Yiyiren), Loranthus parasiticus (Chinese taxillus twig, Sangjisheng), and Stigma maydis (corn silk, Yumixu). Several pharmacological studies have shown that these herbs may have positive effects on gout. For example, Rhizomasmilacis glabrae has anti-inflammatory and analgesic actions.[36] As a flavonoid compound isolated from Rhizoma smilacis Glabrae, astilbin has anti-inflammatory effects.[37] In addition, astilbinis effective in preventing hyperuricemia and nephropathy by increasing the urinary UA level and fractional excretion of urate, preventing renal damage by the expression of transforming growth factor-β1 and connective tissue growth factor, and inhibiting the formation of monosodium urate as well as the production of prostaglandin E2 and interleukin-1.[38] These findings provide strong evidence supporting the use of astilbin as a safe and promising lead compound for the prevention of hyperuricemia and gouty nephropathy. D tokoro Makino and its saponin ingredients, which have been shown to decrease foot swelling in mice and rats and heighten the pain threshold of mice, have positive effects on gouty arthritis induced by sodium urate by reducing sUA.[39,40] Herba siegesbeckiae has anti-inflammatory and analgesic effects.[41–43] Rhizoma corydalis has analgesic effects and is effective for treating gastric ulcer.[44,45]C longa, which contains 2 classes of secondary metabolites namely curcuminoids and essential oils, possesses effective inhibitory activity against xanthine oxidase[46] as well as anti-inflammatory and antiarthritic effects.[47–49] Because of its strong antioxidant capacity and recovery of cisplatin-induced nephrotoxicity, L parasiticus exhibits potent anti-inflammatory and antinephrotoxic activities,[50–52] which may be beneficial for reducing the recurrence of gouty arthritis and preventing urate-induced renal damage. Semen coicis shows analgesic and anti-inflammatory properties.[53,54] Stigma maydis (corn silk), which contain saponin, alkaloid, flavones, volatile oil, mucilage, Vitamins B, C, and K, Silicon, increases the urinary output and the percentage the passage of urinary stones through the urinary tracts without decomposed stones, lower the total amount of acid in the urine,[55,56] and inhibit xanthine oxidase,[57] Therefore, Stigma maydis play an active role in treating gout by inhibiting the production of sUA, promoting the excretion of UA, and preventing the formation of kidney stones.
Therefore, we hypothesized that CoTOL might lower sUA and reduce the recurrence of gouty arthritis. The present study clearly demonstrates that the median sUA levels of patients treated with CoTOL were significantly decreased compared to those of the patients treated with placebo after 12 weeks, whereas the recurrence rate of joint swelling or pain in the treatment group was lower than that in the placebo control group. Moreover, severe AEs did not occur in either group. Only 2 participants experienced mild AEs and three manifested leukopenia in the treatment group, which was unrelated to CoTOL.
8. Conclusions
In conclusion, our findings suggest that CoTOL has significant therapeutic potential not only by reducing the sUA level but also by decreasing the frequency of recurrent joint swelling or pain without obvious AEs. The study has limitations including the fact that the observation period was limited to 12 weeks, and no positive control was used. Future studies should involve a trial with a longer treatment course and a positive control drug to provide further evidence of the efficacy of CoTOL in treating gout. Furthermore, its mechanisms of action also need to be investigated.
Supplementary Material
Acknowledgments
The authors thank the members of the Quality Control Department in the Affiliated Hospitals of Nanjing University of Chinese Medicine, the other investigators and study coordinators, Dr. Wei-dong Lu (Harvard University, Boston. MA, USA), and Dr. Hong-Huang Lin (Boston University, Boston, MA, USA)for their help in completing this study.
