Supplemental Digital Content is available in the text
Keywords: biomarker, Crohn disease, inflammatory bowel diseases, platelet factor 4 (PF4), ulcerative colitis (UC)
Abstract
To investigate the diagnostic utility of serum platelet factor 4 (PF4) levels and to assess its accuracy in detecting inflammatory bowel disease activity.
This study included 45 patients with ulcerative colitis (UC), 45 patients with Crohn disease (CD), and 30 control subjects at Jinling Hospital between May 2014 and July 2015. Laboratory tests measured white blood count, C-reactive protein, erythrocyte sedimentation rate, and platelet count. PF4 was examined by enzyme-linked immunosorbent assays. Patients were divided into 2 groups according to disease activity: active and inactive.
Median PF4 values dramatically increased in UC and CD patients compared with the healthy group (UC: 26.64 [20.00–36.22] mg/mL vs 20.02 [14.63–26.83] mg/mL, P = 0.002; CD: 25.56 [18.57–36.36] mg/mL vs 20.02 [14.63–26.83] mg/mL, P = 0.014); however, the serum PF4 levels between UC and CD failed to show a significant difference (26.64 [20.00–36.22] mg/mL vs 25.56 [18.57–36.36] mg/mL, P = 0.521). Furthermore, serum PF4 levels were elevated in both UC and CD patients with active disease (UC: 20.19 [14.89–23.53] mg/mL vs 28.86 [22.57–37.29] mg/mL, P < 0.001; CD: 18.33 [16.72–25.77] mg/mL vs 34.38 [22.58–39.92] mg/mL, P < 0.001). Multivariate analysis revealed higher PF4 level as an independent predictor of disease activity in UC and CD patients (UC: odds ratio 30.375, P = 0.002; CD: odds ratio 54.167, P < 0.001). The cut-off level of PF4 for distinguishing active from inactive UC patients was 24.1 mg/mL. While in CD patients, the cut-off level of PF4 was 19.24 mg/mL.
Serum PF4 levels could be a potential biomarker for monitoring the disease activity of inflammatory bowel disease.
1. Introduction
Ulcerative colitis (UC) and Crohn disease (CD), collectively named inflammatory bowel diseases (IBDs), are chronic, relapsing, inflammatory disorders of the gastrointestinal tract that can lead to digestive disability.[1,2] Symptoms include abdominal pain, diarrhea, bloody stools, weight loss, and fever. Mucosal lesions for UC patients are confined to the large intestine and rectum, whereas CD is characterized by skip lesions and could involve the whole gastrointestinal tract. Most patients are between the age of 15 and 40 years.[3] The etiology of IBD is unknown, but the disease is thought to involve a complex interplay among genetic and environmental factors.[4] Characterized by its repetitiveness, patients with IBD are often required to continually evaluate disease activity to ensure a prompt therapeutic regimen. Serological markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are routinely tested, but they are nonspecific and minimally reliable for IBD.[5] More recently, fecal calprotectin has been used for diagnosing IBD, predicting clinical relapse and monitoring disease activity.[6–8] However, some studies imply that fecal calprotectin results are less reliable in some cases.[9–12] To date, the initial evaluation of UC activity and CD activity integrates clinical symptoms and physical findings with imaging and laboratory tests, including endoscopic examination.[13] Repeated endoscopic examinations are often needed to accurately evaluate mucosal lesions and assist clinicians in choosing correct therapeutic interventions. In addition to the economic burden on patients and the need for complicated procedures, it is also challenging to monitor ulceration in the distal ileum. Therefore, an easily accessible surrogate marker to accurately reflect disease activity is strongly desirable.
