Abstract
Background:
Pretreatment hematologic parameters of the inflammatory response, including lymphocyte, neutrophil, and platelet counts, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio, have emerged as prognostic factors for patients with cancer. This systematic review and meta-analysis aimed to summarize the association between the hematologic markers and prognosis of nasopharyngeal carcinoma (NPC).
Methods:
A systematic search of PubMed, Google Scholar, MEDLINE, EMBASE, Web of Science, and the Cochrane Library was conducted up to April 2016. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were extracted and synthesized to examine prognostic outcomes including cancer-specific survival (CSS), overall survival (OS), progression-free survival (PFS), distant metastasis-free survival, and local relapse-free survival (LRFS).
Results:
Fourteen studies comprising 11,651 NPC patients were ultimately included, and all eligible studies were conducted in East Asia. The OS, CSS, PFS, distant metastasis-free survival, and LRFS risks differed among patients according to hematologic marker levels. All of the parameters were associated with prognostic outcomes in patients with NPC. NLR and lymphocyte counts were most commonly reported. A high NLR was significantly associated with poor NPC prognosis (pooled HR 1.42, 95% CI 1.21–1.67 for CSS; pooled HR 1.77, 95% CI 1.41–2.23 for OS; pooled HR 1.67, 95% CI 1.36–2.06 for PFS; pooled HR 1.64, 95% CI 1.15–2.34 for LRFS). High lymphocyte count indicated favorable NPC prognosis (pooled HR 0.72, 95% CI 0.64–0.81 for OS; pooled HR 0.71, 95% CI 0.56–0.91 for PFS).
Conclusions:
Meta-analysis indicated that NLR and lymphocyte counts could be prognostic predictors in NPC for East Asian population. Patients with a high NLR or low lymphocyte count had poor prognosis. However, due to the limitation of included population, the conclusion was limited to East Asian patients only.
Keywords: hematologic markers, lymphocyte, nasopharyngeal carcinoma, neutrophil-to-lymphocyte ratio, prognosis
1. Introduction
Nasopharyngeal carcinoma (NPC) is the most frequent cancer originating in the nasopharynx, which exhibits a distinct endemic distribution consisting of a particularly high incidence in Southern China and Southeast Asia.[1] Because of inherent anatomic location and radiosensitivity, radiotherapy with or without chemotherapy is the standard treatment for NPC.[2] Currently, the prognosis of patients with NPC is primarily evaluated using the Tumor, Node, Metastasis (TNM) staging system; however, sometimes there are discrepancies between TNM stages and the clinical outcomes in some cases.[3] Patients within the same staging category showed various survival outcomes because of the inability of the TNM system to reflect biological heterogeneity among tumors.[1,4] Thus, the identification of novel prognosis-related biomarkers may complement the TNM system.
Recent studies have reported a number of additional prognostic markers of NPC, which may be significantly associated with the prognosis, such as circulating Epstein-Barr virus (EBV) DNA loads,[5] microRNA signatures,[6,7] and abnormal expression of some functional proteins,[8,9] but kinds of limitations, such as cost efficiency, detection difficulty, and interlaboratory variability limited the application of these biomarkers in actual clinical use. Therefore, it is of great value to further screen for some easily applicable markers. Apart from these prognostic factors representing tumor status and molecular biology characters mentioned above, emerging evidences showed that the host inflammatory response, in particular, the systemic inflammatory response, plays an important role in the development and progression of cancer and can be implicated as a promoter of various cancers. The connection between inflammation and cancer has led to emerging interest in the prognostic value of inflammatory factors.[10,11] Many hematologic parameters of systemic inflammatory response including leukocyte counts, neutrophil counts, monocyte counts, platelet counts, and the ratios between them such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were found to be prognostic markers in various cancers.[12–14] Importantly, these are cheap and easily acquired markers compared with other reported markers. Hence, we aimed to conduct a systematic review and meta-analysis to reveal the predictive effect of hematologic parameters on NPC prognosis. Identifying these new biomarkers in NPC is essential to the stratification of prognosis, medical treatment, and clinical research.
2. Methods
This meta-analysis was performed in accordance to the PRISMA recommendation.
