Abstract
Pain is prevalent in advanced malignancies; however, some patients cannot get adequate pain relief by conservative routes of analgesic administration or experience serious side effects related to high dose of opioids. For those who have exhausted multimodal conservative analgesic, intrathecal drug delivery is an alternative intervention for truly effective pain management. The objective of this study was to evaluate the clinical efficacy and safety of intrathecal drug delivery system (IDDS) for the treatment of intractable pain in advanced cancer patients.
A prospective cohort study was performed between July 2015 and October 2016. Fifty-three patients undergoing intractable cancer-related pain or intolerable drug-related adverse effects were recruited and received IDDS therapy with a patient-controlled intrathecal analgesia pump. The assessment was conducted during admission, in titration period, and followed up monthly to death by scheduled refill visits. Pain numeric rating scale scores, comprehensive toxicity scores, quality of life scores, systemic opioid use (basal and breakthrough dose), intrathecal morphine use (basal and patient-controlled intrathecal analgesia dose), and complications were recorded to evaluate the curative effect and safety.
Between baseline and all subsequent follow-ups, statistically significant decreases in pain numeric rating scale scores and comprehensive toxicity scores were verified. A statistical improvement in quality of life scores was found after starting IDDS therapy. Both basal and breakthrough doses of systemic opioid showed a significant decrease during the follow-up period. And there was a modest escalation in the intrathecal morphine dose throughout the duration of study. No infective, device-related, and catheter-related complications were observed.
The findings showed that IDDS therapy allowed for rapid and highly effective pain relief with less toxicity in comparison to conservative medications. Patients with advanced malignancies would also benefit from an improvement in the life quality after the procedure. IDDS therapy represented a valuable option for intractable cancer-related pain management.
Keywords: advanced malignancies, intractable pain, intrathecal drug delivery system (IDDS), morphine, patient-controlled intrathecal analgesia (PCIA), ropivacaine
1. Introduction
It had been reported that pain was prevalent in cancer patients with metastatic or advanced stage, and >33% of patients graded their pain as moderate or severe.[1] Although the World Health Organization introduced the Guidelines on Pain Relief Ladder for cancer pain in 1986, adequate pain relief still could not be available in approximately 10%–30% of cancer patients with limited life expectancy.[2–4] Moreover, long-term opioid use could produce tolerance and hyperalgesia, leading to diminishing response to medication and hypersensitivity to pain.[5,6] Opioid doses were constantly escalated to achieve the same analgesic effect; on the contrary, the risk of adverse effects such as respiratory depression, nausea, and constipation was highly increased.[7] For patients who failed to respond to conservative medications, there was increasing evidence that the intrathecal (IT) route provided more effective analgesia, especially when efficacy was limited by dose-dependent toxicity.[8,9] The aim of our study was to observe the clinical response of intrathecal drug delivery system (IDDS) for the treatment of intractable pain in advanced cancer patients who required a more effective management than conservative administration to control pain.