An initial exploration into the role of platelets in IBD came from an awareness of thrombocytosis as a marker of disease activity.[14] Danese et al[15] reported the existence of platelet dysfunction in the peripheral circulation of IBD patients, which may contribute to inflammation. Platelet factor 4 (PF4) belongs to the CXCL chemokine family with anti-heparin activity and is released from platelet a-granules when platelet activation occurs.[16] Dating back to 1987, Simi et al[17] demonstrated that plasma PF4 levels were significantly higher in CD patients compared with that in healthy volunteers, but they failed to find a correlation between PF4 levels and disease activity. Recently, Takeyama et al[18] showed that PF4 levels were highly correlated with the Crohn Disease Activity Index (CDAI) in CD patients with low CRP levels.
Based on these conflicting results, the aim of our study is to investigate serum PF4 levels in patients with UC and CD, and further, to explore the relationships between PF4 levels and disease activity. Afterwards, we would like to compare the accuracy of PF4 with other common serum markers that are currently used in clinical practice.
2. Materials and methods
2.1. Patients
This prospective study of a single-center cohort included 120 subjects who referred to the Department of Gastroenterology and Hepatology of Jinling Hospital from May 2014 to July 2015, including 45 UC patients, 45 CD patients, and 30 healthy controls, and followed both active and inactive phases of the diseases. The IBD diagnosis was documented according to established criteria.[19] Subsequent patients with confirmed IBD were asked to participate in the study. Patients were excluded according to the following criteria: patients who were under the age of 18; those who showed remarkable evidence of systemic infection; those who had Clostridium difficile infection or liver dysfunction or renal dysfunction; those who had hematological diseases; and those who did not agree to participate in the study. As for UC patients, clinical disease activity was evaluated using a modified Mayo score. Patients with a score of 3 or higher were considered to be active.[20] The disease activities of CD patients were evaluated according to Harvey–Bradshaw, and active was defined as the CDAI higher than a score of 150.[21] All included patients were given written informed consent which was organized in accordance with the guidelines written by the Ethics Committee of Jinling Hospital and agreed to participate in the study.
2.2. Laboratory tests
Blood was drawn from each participant at the time of entry. IBD patients and healthy controls were measured for white blood count (WBC), ESR, CRP, platelet count (PLT), and PF4 levels. The PF4 serum concentrations were determined using a specific enzyme-linked immunosorbent assay (ELISA) procedure according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Three samples of known concentration were tested 20 times on 1 plate to assess intra-assay precision, and also tested in 20 separate assays to assess interassay precision. Detailed data on the imprecision of the employed PF4 ELISA were listed in Table S1. The minimum detectable dose of human PF4 ranged from 0.010 to 0.100 ng/mL. Blood samples were collected from a peripheral vein after an overnight fast and were subjected to centrifugation at a speed of 3000 revolutions per minute for 10 minutes at 4°C to obtain serum. Serum samples were stored at −80°C. Also, when measuring PF4 levels, serum samples were thawed on ice and diluted at 1:400. An amount of 50 μL of standard, control, or sample was added to per well. Calibrator diluent was used as the control for PF4 ELISA. The plates were read on a Spectra reader (Bio-Rad) at an optical density of 450 nm. The ELISA kit measures in the ng/mL range, but the data were too large if expressed as ng of PF4 per mL of sera. Thus, the data were expressed as mg of PF4 per mL of sera.