2.1. Study identification and selection
A systematic literature search of the 2 search engines PubMed and Google Scholar, and other 4 electronic databases (MEDLINE, EMBASE, Web of Science, and the Cochrane Library) was conducted to retrieve possible articles relevant to the topic of interest up to April 2016 without restriction to regions and publication types. The following MeSH terms and their combinations were searched to find potential eligible studies: “neutrophil”, “lymphocyte”, “platelet”, “monocyte”, “blood cell”, “blood routine”, “hematologic”, “nasopharyngeal neoplasms” and “head and neck neoplasms”. Two reviewers independently screened the database search for titles and abstracts. The initial selection was performed to eliminate obviously irrelevant articles and retain potentially relevant articles about prognostic role of hematologic parameters in NPC by an analysis of the title and abstract. Thereafter, the full text was reviewed according to the following eligibility criteria: studies should contain an evaluation of the prognosis value of at least 1 hematologic parameter in NPC, including lymphocyte counts, neutrophil counts, monocyte counts, platelet counts, NLR, lymphocyte-to-monocyte ratio (LMR), and PLR; and the hazard ratio (HR) of survival outcomes, along with their 95% confidence intervals (CIs) or P value should be available. When multiple reports describing the same population were published, the most recent or complete report was involved. Studies meeting the following criteria were excluded: duplicated literature; duplicated reported data; no available data; abstract-only laboratory studies; animal experimental studies; letters; review articles; and case reports.
2.2. Data extraction
Two reviewers (L.S. and M.W.Z.) independently extracted the following data from each study by using a standardized data-abstraction form: first author, year of publication, study period, study design, sample size, baseline characteristics of the study cohort, cut-off value of hematologic parameters, prognostic outcomes, and statistical model. The primary outcomes were cancer-specific survival (CSS) and overall survival (OS). The secondary outcomes included progression-free survival (PFS), distant metastasis-free survival (DMFS), and local relapse-free survival (LRFS). The HR was preferred for evaluating the survival outcome since it is time-to-event data. For studies showing only survival curves, the HR values were obtained by contacting the corresponding author to obtain the original data or results, or were estimated by the methods described by Tierney et al.[15]
2.3. Quality assessments
There are no standard quality-assessment tools for prognostic studies in systematic reviews. We chose the relatively widely used “Newcastle-Ottawa Scale (NOS)” to assess the quality of each of the involved studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). This scale contains 3 domains including patient selection, comparability of the study groups, and assessment of outcome, with a total score of 0 to 9; studies achieving a score of ≥6 were considered to be of high quality. The quality of each eligible study was evaluated independently by 2 reviewers using a methodology assessment. The corresponding authors of the eligible studies were contacted to clarify any questions about the methodology to assess each study as accurately as possible. Any disagreement was resolved by the adjudicating the senior author.
2.4. Statistical analysis
Meta-analyses were carried out using Review Manager Version 5.3 for Windows (The Cochrane Collaboration, 2014). HR was selected as effect measure of prognostic outcomes and reported along with the corresponding 95% CI. A P value <0.05 was considered statistically significant. Statistical heterogeneity across studies was explored by inspection of the forest plot, Cochran Q test, and Higgins I2 statistic. Studies with a P < 0.1 and/or I2 >50% had high statistical heterogeneity. Potential publication bias was assessed by visual inspection of inverted funnel plot asymmetry.
2.5. Ethics approval
Since this is a protocol for a systematic review based on available evidences, ethics approval is not required.
3. Results
3.1. Data retrieval
The work flow chart for this study is shown in Fig. 1. Through initial searches of electronic databases and other sources, the systematic search identified 324 relevant references. A total of 128 duplicated articles were removed. After screening titles and abstracts, we excluded 106 articles, including laboratory studies, meeting abstracts, reviews, letters, and other articles irrelevant to our study. After assessment of the full text, 76 additional articles were excluded. Ultimately, 14 retrospective cohort studies[16–29] were included in the following meta-analysis.
Figure 1.
