Abstract
Rationale:
Antiphospholipid syndrome (APS) in pregnancy may trigger the life-threatening catastrophic antiphospholipid syndrome (CAPS). Complement activation is implicated in the pathogenesis, and inhibition of complement factor C5 is suggested as an additional treatment option.
Patient concerns, diagnosis and interventions:
We present a pregnant patient treated with the C5-inhibitor eculizumab due to high risk of developing devastating APS-related complications. The complement inhibitory effects of the treatment were examined both in the patient and the premature infant.
Outcomes:
Complement activity in the mother recovered considerably faster than anticipated; however, no new thrombosis or CAPS developed during the last week of pregnancy or postpartum. Blood sampling from the umbilical vein and artery, and from the infant after delivery showed low complement activity; however, only 0.3% of the eculizumab concentration detected in the mother, consistent with low placental passage of eculizumab.
Lessons:
The data underscore the importance of close monitoring of complement inhibition and individualizing dosage regimens in pregnant patients receiving eculizumab. We document how traditional functional complement activity tests cannot assess the effect of eculizumab in premature infants due to the very low levels of complement factors detected in this infant born in gestational week 33. Only trace amounts of eculizumab passed the placenta. In conclusion, complement C5 inhibition might be a safe candidate treatment option for APS during pregnancy and delivery, and additionally, enables prolongation of pregnancy with important weeks.
Keywords: antiphospholipid syndrome, complement, eculizumab, pregnancy
1. Introduction
Antiphospholipid syndrome (APS) is characterized by arterial, venous, or small-vessel thrombosis and/or pregnancy morbidity in the presence of persistent antiphospholipid antibodies (anticardiolipin antibodies, antibeta2 glycoprotein 1 antibodies, and lupus anticoagulant).[1] Although the pathogenesis is not fully understood, the binding of antiphospholipid antibodies to β2 beta2 glycoprotein 1 promotes endothelial cell activation determined by upregulation of adhesion molecules, tissue factor, and production and secretion of proinflammatory cytokines, which enhance the risk of thrombosis formation.[2] Complement appears to play a significant role in the pathophysiology based on both in vitro and in vivo studies.[3–5] Catastrophic APS (CAPS), although rare, is a devastating and life-threatening syndrome featured by multiorgan thrombosis. Infection, surgery, pregnancy, and puerperium are identified triggers of CAPS.[6,7] Current treatment options in addition to anticoagulation are glucocorticoids, plasma exchange, or intravenous immunoglobulins; however, case reports have reported that inhibition of complement may be lifesaving.[8–10]
2. Case report
A 22-year-old primigravida was admitted to hospital in the 2nd trimester with painful ulcerations of ischemic origin in her right leg. Barely 14 years old, she developed her 1st episode of lower limb arterial thrombosis which was treated with bypass grafting and digital amputations. No arteriosclerosis or vasculitis was detected and she was diagnosed with APS, fulfilling the Sydney criteria[1] with persistent triple positive antiphospholipid antibodies: anticardiolipin immunoglobulin G (IgG) 205GPL-U/L (ref < 10 GPL-U/L), antibeta 2 glycoprotein 1 IgG 125 U/mL (ref < 10 U/mL), and positive lupus anticoagulant 2.41 (ref < 1.3 Silica Clotting time). Lifelong warfarin treatment was commenced. A recurrent episode of thrombosis was treated with percutaneous transluminal angioplasty, and an episode of microemboli resolved with intensified anticoagulant treatment.
In conjunction with pregnancy, warfarin was substituted with low molecular weight heparin adjusted up to 10,000 IU twice daily (antifactor Xa levels of 0.9–1.1 IU/mL) and low dose aspirin (75 mg daily). Ischemia was treated conservatively with analgesia in addition to anticoagulation therapy, and pregnancy was monitored by regular ultrasounds following fetal growth and placental function.