2. Methods
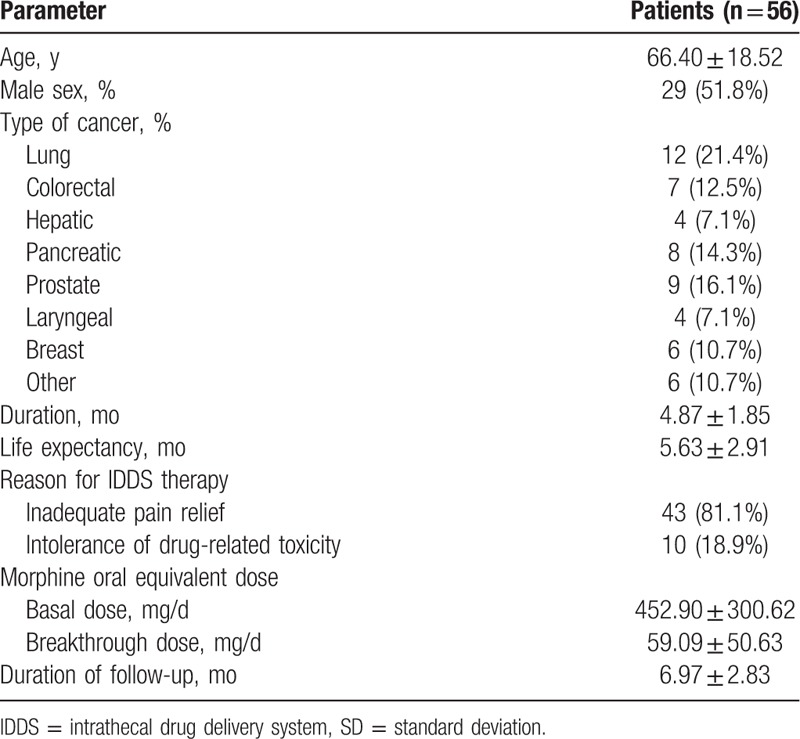
2.1. Patients
A total of 53 patients with intractable cancer-related pain were recruited in the prospective cohort study and scheduled to receive IDDS implantation at our pain center in Central Hospital of China Aerospace Corporation (Aerospace Clinical Medical School of Peking University), Beijing, China, between July 2015 and October 2016 (Fig. 1). Patients were eligible if they had pathologically or clinically diagnosed advanced malignancies with a life expectancy of at least 3 months, failed to respond to conservative management on at least 200 mg/d of oral morphine or the equivalent with pain intensity rated 7 to 10 on numeric rating scale (NRS), or were on lower doses but with poor tolerability of systemic opioids due to serious toxicity. We excluded patients who had coagulation disorders and disturbance of cerebrospinal fluid (CSF) circulation arising from neoplastic invasion of spine or spine disorders shown in magnetic resonance imaging, such as spinal stenosis, spondylolisthesis, and postlaminectomy. Those who simply could not endure the implantation procedure due to serious systemic disease were also excluded. All recruits agreed and signed informed consent prior to procedure. After starting IDDS therapy with an external electronic pump, follow-ups were performed monthly to death by periodical refill visits. Data were collected at baseline (T0), at the time of discharge (T dis), and then at 1 month (T1) and 3 months (T3) after procedure, as well as at the last observation (1 week before death, T death) during follow-up period. The study was authorized by the institution's Ethics Examining Committee of Human Research. And written informed patient consent was obtained in all recruits.
Figure 1.
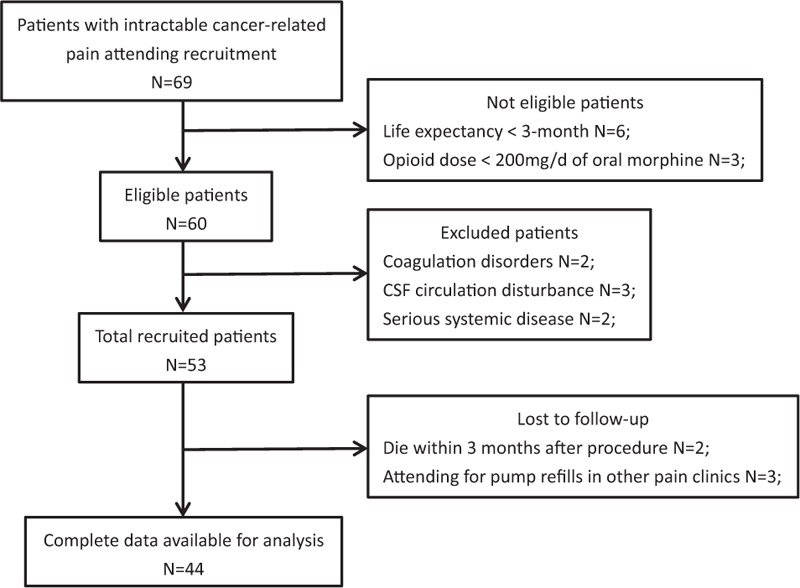
The flowchart of patient recruitment. CSF = cerebrospinal fluid.