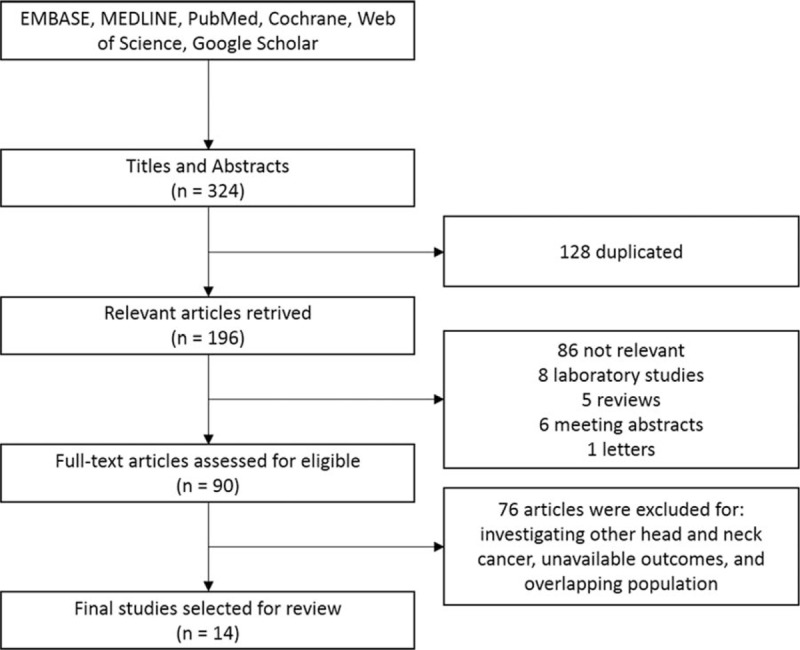
Literature screening flowchart.
3.2. Study description and quality assessment
Table 1 shows the characteristics and quality assessment of the included studies. The 14 eligible studies were published between 2011 and 2015, and all were conducted in Asia. The sample sizes of the included studies ranged from 62 to 1895, and a total of 11,651 cases were included. Four studies focused on metastatic NPC,[21,22,23,27] whereas 10 studies only included patients with nonmetastatic NPC.[16–20,24–26,28,29] The majority of studies assessed the patients’ hematologic parameters before treatment. NLR and lymphocyte counts were the most reported hematologic parameters, followed by PLR and LMR. The majority of the included studies were adjusted for potential confounders using the Cox proportional-hazard model, but the adjusted factors did not conform to each study. Univariate and estimated outcomes were acquired from the article when no multivariate outcomes were reported. The NOS scores of the included studies were around 6 to 7, and the most common inadequacies in methodology were the use of a retrospective study design and incomparability between groups. The cut-off values of hematologic parameters, including NLR, PLR, LMR, lymphocyte counts, and monocyte counts,were determined by receiver-operating characteristic curves to select the most significant points in most studies;[16,18,20,23,25,27,29] Apart from this, the study conducted by He et al[17] and Jiang et al[28] chose quartilevalues as cut-off points artificially. Because the cut-off value of hematologic parameters was artificially chosen to acquire the most significant effect and the clinicopathological features between groups in each study were incomparable, there could be both inter and intrastudy variability; thus, it was reasonable to use a random-effects model. A meta-analysis was performed using a random-effects model in the following pooled analysis.
Table 1.
Characteristics of the included studies.

3.3. Correlation between hematologic parameters and survival outcomes
The included studies focused on several prognostic outcomes, including CSS, OS, PFS, DMFS, and LRFS. Table 2 tabulates the pooled results of the prognostic value of each hematologic parameter on the above outcomes.
Table 2.
Overview of pooled results of the prognostic value of hematologic parameters.

3.4. NLR
The pooled analysis of the prognostic value of NLR is shown in Fig. 2. The effect of NLR on CSS, OS, PFS, DMFS, and LRFS was available in 3 studies,[16,18,25] 4 studies,[17,21,22,29] 4 studies,[17,22,25,29] 1 study,[16] and 2 studies,[16,25] respectively. The synthesized HR value for each prognostic outcomes consistently favored the low NLR patients (pooled HR 1.42, 95% CI 1.21–1.67, P < 0.001 for CSS; pooled HR 1.77, 95% CI 1.41–2.23, P < 0.001 for OS; pooled HR 1.67, 95% CI 1.36–2.06, P < 0.001 for PFS; pooled HR 1.64, 95% CI 1.15–2.34, P = 0.01 for LRFS). The I2 was 0% in each result, which meant there was no heterogeneity in the pooled estimate. The value of NLR for predicting DMFS was available in 1 study,[16] which also favored low NLR patients (HR 2.37, 95% CI 1.37–4.10). The above findings meant that patients with a higher NLR had both higher mortality risk and recurrence risk than those with a low NLR.
Figure 2.