Based on her multiple previous arterial thromboses and ongoing ischemia during pregnancy, the risk of developing CAPS in relation to pregnancy, delivery, and puerperium was considered significant. Ruffatti et al[11] published data suggesting that addition of 2nd-line therapy increases live-birth rates in high risk pregnant patients with APS, although no guidelines are currently available on the ideal treatment strategy. Previous experience with the efficacy of the complement C5 inhibitor eculizumab in treatment of CAPS and described safety in pregnancy[8,12,13] prompted the choice of eculizumab. Thus, 600 mg of eculizumab was administered 8 days before delivery (day 0) in addition to prophylactic antibiotics. Serum (prepared by drawing whole blood into empty tubes, left for clotting 60 minutes followed by centrifugation 15 minutes, 3500 g, 4 °C) and ethylenediaminetetraacetic acid (EDTA) plasma (prepared by drawing blood into K2EDTA tubes, followed by immediate centrifugation 15 minutes, 3500 g, 4 °C) samples were obtained from the patient before and at several time points after eculizumab administration and analyzed directly or stored at −70 °C. Complement activity in plasma (Total Complement System Screen, WIESLAB, Malmo, Sweden) decreased to zero after the 1st eculizumab infusion and remained low at day 2, however had returned to normal levels already by day 7 (Fig. 1A). Eculizumab-C5 (E-C5) complexes in serum (enzyme immunoassay as described in ref[12]) increased from zero to 67 after the 1st eculizumab dose (Fig. 1A). Interestingly, the patient reported decreased ischemic pain following the 1st dose of eculizumab, and opioid analgesia was successfully reduced.
Figure 1.
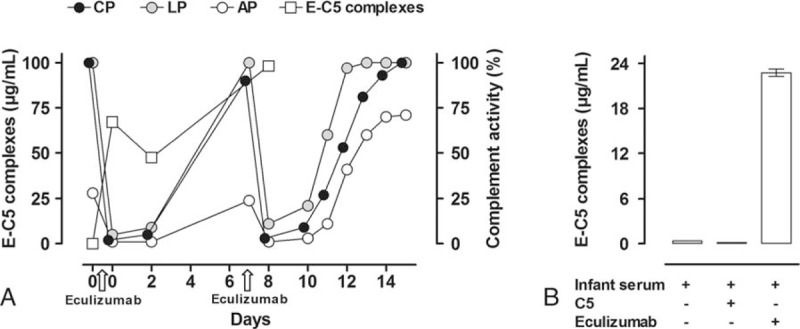
Complement activity and E-C5 complexes in a pregnant patient with APS and the newborn infant. (A) The patient received eculizumab 600 mg day 0 and 7 and a caesarean section was performed on day 8. Effect of eculizumab on complement functional activity was measured as a common readout (C5b-9 formation) for the CP, LP, and AP, by ELISA in patient serum obtained before and repeatedly after the administration of eculizumab. E-C5 complexes were measured by ELISA at day 0, 2, and 8 in the patient serum before and after administration of eculizumab. Complement activity was completely abolished by eculizumab 600 mg, however normalized within 7 days. The increased activity was revealed after 3 days following the 2nd dose. Consistently, E-C5 complexes showed an inverse pattern with high levels following eculizumab administration. (B) The infant's E-C5 complexes were measured by ELISA in infant serum (left column) and subsequently after in vitro challenge with purified complement protein C5 (50 μg/mL) (middle column) and eculizumab (100 μg/mL) (right column). The increased E-C5 complex formation following challenge with eculizumab, but not C5, is consistent with the presence of free C5 in infant serum and negligible levels of eculizumab. AP = alternative pathway, APS = antiphospholipid syndrome, CAPS = catastrophic antiphospholipid syndrome, CP = classical pathway, E-C5 = eculizumab-C5, EDTA = ethylenediaminetetraacetic acid, ELISA = enzyme-linked immunosorbent assay, LP = lectin pathway.