2.3. Data analysis and statistics
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 13 software (SPSS Inc., Chicago, IL). Quantitative results were presented in the study as the median (interquartile range [IQR]). Normal distribution of the variables was assessed by Kolmogorov–Smirnov test. P values below 0.05 indicate these variables have a nonparametric distribution. Data with parametric distribution were analyzed using unpaired Student t tests or 1-way analysis of variance (ANOVA). Data with nonparametric distribution were analyzed by the Mann–Whitney U test or Kruskal–Wallis test. Fisher exact test was used for categorical variables. The Spearman rank correlation coefficient was used to analyze the correlation between PF4 and other markers. Receiver-operating characteristic (ROC) analyses were performed. Active UC or CD patients were defined as “1,” inactive UC or CD patients were defined as “0,” and the value of “state variable” was 1. Then SPSS outputted ROC curves, and also the coordinate points. The best thresholds of PF4, CRP, ESR, PLT, and WBC levels for the evaluation of disease activity were determined using Youden index. Then the maximal areas under the ROC curves were calculated.[22] To identify predictors of disease activity in UC and CD patients, multivariate analysis was performed using binary logistic regression analysis. PF4, CRP, ESR, PLT, and WBC were adjusted by “Forward: LR” method of statistical program as covariates in regression model. P values below 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
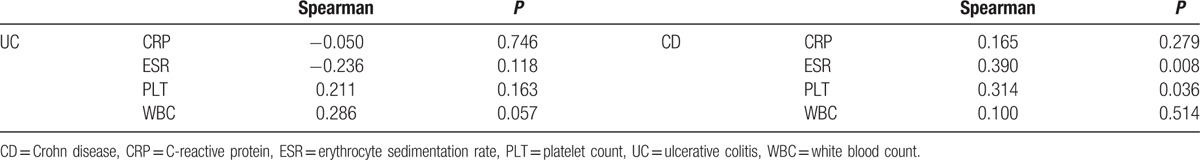
The demographic and clinical characteristics of the enrolled subjects are summarized in Table 1. The groups showed similarity in sex and age, but there were significant differences between the UC and CD groups with regard to disease activity (P = 0.044, Fisher exact test), which likely resulted in statistically significant differences in median CRP and WBC levels between UC and CD patients. Table 1 showed that median CRP in UC and CD were 6.50 and 2.50 mg/L, respectively. The median level of WBC for UC patients was 7.40 × 109 and 5.20 × 109 for CD patients. Median PF4 values were significantly higher in UC and CD patients compared with healthy groups (UC 26.64 mg/mL vs HC 20.02 mg/mL, P = 0.002; CD 25.56 mg/mL vs HC 20.02 mg/mL, P = 0.014, Mann–Whitney U test); however, the difference between UC and CD groups in terms of serum PF4 levels was statistically insignificant (P = 0.521, Mann–Whitney U test). No correlation was found between PF4 and other inflammatory markers for UC patients (Table 2). As for CD patients, both PLT and ESR were correlated with PF4 (PLT: r = 0.314, P = 0.036; ESR: r = 0.390, P = 0.008, Spearman rank correlation coefficient; Table 2).
Table 1.
Characteristics and comparison of PF4 with other laboratory markers between patients and control group (%).
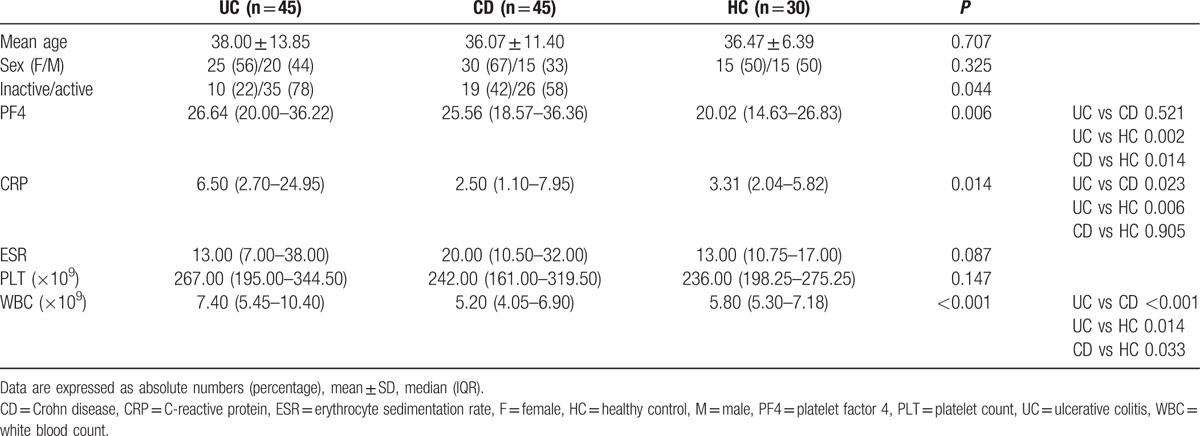
Table 2.