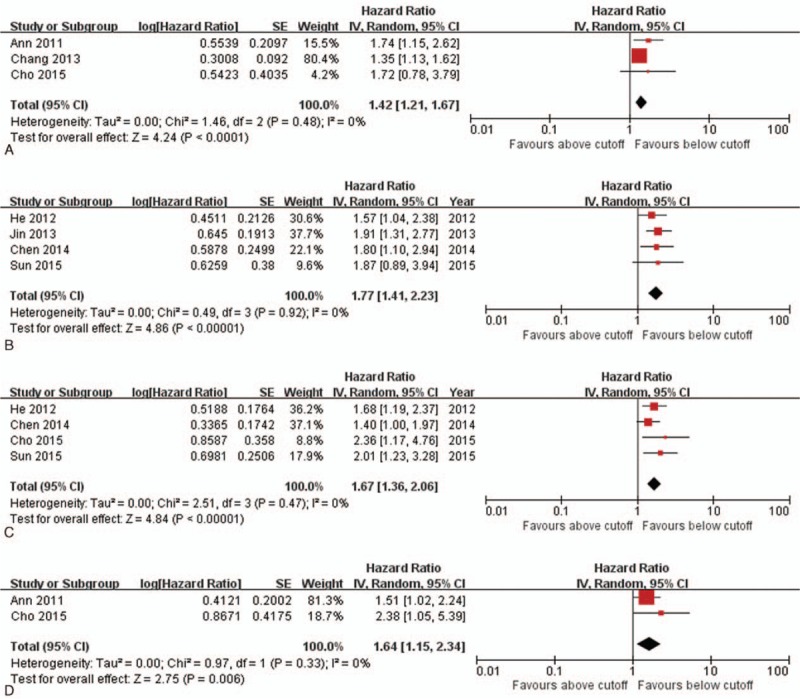
Forest plot and meta-analysis for relationship between pretreatment NLR and CSS, OS, PFS, and LRFS in patients with NPC. (A) Forest plot of the pooled analysis of NLR and CSS. (B) Forest plot of the pooled analysis of NLR and OS. (C) Forest plot of the pooled analysis of NLR and PFS. (D) Forest plot of the pooled analysis of NLR and LRFS. CSS = cancer-specific survival, LRFS = local relapse-free survival, NLR = neutrophil-to-lymphocyte ratio, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression-free survival.
3.5. PLR
Three studies reported the effect of PLR on OS, and 2 studies reported the effect of PLR on PFS.[22,28,29] Pooled results of 3 studies including 1723 patients showed significant superiority of a low PLR on OS (pooled HR 1.68, 95% CI 1.20–2.35, P < 0.001). However, pooled analysis showed that PLR was not associated with PFS (pooled HR 1.46, 95% CI 0.74–2.87, P = 0.27). In addition, 1 study conducted by Jiang et al[28] reported that decreased PLR values predicted better CSS and DMFS. No study reported a correlation between PLR and LRFS.
3.6. LMR
Three studies provided sufficient data on OS outcome for the pooled estimate.[20,23,27] The pooled HR favored patients with a higher LMR (pooled HR 0.50, 95% CI 0.43–0.58, P < 0.001). PFS, DMFS, and LRFS outcomes were available only in 1 study,[20] which demonstrated that higher LMR predicted better PFS and DMFS, but not LRFS.
3.7. Lymphocyte counts
The pooled analysis of the prognostic value of lymphocyte counts is shown in Fig. 3. The effect of lymphocyte counts on OS, PFS, and LRFS was reported in 6 studies,[17,20,21,23,27,29] 4 studies,[17,20,25,29] and 2 studies,[20,25] respectively. The pooled results showed that patients with higher lymphocyte counts had better OS (pooled HR 0.72, 95% CI 0.64–0.81, P < 0.001) and PFS (pooled HR 0.71, 95% CI 0.56–0.91, P = 0.01). However, no significant difference in LRFS was observed (pooled HR 0.71, 95% CI 0.31–1.61, P = 0.41). DMFS was available in 1 study, which reported a borderline significant decreased HR in patients with higher lymphocyte counts.[20] In addition, 1 study assessed the prognostic value of lymphocyte counts on CSS[25] and found that both pretreatment lymphocyte counts and minimum absolute lymphocyte counts during treatment were correlated with CSS.
Figure 3.