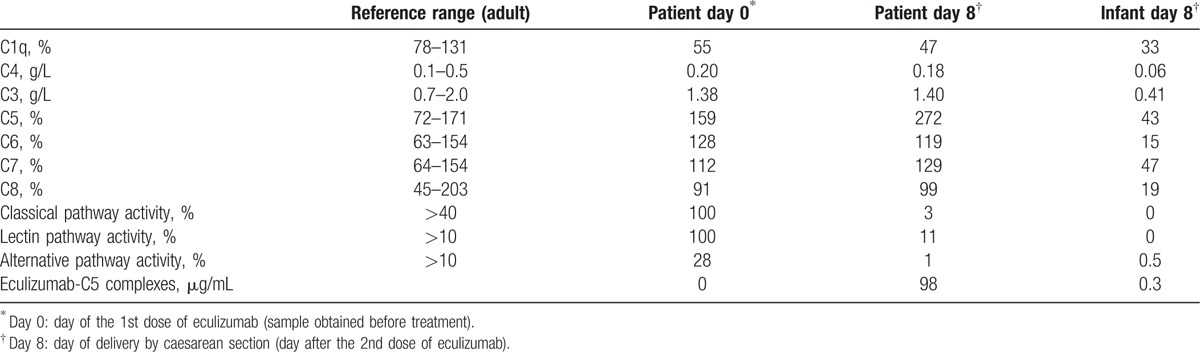
A 2nd dose of eculizumab 600 mg was infused on day 7, and a caesarean section was performed the following day (day 8) in gestational week 32 + 4, resulting in delivery of a healthy infant (Apgar 8/9/9) with a normal weight (1875 g) for gestational age. Serum and EDTA plasma samples were obtained from the umbilical cord artery and vein by careful needle puncture to avoid contamination from mothers’ blood and Wharton jelly, directly after cord clamping during caesarean section. In addition, EDTA-plasma was obtained from the infant 2 hours after delivery. Samples were analyzed directly or stored at −70 °C. The infant's total complement activity was <1%, however consistent with the measured low complement protein concentrations (standard routine immunochemical methods, Department of Clinical Immunology and Transfusion Medicine, Lund, Sweden) as expected at gestational week 32 (Table 1).[14] Umbilical and infant E-C5 complexes were 0.3 μg/mL. Concomitant patient E-C5 complexes were 98 μg/mL consistent with a 0.3% placental passage of eculizumab (Table 1). To further support the findings of low eculizumab passage, we added eculizumab (final concentration of 100 μg/mL) or purified C5 (50 μg/mL), respectively, to the infant serum in vitro and subsequently analyzed for E-C5 complexes. Eculizumab, but not C5, increased the E-C5 complex formation in infant serum substantially (Fig. 1B), consistent with the presence of free C5 (Table 1) and negligible amounts of eculizumab.
Table 1.
Complement protein levels, complement activity, and eculizumab-C5 complexes in samples from a pregnant eculizumab-treated patient with antiphospholipid syndrome before delivery and from the patient and newborn infant immediately after delivery.
Close monitoring of the patient's complement activity after the 2nd dose revealed increased activity already after 3 days and again normal activity within 1 week (Fig. 1A). The patient did not develop new thrombosis or CAPS during the last week of pregnancy or postpartum. The intervention given (eculizumab) was part of normal health care and thus ethical approval was neither obliged nor sought. However, written consent was obtained from the patient in order to publish the case report.
3. Discussion
A history of thrombosis and triple positive antiphospholipid antibodies, as seen in the present patient, is associated with a higher risk of maternal and fetal complications in pregnant women with APS.[11,15] Our patient demonstrated multiple risk factors and showed clinical signs of thrombosis despite intensive anticoagulant treatment. Additionally, the risk of peri- and postoperative hemorrhage due to intense anticoagulant treatment was high, while on the other hand, surgery and cessation of anticoagulant treatment would increase the risk of triggering CAPS. Consequently, additional treatment was warranted. Eculizumab has been suggested in several recent reports as a 2nd-line treatment option in APS and CAPS.[6,8,16] Hence, a short term course of eculizumab was chosen before cesarean section to reduce the risk of CAPS triggered by delivery and puerperium. The ischemic symptoms declined following the 1st dose and no adverse effects were seen.
Complement activity surprizingly increased to normal levels within 7 days after both doses of eculizumab despite complete inhibition of complement activity after each infusion. Others have observed a similar requirement of an increased eculizumab dosage regimen as pregnancy proceeds.[13] Eculizumab is distributed mainly in the plasma volume and degraded by lysosomal enzymes.[17] However, pregnancy-induced changes of the normal physiology may influence the pharmacokinetics and pharmacodynamics of eculizumab, underscoring the importance of individual treatment monitoring.
Eculizumab is a monoclonal antibody based on an IgG subclass 2/4 hybrid. Data from pregnancies in patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab suggest that the treatment is safe.[13] We examined both arterial and venous blood samples from the umbilical cord, as well as a blood sample from the infant 2 hours after delivery. All 3 samples showed similar results, suggesting that virtually no eculizumab (approximately 0.3%) had passed the placenta. To our knowledge the amount of eculizumab has previously not been examined in preterm newborns at this stage of gestation. Collectively, these data indicate that the IgG subclass 2/4 chimeric structure of eculizumab largely prevents the molecule from passing the placental barrier.