Correlations of serum PF4 with other serum regular markers.
3.2. Median PF4 levels of active and inactive IBD patients
As shown in Tables 3 and 4, median PF4 values were significantly higher in active patients when compared with patients in remission (UC: 20.19 vs 28.86 mg/mL, P < 0.001; CD: 18.33 vs 34.38 mg/mL, P < 0.001, Mann–Whitney U test). For UC patients, other inflammatory markers were not significantly higher in active patients (CRP: 6.35 vs 6.50 mg/L, P = 0.718; ESR: 11.00 vs 15.00 mm/h, P = 0.606; PLT: 253.00 × 109 vs 267.00 × 109, P = 0.219; WBC: 6.60 × 109 vs 7.70 × 109, P = 0.469, Mann–Whitney U test). For CD patients, both median ESR and PLT levels were significantly elevated in active patients (ESR: 12.00 vs 23.50 mm/h, P = 0.047; PLT: 199.00 × 109 vs 267.00 × 109, P = 0.018, Mann–Whitney U test). Serum PF4 level of each individual was shown in Fig. 1.
Table 3.
Median levels of PF4 and other laboratory markers in UC patients in relation to disease activity.
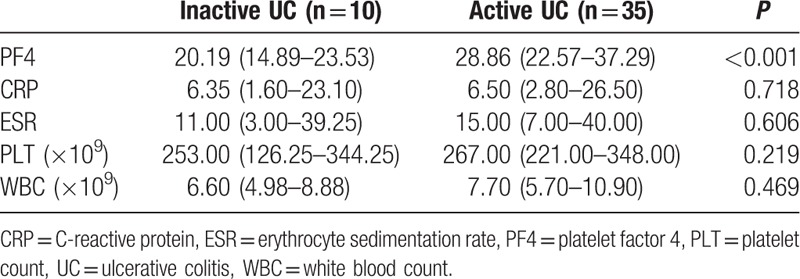
Table 4.
Median levels of PF4 and other laboratory markers in CD patients in relation to disease activity.
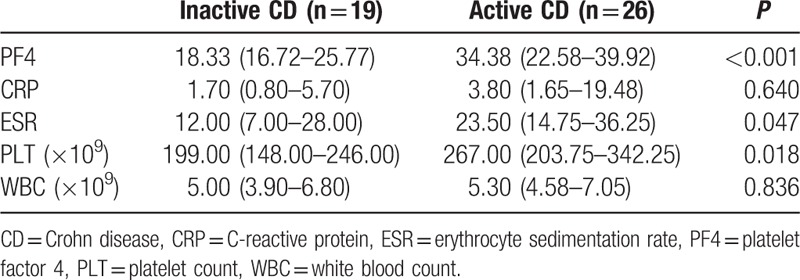
Figure 1.
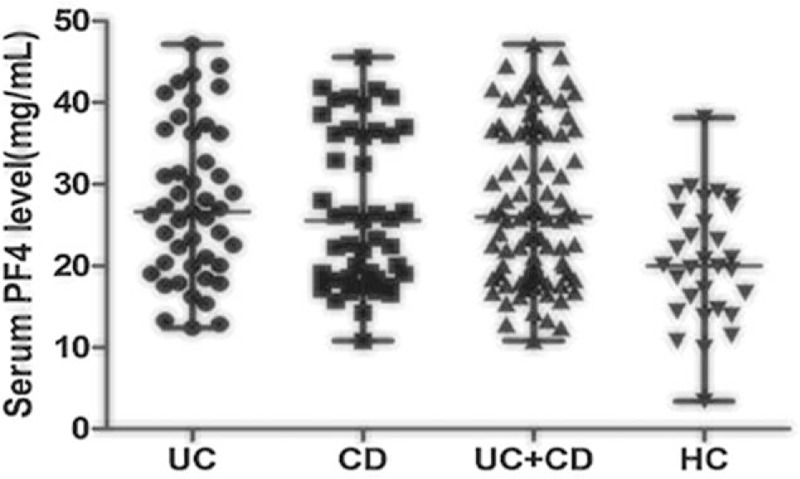
Serum PF4 level of each patient in the 4 groups. The horizontal line in the middle is the median, and the lower line represents the lower quartiles, whereas the upper line represents the upper quartiles. PF4 = platelet factor 4.