Footnotes
Abbreviations: ADRs = adverse drug reactions, AEs = adverse events, ALT = alanine transaminase, AST = aspartate transaminase, CoTOL = compound tufuling oral liquid, CRF = case report form, GLU = glucose, HDL-c = high-density lipoprotein cholesterol, ITT = intention-to-treat, NSAIDs = nonsteroidal anti-inflammatory drugs, PPS = per-protocol population set, RCTs = randomized controlled trials, Scr = serum creatinine, SS = safety set, sUA = serum uric acid, TC = total cholesterol, TCMs = traditional Chinese medicines, TG = triglyceride.
ZX and HW have contributed equally to this study.
This study was supported by Zhejiang Province Natural Science Fund Committee (LQ16H270004) and Ministry of Science and Technology of China (2007BAI20B06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was funded by the Ministry of Science and Technology of China.
Participating clinical centers: Zhejiang Chinese Medical University, Hangzhou, P.R. China. The Second Affiliated Hospital of Zhejiang University Medical College, Hangzhou, P.R.China. Zhejiang Hospital, Hangzhou, P.R. China. Medical College, Zhejiang University, Hangzhou, P.R. China. Zhejiang People's Hospital, Hangzhou, P.R.China. Sir Run Run Shaw Hospital of Zhejiang University Medical College, Hangzhou, P.R. China. The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, P.R.China. Chinese Medical Academy Gate Hospital, Beijing, P.R. China. The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, P.R. China. Author Contributions: CW, YF, and ZX conceived and designed the experiments; CW, YF, HW, XJ, XT, YL, YH, XG, JS, and ZX performed the experiments; XL performed statistical analysis; CW contributed to reagents/materials/analysis tools; ZX wrote the manuscript.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Tu HP, Chen CJ, Tovosia S, et al. Associations of a non-synonymous variant in SLC2A9 with gouty arthritis and uric acid levels in Han Chinese subjects and Solomon Islanders. Ann Rheum Dis 2010;69:887–90. [DOI] [PubMed] [Google Scholar]
- [2].Dehghan A, Köttgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008;372:1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mukhin I, Ignatenko G. Changes in blood hormone levels in gout and methods of their correction (an experimental and clinical study). Klin Lab Diagn 2003;1:19–22. [PubMed] [Google Scholar]
- [4].Zhang YQ, Chen C, Choi H, et al. Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis 2012;71:1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miao ZM, Li CG, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 2008;35:1859–64. [PubMed] [Google Scholar]
- [6].Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004;31:1582–7. [PubMed] [Google Scholar]
- [7].Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Ann Rheum Dis 2008;67:960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Trifirò G, Morabito P, Cavagna L, et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005-2009: a nationwide population-based study. Ann Rheum Dis 2013;72:694–700. [DOI] [PubMed] [Google Scholar]
- [9].Chang S, Ko YC, Wang T, et al. High prevalence of gout and related risk factors in Taiwan's Aborigines. J Rheumatol 1997;24:1364–9. [PubMed] [Google Scholar]
- [10].Nan H, Qiao Q, Dong Y, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. The Journal of rheumatology 2006;33:1346–50. [PubMed] [Google Scholar]
- [11].Burke A, Smyth E, FitzGerald GA. Analgesic-antipyretic agents; pharmacotherapy of gout. 2006;New York: McGraw-Hill Medical Publishing Division, 671–715. [Google Scholar]
- [12].Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, et al. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis 1998;57:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- [14].Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chattopadhyay I, Shetty H, Routledge P, et al. Colchicine induced rhabdomyolysis. Postgrad Med J 2001;77:191–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Altman A, Szyper-Kravitz M, Shoenfeld Y. Colchicine-induced rhabdomyolysis. Clin Rheumatol 2007;26:2197–9. [DOI] [PubMed] [Google Scholar]