2.2. Procedure
According to the expert consensus guidelines, the IT morphine screening trial and psychological assessment were not necessarily required in advanced cancer patients before implantation.[10] After carefully weighing the benefits and risks of procedure and IT analgesia, all the 53 patients were elected to undergo the procedure under a local anesthesia in the operating room. The spine area was sterilely prepped and draped, with patients being in the lateral position. Under the guidance of X-ray fluoroscopy, an introducer needle of 15-gauge was advanced obliquely with an angle of approximately 30° at the skin entry point. The point was parallel to vertebral pedicle, 1 to 2 cm lateral to the midline, and equivalent 1 to 1.5 spinous process distance subjacent to the L2 to L3 interspace. After the needle successfully got access to the CSF, the IT catheter was implanted into the IT space through the trocar. The tip of catheter was finally placed between 7 and 10 thoracic vertebral level. After confirmation by fluoroscopy, a subcutaneous tunnel was built and the catheter was attached to a subcutaneous port via the tunnel. The subcutaneous port was placed in the subcutaneous pouch that was parallel to the costal arch on the anterior axillary line in the lower abdomen. Finally, the port was fixed beneath the fascia by suturing the pouch. The IT infusion system was connected to a patient-controlled external drug infusion pump by a butterfly-shaped special needle vertically inserting into the subcutaneous port. All procedures were performed by the same doctor.
2.3. Drug use
All patients received IT application of morphine–ropivacaine mixtures that were diluted with 0.9% sodium chloride solution to 100 mL and filled into the electronic patient-controlled intrathecal analgesia (PCIA) pump. The initial IT morphine dose was calculated from the baseline opioid use with an oral–IT morphine conversion ratio of 300:1.[11] The initial infusion was started at a flow rate of 0.1 mL/h without PCIA dose for safety reasons. IT morphine titration was conducted 1 day after the procedure. Patients were suggested to stop taking opioids by other routes. The IDDS parameters programmed by pump were adjusted every 12 hours until sufficient pain relief (≥50% pain reduction in NRS and breakthrough pain ≤3 times a day) and improvements in side effects (≥50% reduction in toxicity) were achieved. The parameters contained basal morphine dose, delivery velocity, bolus dose, lockout interval, number of bolus allowed, and maximum dose in a 24-hour period. After successful titration, patients were discharged. Scheduled refill visits were conducted monthly during follow-up period. And pump refill would be performed by doctors from the same hierarchy according to the same criteria for patient evaluation to control bias. Moreover, rescue oral opioid medication was allowed to cope with flare-up pain over the duration of follow-up period.
2.4. Patient assessment
The patient's pain intensity was assessed using 0 to 10 pain NRS (0 = no pain and 10 = worst pain). Drug-related adverse effects were evaluated by comprehensive toxicity scores extracted from National Cancer Institute Common Toxicity Criteria.[12] A total of 15 individual drug toxicity scores (0–4) according to the National Cancer Institute Common Toxicity Criteria standard scales were measured. The comprehensive toxicity score was calculated by summing the grade of all the 15 toxicities for each patient at each follow-up visit.[13] The European Organization for the Research and Treatment of Cancer core quality of life (QOL) questionnaire, European Organization for the Research and Treatment questionnaire QLQ-C30, was used for the assessment of patients’ QOL.[14] According to the questionnaire, excellent QOL was defined by scores ranging from 51 to 60, good QOL from 41 to 50, fair QOL from 31 to 40, poor QOL from 21 to 30, and very poor QOL <30. Opioid use was calculated by morphine oral equivalent dose.[11] Systemic opioid use consisted of controlled-release opioid use (basal dose) and immediate-release as-needed opioid use (breakthrough dose). IT opioid use included continuous infusion morphine use (basal dose) and PCIA morphine use (PCIA dose).
2.5. Statistical analysis
Statistical analyses were performed by SPSS software, version 19.0 (SPSS Inc, Chicago, IL). Statistical significance was set at the 5% level (2-tailed). Normal quantitative data were reported as mean ± standard deviation. The repeated-measures analysis of variance test was used to investigate the changes in IT morphine dose over the duration of the follow-up period, followed by Bonferroni test for post hoc analysis. Other non-normal quantitative data including pain NRS scores, comprehensive toxicity scores, and systemic opioid dose, as well as the categorical variable (QOL scores), were analyzed by Wilcoxon signed-rank test.