The observed complement activity of less than 1% in the infant could have been misinterpreted as an eculizumab effect. Eculizumab blocks cleavage of C5 and thus prevents assembly of the terminal C5b-9 complement complex detected in the complement function enzyme-linked immunosorbent assay (ELISA). However, a normal complement activity test requires adequate levels of complement proteins to produce sufficient amounts of TCC for assay detection. Premature infants have low levels of most complement proteins,[14] as did the infant in this report. The detection of merely trace levels of E-C5 complexes in the infant samples and the results from the in vitro assay demonstrating that only the addition of eculizumab, but not purified C5, could increase the E-C5 complex formation in infant serum, indicate that the low complement activity resulted from the low levels of most of the complement proteins (eg, C4, C3, C5, C6, and C8), and was not due to the presence of eculizumab. Consequently, abnormal results in complement activity assays, such as in premature infants, must be carefully interpreted to avoid incorrect conclusions.
4. Conclusion
In conclusion, these results add up to the increasing body of literature stating that eculizumab treatment can be considered safe in pregnancy as negligible amounts pass over the placental barrier. Our data suggest complement inhibition as a treatment option to safely prolong pregnancy and reduce the risk of CAPS triggered by pregnancy, surgery, and puerperium without affecting the infant. In addition, we stress the importance of monitoring treatment effects of eculizumab using reliable methods to ensure individual adequate complement inhibition.
Footnotes
Abbreviations: APS = antiphospholipid syndrome, CAPS = catastrophic antiphospholipid syndrome, E-C5 = eculizumab-C5, EDTA = ethylenediaminetetraacetic acid, IgG = immunoglobulin G.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- [2].Allen KL, Fonseca FV, Betapudi V, et al. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2012;119:884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med 2002;195:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Romay-Penabad Z, Carrera Marin AL, Willis R, et al. Complement C5-inhibitor rEV576 (coversin) ameliorates in-vivo effects of antiphospholipid antibodies. Lupus 2014;23:1324–6. [DOI] [PubMed] [Google Scholar]
- [5].Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:1033–44. [DOI] [PubMed] [Google Scholar]
- [6].Gomez-Puerta JA, Espinosa G, Cervera R. Catastrophic antiphospholipid syndrome: diagnosis and management in pregnancy. Clin Lab Med 2013;33:391–400. [DOI] [PubMed] [Google Scholar]
- [7].Cervera R, Rodriguez-Pinto I. Catastrophic antiphospholipid syndrome: task force report summary. Lupus 2014;23:1283–5. [DOI] [PubMed] [Google Scholar]
- [8].Barratt-Due A, Floisand Y, Orrem HL, et al. Complement activation is a crucial pathogenic factor in catastrophic antiphospholipid syndrome. Rheumatology (Oxford) 2016;55:1337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kronbichler A, Frank R, Kirschfink M, et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine (Baltimore) 2014;93:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez-Pintó I, Espinosa G, Cervera R. Catastrophic antiphospholipid syndrome: the current management approach. Best Pract Res Clin Rheumatol 2016;30:239–49. [DOI] [PubMed] [Google Scholar]
- [11].Ruffatti A, Salvan E, Del Ross T, et al. Treatment strategies and pregnancy outcomes in antiphospholipid syndrome patients with thrombosis and triple antiphospholipid positivity. A European multicentre retrospective study. Thromb Haemost 2014;112:727–35. [DOI] [PubMed] [Google Scholar]
- [12].Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015;220:452–9. [DOI] [PubMed] [Google Scholar]
- [13].Kelly RJ, Hochsmann B, Szer J, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 2015;373:1032–9. [DOI] [PubMed] [Google Scholar]
- [14].McGreal EP, Hearne K, Spiller OB. Off to a slow start: under-development of the complement system in term newborns is more substantial following premature birth. Immunobiology 2012;217:176–86. [DOI] [PubMed] [Google Scholar]
- [15].Bramham K, Hunt BJ, Germain S, et al. Pregnancy outcome in different clinical phenotypes of antiphospholipid syndrome. Lupus 2010;19:58–64. [DOI] [PubMed] [Google Scholar]
- [16].Erkan D, Aguiar CL, Andrade D, et al. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014;13:685–96. [DOI] [PubMed] [Google Scholar]
- [17].Dmytrijuk A, Robie-Suh K, Cohen MH, et al. FDA report: eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist 2008;13:993–1000. [DOI] [PubMed] [Google Scholar]