- [17].Blower A. NSAIDs and peptic ulcers. BMJ 1990;300:815. [PMC free article] [PubMed] [Google Scholar]
- [18].Hoskison KT, Wortmann RL. Management of gout in older adults. Drugs Aging 2007;24:21–36. [DOI] [PubMed] [Google Scholar]
- [19].White WB. Cardiovascular risk, hypertension, and NSAIDs. Curr Pain Headache Rep 2007;11:428–35. [DOI] [PubMed] [Google Scholar]
- [20].Christensen S, Riis A, Nørgaard M, et al. Perforated peptic ulcer: use of pre-admission oral glucocorticoids and 30-day mortality. Aliment Pharmacol Ther 2006;23:45–52. [DOI] [PubMed] [Google Scholar]
- [21].Choi H, De Vera M, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology 2008;47:1567–70. [DOI] [PubMed] [Google Scholar]
- [22].Esposito M, Ruberto M, Pascotto A, et al. Nutraceutical preparations in childhood migraine prophylaxis: effects on headache outcomes including disability and behaviour. Neurol Sci 2012;33:1365–8. [DOI] [PubMed] [Google Scholar]
- [23].Toldo I, Rattin M, Perissinotto E, et al. Survey on treatments for primary headaches in 13 specialized juvenile Headache Centers: the first multicenter Italian study. Eur J Paediatr Neurol 2016. [DOI] [PubMed] [Google Scholar]
- [24].Zhou L, Liu L, Liu X, et al. Systematic review and meta-analysis of the clinical efficacy and adverse effects of chinese herbal decoction for the treatment of gout. PloS One 2014;9:e85008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aziz MA. Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J Integr Med 2015;13:173–84. [DOI] [PubMed] [Google Scholar]
- [26].Li T, Liu X, Zhang M, et al. Assessment of systematic reviews and meta-analyses on traditional Chinese medicine published in Chinese journals. Chinese J Evid Based Med 2007;7:180–8. [Google Scholar]
- [27].Xu Y, Wen CP, Jin CY, et al. [Clinical observation of ‘Qu-zhuo-tong-bi’ particle in treating acute gout]. Journal of Zhejiang University of TCM 2004;28:41–2. [Google Scholar]
- [28].Chen J, Zhou J, Wei S, et al. Effect of a traditional Chinese medicine prescription Quzhuotongbi decoction on hyperuricemia model rats studied by using serum metabolomics based on gas chromatography–mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1026:272–8-. [DOI] [PubMed] [Google Scholar]
- [29].Li H, Wen C, Xie Z, et al. Literature research of traditional Chinese medical prescriptions of gout in lntermission and chronic phase. Chin Arch Tradit Chin Med 2013;31:252–5. [Google Scholar]
- [30].Xie ZJ, Wen CP, Bao HJ, et al. [Effect of Qu-zhuo-tong-bi Recipe on The Levels of Xanthine Oxidase in Hyperuricemia Rats]. China Journal of Traditional Chinese Medicine and Pharmacy 2011;26:1398–400. [Google Scholar]
- [31].Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ 2008;86:650–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arth Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- [33].Reinders M, Van Roon E, Jansen TTA, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis 2009;68:51–6. [DOI] [PubMed] [Google Scholar]
- [34].Dalbeth N, Kumar S, Stamp L, et al. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol 2006;33:1646–50. [PubMed] [Google Scholar]
- [35].Panomvana D, Sripradit S, Angthararak S. Higher therapeutic plasma oxypurinol concentrations might be required for gouty patients with chronic kidney disease. J Clin Rheumatol 2008;14:6–11. [DOI] [PubMed] [Google Scholar]
- [36].Man MQ, Shi YJ, Man M, et al. Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and anti-inflammatory activity in murine disease models. Exp Dermatol 2008;17:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cai Y, Chen T, Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res 2003;52:334–40. [DOI] [PubMed] [Google Scholar]