3. Results
Complete data for analysis were available in 44 subjects. Seven patients (13.2%) died within 3 months after starting IT therapy due to tumor progression. Two patients were lost to follow-up, both of them attended for pump refills in another pain clinic close to their home (Fig. 1). The losses to follow-up were excluded from statistical analysis. Baseline characteristics of all the 53 recruits are shown in Table 1.
Table 1.
Baseline characteristics of patients (mean ± SD).
Statistically significant decreases in pain NRS scores and comprehensive toxicity scores were verified between baseline and all subsequent follow-ups (Fig. 2). Prior to procedure, patients’ median pain NRS score was 8.5 with interquartile range (IQR) from 8 to 9. The pain NRS score decreased to 3 (IQR: 2–3, P < 0.05), 3 (IQR: 2–3, P < 0.05), 3 (IQR: 3–4, P < 0.05), and 4 (IQR: 3.25–5, P < 0.05) at discharge time (T dis), 1 month (T1) and 3 months (T3) after procedure, and 1 week before death (T death), respectively (Fig. 2). And significant pain relief (≥50% pain reduction in NRS) was observed in 86.4% (38/44), 79.5% (35/44), 63.6% (28/44), and 52.3% (20/44) of patients at T dis, T1, T3, and T death follow-up, respectively (Fig. 3). The median comprehensive toxicity scores also significantly decreased to 2 (IQR: 2–3, P < 0.05), 3 (IQR: 2–3, P < 0.05), 3 (IQR: 3–4, P < 0.05), and 5 (IQR: 4–5, P < 0.05) at T dis, T1, T3, and T death follow-up, respectively, compared with baseline value 6 (IQR: 5–7) (Fig. 2).
Figure 2.
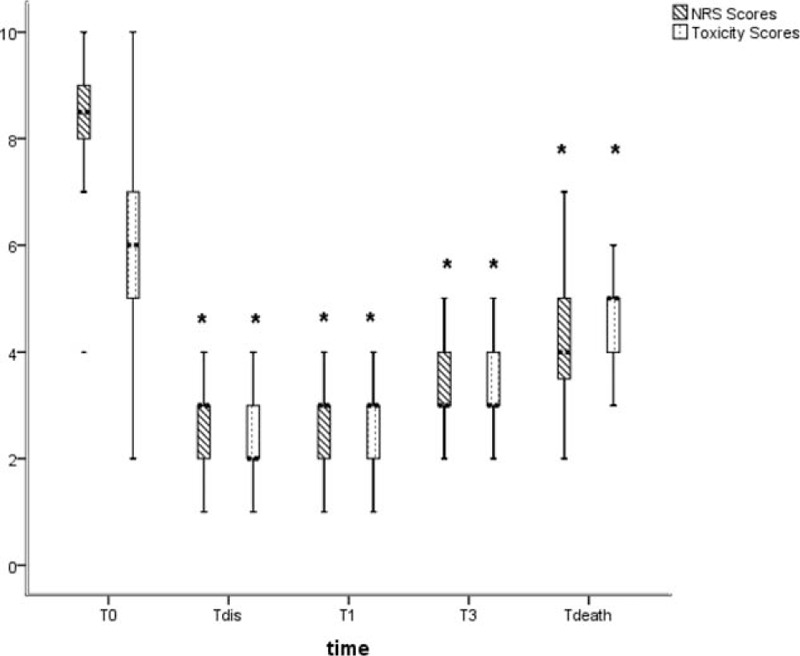
A boxplot of pain NRS scores and toxicity scores at baseline and different follow-up visits after procedure. (∗) A significant difference compared with baseline value (P < 0.05). NRS = numeric rating scale; T death = 1 week before death; T dis = the time of discharge, T0 = baseline, T1 = 1 month after procedure, T3 = 3 months after procedure.
Figure 3.