3.3. ROC analysis
The ROC curve analyses suggested that the optimum serum PF4 cut-off point to distinguish active patients from inactive patients was 24.1 mg/mL for UC patients and 19.24 mg/mL for CD patients (Table 5). This cut-off value had a sensitivity of 74.3% and 96.2% for UC and CD patients, respectively, with a specificity of 90.0% and 68.4%, respectively. Moreover, for UC patients, a cut-off level of 11.4 mg/mL provided 100% sensitivity, and serum PF4 with a cut-off of ≥29.6 mg/mL provided 100% specificity. Whereas in CD patients, a cut-off level of 9.8 mg/mL provided 100% sensitivity, and when serum PF4 levels were higher than 37.8 mg/mL, the specificity could reach 100%. The same analyses for other inflammation markers are summarized in Table 5. The area under the curve (AUC) values for PF4 were significantly higher than those for other markers in both UC and CD patients (UC: AUC = 0.831; CD: AUC = 0.868) (Figs. 2 and 3, Table 5), which showed that there was a favorable relationship between PF4 levels and disease activity. Moreover, multivariate analysis revealed that a higher serum PF4 level than optimal cut-off level for diagnosing disease activity for UC and CD was an independent predictor (UC: odds ratio [OR] 30.375, 95% confidence interval [CI] 3.327–277.306, P = 0.002; CD: OR 54.167, 95% CI 5.880–499.006, P < 0.001). Inflammatory biomarkers, including CRP, ESR, PLT, and WBC, did not show statistical significance.
Table 5.
Receiver-operating characteristic curve analyses indicate the ability of PF4 to distinguish active from inactive patients.
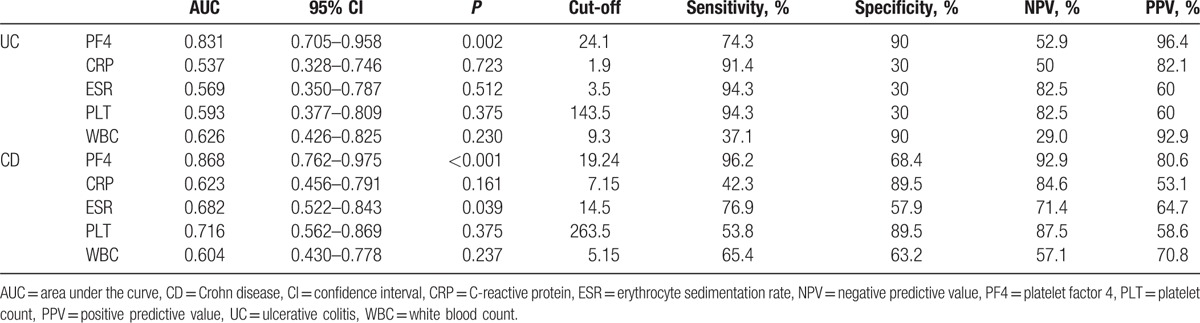
Figure 2.
Receiver-operating characteristic (ROC) plot compares specificity and sensitivity of PF4 and 4 other serum markers in UC patients. Areas under the curves show the accuracy of PF4 and the other 4 serum markers for predicting clinically active UC. UC = ulcerative colitis.
Figure 3.