- [38].Chen L, Lan Z, Zhou Y, et al. Astilbin attenuates hyperuricemia and ameliorates nephropathy in fructose-induced hyperuricemic rats. Planta Med 2011;77:1769–73. [DOI] [PubMed] [Google Scholar]
- [39].Zhao LM. Traditional Chinese medicine treatment of hyperuricemia 27 cases. Journal of Traditional Chinese Medicine 2007;48:533–4. [Google Scholar]
- [40].Chen GL, Lv HX, Wang YY, L IL. Protective effect of total sponin of dioscorea and aehyranthe on rats with acute gouty arthritis induced by uric acid sodium. Pharmacol Clin Chin Mater Med 2010;26:34–7. [Google Scholar]
- [41].Li H, Kim JY, Hyeon J, et al. In vitro antiinflammatory activity of a new sesquiterpene lactone isolated from Siegesbeckia glabrescens. Phytother Res 2011;25:1323–7. [DOI] [PubMed] [Google Scholar]
- [42].Song YP, Yu QH. The mechanism of therapeutic effect of Herba Siegesbeckiae on arthritis. China Med Eng 2012;11:007. [Google Scholar]
- [43].Qian R, Zhang C, Fu H. Study on therapeutic mechanism of anti-rheumatism action of herba siegesbeckiae. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20:192–5. [PubMed] [Google Scholar]
- [44].Figueroa-Guzman Y, Mueller C, Vranjkovic O, et al. Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend 2011;116:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang YQ, Geng BQ, Yong DG, JING Z. Effect of dl-tetrahydropalmatine on rat gastric ulcer. Chinese PHARMACEUTICAL JOURNAL-BEIJING 2005;40:902. [Google Scholar]
- [46].Shen L, Ji HF. Insights into the inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett 2009;19:5990–3. [DOI] [PubMed] [Google Scholar]
- [47].Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med 2003;9:161–8. [DOI] [PubMed] [Google Scholar]
- [48].Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2009;41:40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Funk JL, Frye JB, Oyarzo JN, et al. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.). J Agric Food Chem 2009;58:842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gan RY, Kuang L, Xu XR, et al. Screening of natural antioxidants from traditional Chinese medicinal plants associated with treatment of rheumatic disease. Molecules 2010;15:5988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sohn SH, Lee H, Nam JY, et al. Screening of herbal medicines for the recovery of cisplatin-induced nephrotoxicity. Environ Toxicol Pharmacol 2009;28:206–12. [DOI] [PubMed] [Google Scholar]
- [52].Moghadamtousi SZ, Kamarudin MNA, Chan CK, et al. Phytochemistry and Biology of Loranthus parasiticus Merr, a Commonly Used Herbal Medicine. Am J Chinese Med 2014;42:23–35. [DOI] [PubMed] [Google Scholar]
- [53].Tatsumi S, Mabuchi T, Abe T, et al. Analgesic effect of extracts of Chinese medicinal herbs Moutan cortex and Coicis semen on neuropathic pain in mice. Neurosci Lett 2004;370:130–4. [DOI] [PubMed] [Google Scholar]
- [54].Seo WG, Pae HO, Chai KY, et al. Inhibitory effects of methanol extract of seeds of job's tears (Coix Lachryma-Jobi L Var. Ma-Yuen) on nitric oxide and superoxide production in raw 264.7 macrophages. Immunopharmacol Immunotoxicol 2000;22:545–54. [DOI] [PubMed] [Google Scholar]
- [55].Al-Ali M, Wahbi S, Twaij H, et al. Tribulus terrestris: preliminary study of its diuretic and contractile effects and comparison with Zea mays. J Ethnopharmacol [Comparative Study] 2003;85:257–60. [DOI] [PubMed] [Google Scholar]
- [56].Shamki AW, Al-Amery H. Effect of Corn Silk Extract on Kidney Stone Decomposition in Comparison With Alkalinizeragent (uralyte). Int J Health Nutr 2012;3:1–5. [Google Scholar]
- [57].Jiang TW, Xu J, Wang XM, et al. [Screening of active ingredient of Stigmata maydis inhibitrg xanthine oxidase and its effect]. Journal of Jilin University Medicine Edition 2011;37:433–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