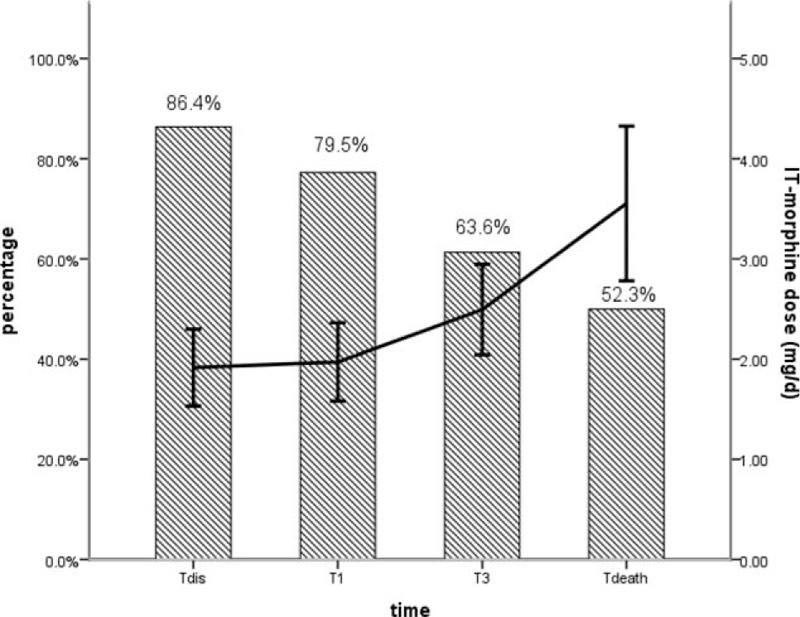
The proportion of patients with significant pain relief (≥50%) and intrathecal morphine dose over the follow-up period. Error bars represent 95% CI for the mean. CI = confidence interval, IT = intrathecal; T death = 1 week before death; T dis = the time of discharge, T0 = baseline, T1 = 1 month after procedure, T3 = 3 months after procedure.
At baseline, 53 patients took a mean morphine oral equivalent of 452.90 mg/d (range: 219–1600 mg/d) (Table 1). However, the majority of patients (90.1%, 48/53) were off all controlled-release opioid use over the follow-up period (Table 2). Prior to IT therapy, 92.5% (49/53) of patients used immediate-release opioid to control breakthrough pain with an average of 59.09 mg/d (range: 10–300 mg/d) (Table 1). At T dis, T1, T3, and T death follow-up, the respective number of patients who used rescue oral opioid for breakthrough pain was 6.8% (3/44), 25% (11/44), 38.6% (17/44), and 47.7% (21/44) (Table 2). Both basal and breakthrough doses of systemic opioid showed a significant decrease after IT therapy.
Table 2.
Systemic opioid daily use over the study period.
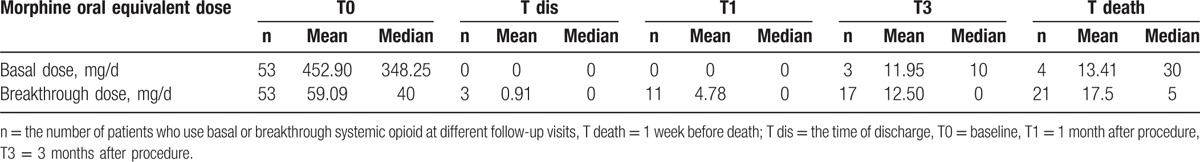
All of the 53 patients were treated with IT morphine–ropivacaine mixtures after procedure. Increases in basal IT morphine dose started to be significant at 3-month follow-up, remaining the rest of study. The basal IT morphine dose of 1.69 ± 1.18 mg/d (95% confidence interval [CI]: 1.35–2.06) at T dis increased gradually to 1.71 ± 1.16 mg/d (95% CI: 1.33–2.05) at T1, 2.13 ± 1.36 mg/d (95% CI: 1.72–2.55) at T3, and 3.02 ± 2.33 mg/d (95% CI: 2.31–3.73) at last follow-up (Table 3). And from 3-month follow-up onward, significant differences were verified with subsequent doses (Table 3). Although significant differences were found between T dis follow-up and all subsequent observations, there was a slow increase in PCIA morphine dose with the average rising to 0.54 mg/d (95% CI: 0.44–0.63) from 0.21 mg/d (95% CI: 0.17–0.25) (Table 3). The morphine dose escalations were modest throughout the duration of IT therapy (Fig. 3).