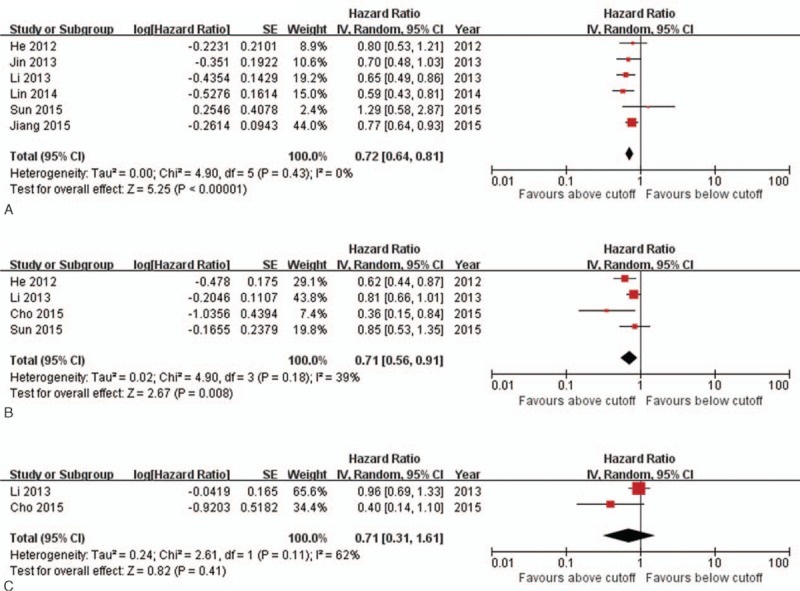
Forest plot and meta-analysis for relationship between pretreatment lymphocyte counts and OS, PFS, and LRFS in patients with NPC. (A) Forest plot of the pooled analysis of lymphocyte counts and OS. (B) Forest plot of the pooled analysis of lymphocyte counts and PFS. (C) Forest plot of the pooled analysis of lymphocyte counts and LRFS. LRFS = local relapse-free survival, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression-free survival.
3.8. Neutrophil counts
In terms of prognostic value of neutrophil counts, only 2 studies [17,21] reported OS outcome and 1 study[17] reported PFS outcome. The pooled HR for OS favored patients with a low neutrophil counts (pooled HR 1.65, 95% CI 1.24–2.20, P < 0.001). The study conducted by He et al[17] reported that the PFS rate was not significantly different between the low and high neutrophil count groups.
3.9. Platelet counts
The prognostic value of platelet count on survival outcomes of patients with NPC is summarized in Table 3. One study[18] evaluated the effect of platelet counts during treatment on survival, whereas 3 other studies[19,24,26] evaluated that of the pretreatment platelet count. Most studies reported that patients with high platelet counts showed poor prognosis. However, it is worth noting that Chen et al[24] found that patients those with lowest platelet counts (<150 × 109) also demonstrated poor OS; additionally, the OS of patients with highest platelet counts (>300 × 109) was not significantly lower than those with lowest platelet counts (<150 × 109). These inconsistent results across studies suggest that patients with a very low platelet count may also have a poor prognosis.
Table 3.
Summary of multivariate analyses results of prognostic value of platelet counts.

3.10. Sensitivity analysis
The sensitivity analysis was performed of the pooled estimates that involved more than 2 studies. As shown in Table 2, in these pooled analyses, most of the I2 values were 0%, which indicated a lack of heterogeneity. A sensitivity analysis performed by removing individual studies, and we found that both the I2 values and statistical difference of pooled HR valued did not change significantly, indicating there was no heterogeneity caused by a single study.
3.11. Publication bias
The above analysis showed that NLR and lymphocyte counts were the 2 most commonly reported hematologic parameters. Therefore, funnel plots of the pooled analysis of these 2 parameters were created. As shown in Fig. 4, the funnel plots showed a symmetrical distribution of studies around the vertical axis. However, because the number of included studies in each pooled analysis was small, the funnel plots may not be significant.
Figure 4.