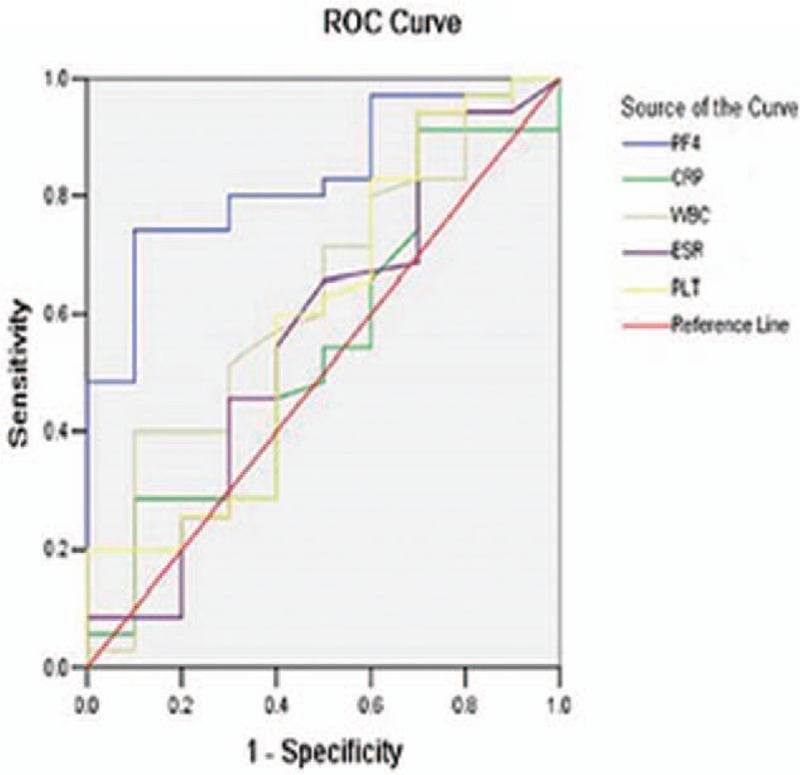
Receiver-operating characteristic (ROC) plot compares specificity and sensitivity of PF4 and 4 other serum markers in CD patients. Areas under the curves show the accuracy of PF4 and the other 4 serum markers for predicting clinically active CD. CD = Crohn disease.
4. Discussion
4.1. Key findings
To the best of our knowledge, this is the first pilot study reporting the clinical significance of PF4 in diagnosing the disease activity for IBD patients. First, we found that serum PF4 levels were significantly elevated in patients when compared with healthy controls. In contrast, PLT levels were not increased in patients with IBD, which is inconsistent with some published studies.[23,24] Similarly, ESR, a typical parameter for assessing inflammation, was not significantly different between patients and controls. Moreover, we explored the correlations between PF4 and 4 additional serum markers. No correlation was found in UC patients, and a weak correlation between PF4 and PLT or ESR was found in CD patients. ROC analysis showed a relatively high accuracy of PF4 in detecting disease activity in IBD patients (ACU for UC = 0.868; AUC for CD = 0.831). In addition, multivariate analysis also confirmed the independent role of PF4 in monitoring the disease activity of IBD patients. These results strongly supported that PF4 was highly reflective of disease activity.
Among the laboratory markers used in practice, CRP is recognized as a promising predictor of disease activity in IBD patients. Nevertheless, it has been reported that CD patients are able to mount a major acute disease phase; thus, CRP levels are better associated with clinical disease activity in CD patients, but not UC patients.[25,26] For CD patients, studies also showed that CRP was not always consistent with clinical scores.[27–30] Likewise, we did not detect an elevated CRP level in active patient. Furthermore, our study failed to find a correlation between PF4 and CRP, which indicated that PF4 could be an additional independent factor to assess inflammation associated with IBD.
4.2. Previous studies
Patients with active IBD are 3-fold more likely to develop thrombosis compared with the healthy population.[31] Thromboembolic events are life-threatening in IBD patients. According to an epidemiological review, patients with venous thromboembolism have a 2-fold higher morbidity rate than those without venous thromboembolism.[32] Current evidence demonstrated that thrombosis was closely linked with IBD activity.[33] Among various factors contributing to thrombosis formation, platelets have been reported to express a series of molecules that possibly result in thrombosis and inflammation in IBD.[34] Hence, it is reasonable to hypothesize that platelet factors are involved in the pathophysiology of IBD. PF4 is released from alpha-granules of activated platelets and has been confirmed to participate in the thrombosis formation.[35] Previous studies explored the course of PF4 in IBD patients, but the results are conflicting.[16,17,36] Moreover, these studies included small numbers of patients, and none of them systematically explored the relationship between serum PF4 and disease activity.