Table 3.
Intrathecal morphine daily use after procedure (mean ± SD).
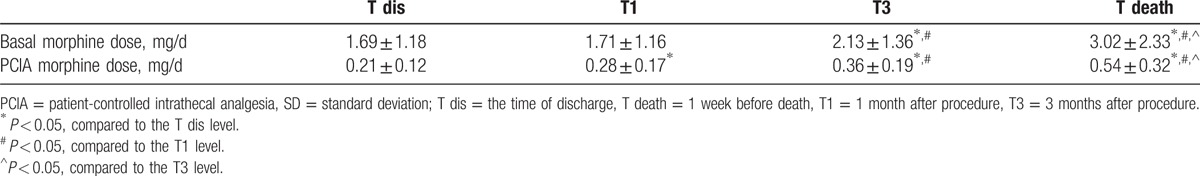
Figure 4 shows a statistical improvement in QOL after starting IT therapy. Compared with baseline value, the QOL scores were better at discharge time and sustained for the whole follow-up period (P < 0.05). At T dis, T1, and T3 follow-up, the proportion of patients who reported “excellent” and “good” ratings were 79.5%, 47.7%, and 18.2%, respectively.
Figure 4.
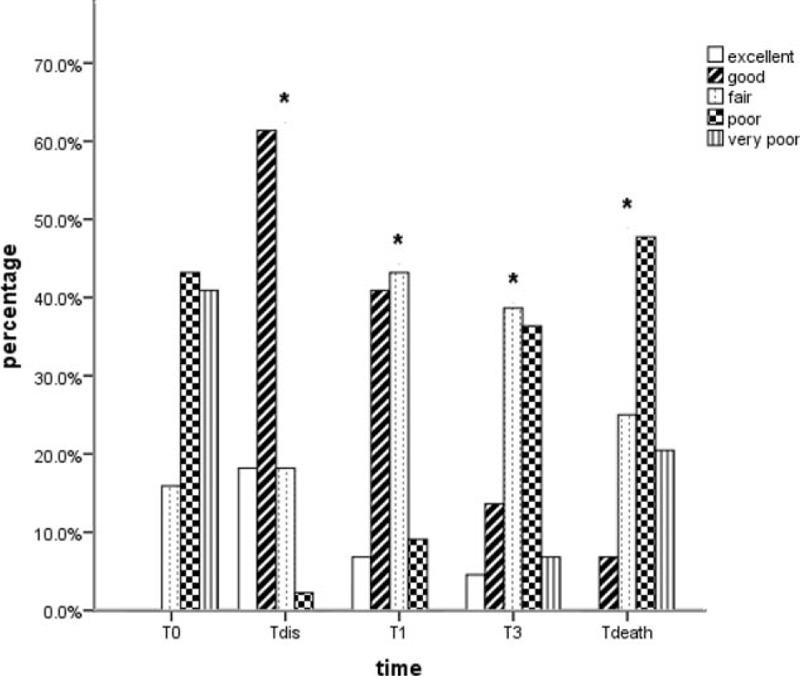
Bar chart of QOL scores at baseline and all subsequent observations in follow-up period. (∗) A significant difference compared with baseline value. QOL = quality of life; T death = 1 week before death; T dis = the time of discharge, T0 = baseline, T1 = 1 month after procedure, T3 = 3 months after procedure.
There were no infective, device-related, and catheter-related complications in our study. Mild lower extremity weakness was reported in 3 patients. All of them achieved remission after reducing the dose of ropivacaine. Five patients developed urinary hesitancy, but only 1 of them required bladder catheterization for a few days.
4. Discussion
In the study, statistically significant decreases in pain NRS scores and comprehensive toxicity scores were verified with a statistical improvement in QOL scores after procedure. Systemic opioid showed a significant decrease; moreover, there was a modest escalation in the IT morphine dose over the follow-up period.