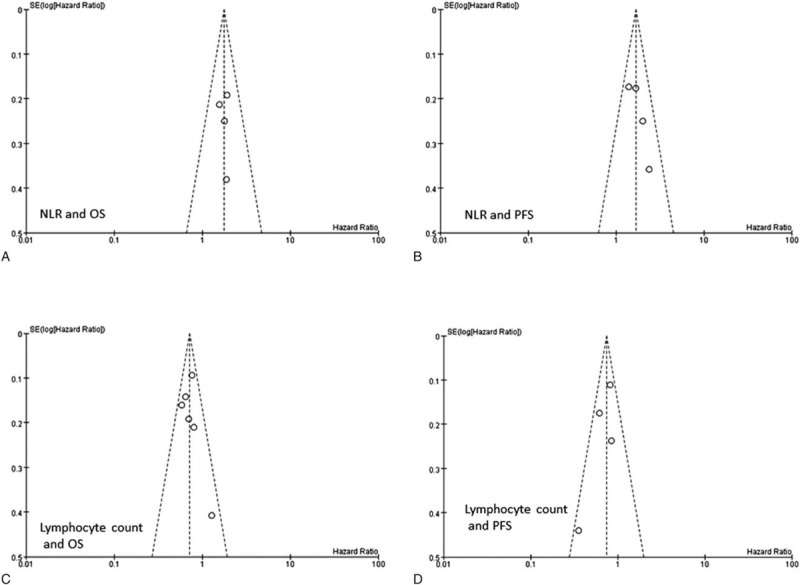
Funnel plots based on prognostic value of NLR and lymphocyte counts. (A) Funnel plot based on the pooled analysis of NLR and OS. (B) Funnel plot based on the pooled analysis of NLR and PFS. (C) Funnel plot based on the pooled analysis of lymphocyte counts and OS. (D) Funnel plot based on the pooled analysis of lymphocyte counts and PFS. NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PFS = progression-free survival.
4. Discussion
Several hematologic parameters of systemic inflammation, such as lymphocyte counts, neutrophil counts, monocyte counts, platelet counts, NLR, LMR, and PLR, have emerged as prognostic factors for a variety of cancer types. These markers can be measured easily and inexpensively; therefore, they may become prognostic markers with widespread actual clinical use. Identifying these easily applicable markers related to NPC prognosis may help clinicians predict individual outcome and guide clinical treatments. In this study, we identified and summarized the published articles that reported the association between the hematologic parameters and NPC prognosis. Our pooled analysis showed that all of the above parameters, including NLR, PLR, LMR, lymphocyte counts, neutrophil counts, and platelet counts, were associated with survival outcomes of East Asian patients with NPC. NLR and lymphocyte counts were the 2 most commonly reported parameters that could be prognostic predictors for NPC. Patients with a high NLR or low lymphocyte count were deemed to have a poor prognosis.
It is now generally recognized that inflammation response plays a critical role in tumor progression and may influence survival outcomes in patients with cancer.[30] Hanahan and Weinberg[31] stated that an important hallmark of cancer is that tumor cells evade immunological attack from lymphocytes, macrophages, and natural killer cells. Lymphocytes are crucial components of the adaptive immune system, which eliminates cancer cells. The presence of lymphocyte infiltrate in tumors is correlated with good prognosis, and T cells have been used to target cancers.[32,33] On the contrary, neutrophilia acts as an important component of inflammatory response and inhibits the immune system by suppressing the cytotoxicity of immune cells such as lymphocytes and activated T cells.[34] Apart from this, neutrophils in the tumor microenvironment have been shown to produce cytokines and chemokines, such as epidermal growth factor (EGF), vascular endothelial growth factor(VEGF), interleukin (IL)-6, and IL-8, which promote tumor cell growth, angiogenesis, and metastasis.[30,35,36] A high NLR indicates systemic and local inflammation that provides a favorable microenvironment for tumor growth, migration, invasion, and metastasis.[30] As systematic inflammatory markers, both lymphocyte counts and neutrophil counts, and also NLR have been recognized to be associated with solid tumor prognosis.[37–39]
We found lymphocyte counts and NLR were the 2 most reported prognostic hematologic parameters of NPC. Our pooled analysis demonstrated that a high NLR or low lymphocyte count was associated with poor prognosis in patients with NPC, which was inconsistent with these above theories. These 2 parameters, lymphocyte count and NLR, are easily reproducible and widely available markers. However, it is worth noting that all of the involved studies in this meta-analysis measured cell counts before treatment, but none evaluated the volatility of the pretreatment counts. Meanwhile, the relationship between changes in cell counts during treatment and prognosis was seldom examined. Therefore, the clinical practicability of these hematologic parameters was limited. Further data on the pretreatment volatility and continuous change during treatment of these parameters are warranted.