4.3. Strengths and limitations
Our study enrolled a total of 120 subjects and confirmed a raised level of PF4 in IBD patients. We further compared the diagnostic benefit of using PF4 as a disease activity marker with other serum biological markers. ROC and multivariate analyses demonstrated that PF4 was a good parameter of disease activity in both UC and CD patients. To date, patients with IBD could not be radically cured and need to be monitored disease activity. However, it is not easy to quantify disease activity due to its subjective nature. Endoscopy examinations are invasive. It is both inconvenient and expensive for patients to take repeated endoscopy examinations. Thus, PF4 is an easy and cheap serum biomarker that has great advantage over regular serum markers. Furthermore, we did not classify patients into subgroups in accordance with ulcers locations and durations. The conclusions could be applied to overall patients regardless of disease locations and durations, which may further save a lot of work in clinical practice.
There are some limitations to this study. The number of patients is relatively small, which clearly limits the conclusions. Nevertheless, these results suggest that measuring serum PF4 is feasible, and the encouraging data aid designing future studies. Moreover, since stool markers like calprotectin have become a standard in IBD disease monitoring,[11] comparisons between serum PF4 and stool markers should been made. A modified Mayo score and the Harvey–Bradshaw Index were used as indicators of UC disease activity and CD disease activity, respectively, but they also have drawbacks and may be affected by the subjective descriptions provided by patients. On the contrary, endoscopic activity is still the gold standard for assessing disease activity. However, for IBD patients who were in clinical remission, they would like to avoid the painful examination. While for those whose symptoms were too serious, they may be not suitable to take endoscopic examinations. Therefore, clinical scores were difficult to be matched with endoscopic scores for each patient, and the modified Mayo score and the simplified CDAI were used as indicators in the study. Though mucosal healing is the therapeutic goal, symptom-based therapeutic strategies are still widely accepted in clinical practice. Thus, serum PF4 levels have potential application in guiding clinical decisions.
5. Conclusions
In summary, our work suggests that serum PF4 plays an important role as a marker of disease activity in IBD patients. More researches are warranted to address the potential applications of PF4, like its ability to predict clinical relapse.
Supplementary Material
Acknowledgment
The authors thank the research team from the Life Science School of Nanjing University.
Footnotes
Abbreviations: AUC = the area under the curve, CD = Crohn disease, CDAI = Crohn Disease Activity Index, CRP = C-reactive protein, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, IBD = inflammatory bowel disease, PF4 = serum platelet factor 4, PLT = platelet count, ROC = receiver-operating characteristic, UC = ulcerative colitis, WBC = white blood count.