Pain was one of the most unbearable suffering and torture in advanced cancer patients.[1] Poor pain control not only worsened life quality but also had negative effect on patient long-term survival rate.[15] Therefore, rapid and adequate analgesia was of paramount importance to those with advanced cancer with limited life expectancy. Although most patients got satisfactory pain control by the systemic administration of opioids, there were still some patients who failed conservative therapy with little benefit or intolerable side effects in clinical practice.[16] Thus, as one of the interventional treatments, IT delivery was considered as an alternative route for opioid use to alleviate pain more effectively.[17] We performed this prospective study to evaluate the clinical efficacy and safety of IDDS for the treatment of advanced cancer-related pain.
Studies had demonstrated that the dorsal horn of the spinal cord within IT space played an important role in pain processing. There were various receptor targets including opioid receptors (μ, κ, and δ) that could produce powerful analgesic effect after being activated. Once morphine was delivered directly into the CSF, it could get in close proximity to and bind to opioid receptors on the postsynaptic membrane. The activated receptors could change the transmembrane distribution of calcium and potassium ions to inhibit the release of presynaptic neurotransmitter such as substance P and calcitonin gene-related peptide.[18] And then the activity of the postsynaptic neurons was prevented due to hyperpolarization of the postsynaptic membrane. Accordingly, pain signal transmission was successfully blocked.[19]
The IT drug delivery to the site of action could avoid the first-pass metabolism and crossing the blood–brain barrier.[20,21] Consequently, it allowed smaller use of opioids with a more rapid and effective response than oral or parenteral administration, resulting in reducing serious toxicity of high dose of systemic opioids. In accordance with what was expected, our results showed that statistical decreases were found in pain NRS scores and drug-related toxicity scores after starting IT therapy. There were always >50% of patients maintaining their pain score at a value <50% of the initial value during the follow-up period (Figs. 2 and 3). The modest increase in pain during the late period of treatment could be related to the progression of the disease or the development of opioid tolerance. The IT therapy significantly alleviated intractable cancer-related pain and offered a long-term effective analgesia with a decrease in drug-related side effects until death. And the benefits of adequate pain relief with less toxicity translated into improvements in patient life quality with significantly better QOL scores (Fig. 4).
Except for very much smaller use of morphine (one three-hundredth of oral morphine dose), there was a slow increase in IT morphine dose according to our results (Fig. 3; Table 3). Statistical differences were found in daily IT morphine dose until 3 months after procedure, with a <1-fold increase during the 6.97 ± 2.83–month follow-up duration (Table 3), consistent with previously reported results.[22] The small dose escalation may be attributed to the addition of ropivacaine to IT morphine. Previous research confirmed that IT local anesthetics had synergetic effect with morphine to enhance the analgesic efficacy and reduce the dose of IT opioid.[23] Furthermore, the IDDS delivered analgesic continuously into the CSF to keep drug consistently stable in blood. Without frequent appearance of peak plasma concentration, the development of opioid tolerance or addition could be delayed, leading to the modest escalation in IT morphine dose. Thus, the use of continuous infusion appeared to contribute to a morphine-sparing effect. Generally speaking, breakthrough pain could not be adequately controlled by only basal IT delivery. In our study, all the patients were permitted to give an additional dose to cover transitory pain fluctuations using the external electronic pump with a programmable bolus option by themselves. With the on-demand bolus dose, averaging about three to six times per day, the unpredictable breakthrough pain was rapidly intervened and effectively controlled. And after the initiation of IT therapy, the rescue oral opioid medications were performed only to cope with fluctuations in pain intensity, especially breakthrough pain. Therefore, the systemic opioid use during follow-up period was observed in very small dose and had limited effect on the results (Table 2).
We concluded that IT administration of morphine–ropivacaine mixtures by IDDS for the treatment of cancer-related pain could provide rapid and highly effective pain relief. Patients who were suffering from intractable pain in advanced malignancies would benefit from long-term improvements of analgesia and life quality with less toxicity and opioid consumption. IDDS represented a valuable option for cancer-related pain management.