Platelets, another blood component, have been well-known to mediate tumor cell growth, metastasis, and angiogenesis. Activated platelets are able to interact with cancer cells through paracrine signaling or direct contact, thereby promoting tumor cell growth and survival.[40–42] Elevated blood platelet count is a common phenomenon in kinds of malignancies and has been reported to be associated with prognosis in these cancer patients. In this meta-analysis, we found only 3 studies examined here evaluated the prognosis value of platelet counts: Du et al,[26] Gao et al,[19] and Chang et al[18] reported that thrombocytosis was associated with poor survival outcome in patients with NPC. Differently, the study conducted by Chen et al[24] stated that low and high platelet counts may predict poor survival and distant metastasis in NPC. The contradicting conclusions among these studies revealed that the prognostic value of platelet counts in NPC requires further study and that there may be a potential “J-shape” correlation between platelet count and surviving HR.
There were some limitations that should be addressed in this study. First, all of the studies included in our meta-analysis were retrospective. Second, all of the included studies used dichotomous variables to determine the prognostic value. The cut-off values differed between the different studies, and the cut-off value for each parameter seemed to be calculated in each study to acquire the most significant effect; thus, the final significance of the outcomes seemed to be created rather than intrinsic. The other side-effect of using an artificial cut-off value was the incomparability between groups. Thus, we recommend using a continuous rather than categorical variable in future studies. Third, a number of the included studies did not report CSS, which is a critical outcome of a cancer survival analysis. Fourth, the correlation between hematologic cells and other systemic inflammatory markers should be noted, which may result in high colinearity in a multivariate analysis and affect the estimation of HR in the Cox regression model. Moreover, almost all of the studies chose a dichotomous cut-off value; therefore, the survival outcomes of patients with very low level of these hematologic parameters may be ignored. In addition, almost all of the included studies were from Chinese population, and 1 study was conducted in Korea; therefore, the conclusion might be limited to East Asian population. This might be explained by the much higher incidence of NPC and much more number of cases in China and Southeast Asia compared with other regions of the world.[43]
5. Conclusions
To our knowledge, this is the first meta-analysis to evaluate the hematologic parameters as prognostic markers for patients with NPC. Our meta-analysis summarized the prognostic value of hematologic parameters in patients with NPC examined in the articles published to date, and the pooled results suggested that these hematologic parameters mentioned above were closely correlated with the survival outcomes of patients with NPC. NLR and lymphocyte counts were the 2 most reported parameters and could be prognostic predictors for patients with NPC. Patients with a high NLR or low lymphocyte count were deemed to have a poor prognosis. The conclusion should be limited to East Asians due to the limitation of included populations, and further well-designed, prospective studies, and also researches from other parts of the world, are needed.
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, DMFS = distant metastasis-free survival, HR = hazards ratio, L count = lymphocyte count, LMR = lymphocyte-to-monocyte ratio, LRFS = local relapse-free survival, Md = median, Mn = mean, Multi = multivariate analysis, N count = neutrophil count, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle-Ottawa Scale, NPC = nasopharyngeal carcinoma, OS = overall survival, P count = platelet count, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, Qua = quartile, Ran = range, SD = standard deviation, Uni = univariate analysis.
Funding: This study was funded by Medical Elite Cultivation Program of Fujian, P.R.C. (Grant no: 2015-ZQN-ZD-19), Health and Family Planning Commission of Fujian Province, Youth Research Projects (Grant no: 2014–1–57), Chinese Medicine Research Projects of Fujian Province (Grant no: wzzy201314), and the Natural Science Foundation of Fujian Province (Grant no: 2014J01308, Grant no: 2016J01541).
The authors have no conflicts to disclose.