Funding: The work was supported by National Natural Science Foundation of China (No.81570506). The Foundations providing grants had no role in the study design, the collection, analysis and interpretation of the data, writing the report, or the decision to submit the paper.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- [2].Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012;61:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015;50:942–51. [DOI] [PubMed] [Google Scholar]
- [4].Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380:159–605. [DOI] [PubMed] [Google Scholar]
- [5].Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18:1894–9. [DOI] [PubMed] [Google Scholar]
- [6].Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut 2005;54:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Louis E. Fecal calprotectin: towards a standardized use for inflammatory bowel disease management in routine practice. J Crohns Colitis 2015;9:1–3. [DOI] [PubMed] [Google Scholar]
- [9].D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- [10].Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing fecal calprotectin value in Crohn's disease. J Crohns Colitis 2015;9:1113–9. [DOI] [PubMed] [Google Scholar]
- [11].Dumoulin EN, Van Biervliet S, De Vos M, et al. Faecal leukocyte esterase activity is an alternative biomarker in inflammatory bowel disease. Clin Chem Lab Med 2015;53:2003–8. [DOI] [PubMed] [Google Scholar]
- [12].Dumoulin EN, Van Biervliet S, Langlois MR, et al. Proteolysis is a confounding factor in the interpretation of faecal calprotectin. Clin Chem Lab Med 2015;53:65–71. [DOI] [PubMed] [Google Scholar]
- [13].Meuwis MA, Fillet M, Geurts P, et al. Biomarker discovery for inflammatory bowel disease, using proteomic serum profiling. Biochem Pharmacol 2007;73:1422–33. [DOI] [PubMed] [Google Scholar]
- [14].Harries AD, Fitzsimmons E, Fifeld R, et al. Platelet count; a simple measure of activity in Crohn's disease. Br Med J 1983;286:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol 2004;99:938–45. [DOI] [PubMed] [Google Scholar]
- [16].Wang Z, Huang H. Platelet factor-4 (CXCL4/PF-4): an angiostatic chemokine for cancer therapy. Cancer Lett 2013;331:147–53. [DOI] [PubMed] [Google Scholar]
- [17].Simi M, Leardi S, Tebano MT, et al. Raised plasma concentrations of platelet factor 4 (PF4) in Crohn's disease. Gut 1987;28:336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takeyama H, Mizushima T, Iijima H, et al. Platelet activation markers are associated with Crohn's disease activity in patients with low C-reactive protein. Dig Dis Sci 2015;60:3418–23. [DOI] [PubMed] [Google Scholar]
- [19].Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989;170:2–6. [DOI] [PubMed] [Google Scholar]
- [20].D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–86. [DOI] [PubMed] [Google Scholar]
- [21].Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- [22].Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- [23].Yan SL, Russell J, Harris NR, et al. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis 2013;19:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan SL, Russell J, Granger DN. Platelet activation and platelet leukocyte aggregation elicited in experimental colitis are mediated by interleukin-6. Inflamm Bowel Dis 2014;20:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Solem CA, Loftus EV, Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:707–12. [DOI] [PubMed] [Google Scholar]
- [26].Saverymuttu SH, Hodgson HJ, Chadwick VS, et al. Differing acute phase responses in Crohn's disease and ulcerative colitis. Gut 1986;27:809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn's disease. Dig Dis Sci 2007;52:2063–8. [DOI] [PubMed] [Google Scholar]
- [28].Florin TH, Paterson EW, Fowler EV, et al. Clinically active Crohn's disease in presence of low C-reactive protein. Scand J Gastroenterol 2006;41:306–11. [DOI] [PubMed] [Google Scholar]
- [29].Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther 2007;25:247–55. [DOI] [PubMed] [Google Scholar]
- [30].Denis MA, Reenaers C, Fontaine F, et al. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn's disease with normal C-reactive protein serum level. Inflamm Bowel Dis 2007;13:1100–5. [DOI] [PubMed] [Google Scholar]
- [31].Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63. [DOI] [PubMed] [Google Scholar]
- [32].Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol 2011;106:713–8. [DOI] [PubMed] [Google Scholar]
- [33].Bollen L, Vande Casteele N, Ballet V, et al. Thromboembolism as an important complication of inflammatory bowel disease. Eur J Gastroenterol Hepatol 2016;28:1–7. [DOI] [PubMed] [Google Scholar]
- [34].Senchenkova E, Seifert H, Granger DN. Hypercoagulability and platelet abnormalities in inflammatory bowel disease. Semin Thromb Hemost 2015;41:582–9. [DOI] [PubMed] [Google Scholar]
- [35].Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010;125:292–6. [DOI] [PubMed] [Google Scholar]
- [36].Vrij AA, Rijken J, Van Wersch JW, et al. Platelet factor 4 and beta-thromboglobulin in inflammatory bowel disease and giant cell arteritis. Eur J Clin Invest 2000;30:188–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