The limitation of the present study was that this was a nonrandom observational study with a small number of investigated patients.
Acknowledgments
The authors thank Professor Jiaxiang Ni for help in procedure technology consulting. And Professor Ni gave his permission to be named.
Footnotes
Abbreviations: CI = confidence interval, CSF = cerebrospinal fluid, IDDS = intrathecal drug delivery system, IQR = interquartile range, IT = intrathecal, NRS = numeric rating scale, PCIA = patient-controlled intrathecal analgesia, QOL = quality of life.
The authors have no conflicts of interest to disclose.
References
- [1].van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49. [DOI] [PubMed] [Google Scholar]
- [2].Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA 1995;274:1870–3. [PubMed] [Google Scholar]
- [3].Walker VA, Hoskin PJ, Hanks GW, et al. Evaluation of WHO analgesic guidelines for cancer pain in a hospital-based palliative care unit. J Pain Symptom Manage 1988;3:145–9. [DOI] [PubMed] [Google Scholar]
- [4].Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65–76. [DOI] [PubMed] [Google Scholar]
- [5].Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014;10:383–93. [DOI] [PubMed] [Google Scholar]
- [6].Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology 2016;124:483–8. [DOI] [PubMed] [Google Scholar]
- [7].Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Newsome S, Frawley BK, Argoff CE. Intrathecal analgesia for refractory cancer pain. Curr Pain Headache Rep 2008;12:249–56. [DOI] [PubMed] [Google Scholar]
- [9].Smyth C, Ahmadzai N, Wentzell J, et al. Intrathecal analgesia for chronic refractory pain: current and future prospects. Drugs 2015;75:1957–80. [DOI] [PubMed] [Google Scholar]
- [10].Deer TR, Prager J, Levy R, et al. Polyanalgesic consensus conference—2012: recommendations on trialing for intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation 2012;15:420–35. [discussion 435]. [DOI] [PubMed] [Google Scholar]
- [11].Bhatia G, Lau ME, Koury KM, et al. Intrathecal drug delivery (ITDD) systems for cancer pain. F1000Res 2013;2:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kautio AL, Haanpaa M, Kautiainen H, et al. Oxaliplatin scale and National Cancer Institute-Common Toxicity Criteria in the assessment of chemotherapy-induced peripheral neuropathy. Anticancer Res 2011;31:3493–6. [PubMed] [Google Scholar]
- [13].Smith TJ, Coyne PJ, Staats PS, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol 2005;16:825–33. [DOI] [PubMed] [Google Scholar]
- [14].King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 1996;5:555–67. [DOI] [PubMed] [Google Scholar]
- [15].Dobosz L, Stefaniak T, Dobrzycka M, et al. Invasive treatment of pain associated with pancreatic cancer on different levels of WHO analgesic ladder. BMC Surg 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lordon SP. Interventional approach to cancer pain. Curr Pain Headache Rep 2002;6:202–6. [DOI] [PubMed] [Google Scholar]
- [17].Ghosh A, Berger A. Opioids, adjuvants, and interventional options for pain management of symptomatic metastases. Ann Palliat Med 2014;3:172–91. [DOI] [PubMed] [Google Scholar]
- [18].Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 2001;81:299–343. [DOI] [PubMed] [Google Scholar]
- [19].McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev 2013;CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand 1997;41(1 pt 2):116–22. [DOI] [PubMed] [Google Scholar]
- [21].D’Honneur G, Gilton A, Sandouk P, et al. Plasma and cerebrospinal fluid concentrations of morphine and morphine glucuronides after oral morphine. The influence of renal failure. Anesthesiology 1994;81:87–93. [DOI] [PubMed] [Google Scholar]
- [22].Malhotra VT, Root J, Kesselbrenner J, et al. Intrathecal pain pump infusions for intractable cancer pain: an algorithm for dosing without a neuraxial trial. Anesth Analg 2013;116:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain 1993;55:119–23. [DOI] [PubMed] [Google Scholar]