References
- [1].Chua ML, Wee JT, Hui EP, et al. Nasopharyngeal carcinoma. Lancet (London, England) 2016;387:1012–24. [DOI] [PubMed] [Google Scholar]
- [2].Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 2015;33:3356–64. [DOI] [PubMed] [Google Scholar]
- [3].Sun R, Qiu HZ, Mai HQ, et al. Prognostic value and differences of the sixth and seventh editions of the UICC/AJCC staging systems in nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2013;139:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 2005;5:845–56. [DOI] [PubMed] [Google Scholar]
- [5].Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004;350:2461–70. [DOI] [PubMed] [Google Scholar]
- [6].Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol 2012;13:633–41. [DOI] [PubMed] [Google Scholar]
- [7].Yang S, Li Y. MicroRNAs: novel factors in clinical diagnosis and prognosis for nasopharyngeal carcinoma. Acta Pharmacol Sin 2012;33:981–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cai Y, Li J, Lu A, et al. Increased serum levels of macrophage inflammatory protein-3alpha and cystatin a predict a poor prognosis of nasopharyngeal carcinoma. Medicine (Baltimore) 2014;93:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ren M, Wang Z, Gao G, et al. Impact of X-linked inhibitor of apoptosis protein on survival of nasopharyngeal carcinoma patients following radiotherapy. Tumour Biol 2016;37:11825–33. [DOI] [PubMed] [Google Scholar]
- [10].Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Payne JK. State of the science: stress, inflammation, and cancer. Oncol Nurs Forum 2014;41:533–40. [DOI] [PubMed] [Google Scholar]
- [12].Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers 2016;2016:8345286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki R, Takagi T, Hikichi T, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett 2016;11:3441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].You J, Zhu GQ, Xie L, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget 2016;7:25516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].An X, Ding PR, Wang FH, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma. Tumour Biol 2011;32:317–24. [DOI] [PubMed] [Google Scholar]
- [17].He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 2012;34:1769–76. [DOI] [PubMed] [Google Scholar]
- [18].Chang H, Gao J, Xu BQ, et al. Haemoglobin, neutrophil to lymphocyte ratio and platelet count improve prognosis prediction of the TNM staging system in nasopharyngeal carcinoma: development and validation in 3,237 patients from a single institution. Clin Oncol (R Coll Radiol) 2013;25:639–46. [DOI] [PubMed] [Google Scholar]
- [19].Gao J, Zhang HY, Xia YF. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol 2013;34:39–45. [DOI] [PubMed] [Google Scholar]
- [20].Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One 2013;8:e83069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin Y, Ye X, He C, et al. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck 2015;37:69–75. [DOI] [PubMed] [Google Scholar]
- [22].Chen C, Sun P, Dai QS, et al. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One 2014;9:e112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin GN, Peng JW, Liu DY, et al. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumour Biol 2014;35:10849–54. [DOI] [PubMed] [Google Scholar]
- [24].Chen YP, Chen C, Mai ZY, et al. Pretreatment platelet count as a predictor for survival and distant metastasis in nasopharyngeal carcinoma patients. Oncol Lett 2015;9:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cho O, Oh YT, Chun M, et al. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck 2016;38(Suppl 1):E1061–7. [DOI] [PubMed] [Google Scholar]
- [26].Du XJ, Tang LL, Chen L, et al. Neoadjuvant chemotherapy in locally advanced nasopharyngeal carcinoma: defining high-risk patients who may benefit before concurrent chemotherapy combined with intensity-modulated radiotherapy. Sci Rep 2015;5:16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jiang R, Cai XY, Yang ZH, et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer 2015;34:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jiang R, Zou X, Hu W, et al. The elevated pretreatment platelet-to-lymphocyte ratio predicts poor outcome in nasopharyngeal carcinoma patients. Tumour Biol 2015;36:7775–87. [DOI] [PubMed] [Google Scholar]
- [29].Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016;38(Suppl 1):E1332–40. [DOI] [PubMed] [Google Scholar]
- [30].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [32].Basso S, Zecca M, Merli P, et al. T cell therapy for nasopharyngeal carcinoma. J Cancer 2011;2:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duong CP, Yong CS, Kershaw MH, et al. Cancer immunotherapy utilizing gene-modified T cells: from the bench to the clinic. Mol Immunol 2015;67(2 Pt A):46–57. [DOI] [PubMed] [Google Scholar]
- [34].el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 1987;139:2406–13. [PubMed] [Google Scholar]
- [35].Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [37].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [38].Bahig H, Taussky D, Delouya G, et al. Neutrophil count is associated with survival in localized prostate cancer. BMC Cancer 2015;15:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sharma D, Brummel-Ziedins KE, Bouchard BA, et al. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 2014;229:1005–15. [DOI] [PubMed] [Google Scholar]
- [41].Rafaty FM. Cervical adenopathy secondary to toxoplasmosis. Arch Otolaryngol 1977;103:547–9. [DOI] [PubMed] [Google Scholar]
- [42].Okamoto E, Osaki M, Kase S, et al. Thymidine phosphorylase expression causes both the increase of intratumoral microvessels and decrease of apoptosis in human esophageal carcinomas. Pathol Int 2001;51:158–64. [DOI] [PubMed] [Google Scholar]
- [43].Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 2015;32:54–73. [DOI] [PubMed] [Google Scholar]


