Supplemental Digital Content is available in the text
Keywords: Crohn disease, efficacy, low bone mineral density, medical therapy, network meta-analysis, safety
Abstract
Background:
Low bone mineral density (BMD) is a frequent complication of inflammatory bowel disease (IBD), particularly in patients with Crohn disease (CD). The aim of our study is to determine the efficacy and safety of different drugs used to treat low BMD in patients with CD.
Methods:
PUBMED/MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials were searched for eligible studies. A random-effects model within a Bayesian framework was applied to compare treatment effects as standardized mean difference (SMD) with their corresponding 95% credible interval (CrI), while odds ratio (OR) was applied to compare adverse events with 95% CrI. The surface under the cumulative ranking area (SUCRA) was calculated to make the ranking of the treatments for outcomes.
Results:
Twelve randomized controlled trials (RCTs) were eligible. Compared with placebo, zoledronate (SMDs 2.74, 95% CrI 1.36–4.11) and sodium-fluoride (SMDs 1.23, 95% CrI 0.19–2.26) revealed statistical significance in increasing lumbar spine BMD (LSBMD). According to SUCRA ranking, zoledronate (SUCRA = 2.5%) might have the highest probability to be the best treatment for increasing LSBMD in CD patients among all agents, followed by sodium-fluoride (27%). For safety assessment, the incidence of adverse events (AEs) demonstrated no statistical difference between agents and placebo. The corresponding SUCRA values indicated that risedronate (SUCRA = 77%) might be the most safe medicine for low BMD in CD patients and alendronate ranked the worst (SUCRA = 16%).
Conclusions:
Zoledronate might have the highest probability to be the best therapeutic strategy for increasing LSBMD. For the safety assessment, risedronate showed the greatest trend to decrease the risk of AEs. In the future, more RCTs with higher qualities are needed to make head-to-head comparison between 2 or more treatments.
1. Introduction
Crohn disease (CD), one of major phenotype of inflammatory bowel disease (IBD), is characterized by chronic relapsing inflammatory disorder of the gastrointestinal tract.[1] In the past 50 years, the incidence and prevalence of CD have increased up to 6 to 15/100,000 and 50 to 200/100,000 persons, respectively, in the west.[2] Various extra-intestinal damages have been reported in CD patients, of these, low bone mineral density (BMD) is considered a common extra-intestinal manifestation.[3] Low BMD is often referred to as osteopenia and osteoporosis in the clinic. The prevalence of osteopenia in patients with IBD was up to 62% while osteoporosis occurred in 38%.[4,5] Although some studies showed that ulcerative colitis was also associated with low BMD,[6] a majority of studies demonstrated that the lower bone density was more likely to be involved in CD.[5,7,8] In patients with CD, the risk factors contributing to the development of low BMD mainly include: sex, malabsorption of vitamin D, calcium, and other nutrients, the use of corticosteroids, inflammatory cytokines, and bowel resection.[9–14] Moreover, it has been reported that low BMD was tightly associated with osteoporosis-related fractures,[15] thus, it is necessary for CD patients to receive the treatment against osteopenia and osteoporosis.
Some randomized controlled trials (RCTs) have demonstrated the effectiveness and safety of several drugs, such as the antiresorptive and bone-anabolic drugs. These clinical trials would benefit the decision-making strategies in clinical practices.[16–27] Recently, a meta-analysis concluded that both bisphosphonates (e.g., alendronate, risedronate, ibandronate, and zoledronate) and sodium fluoride were effective in improvement of low BMD in patients with IBD.[28] However, it was only conducted on the basis of pairwise evidence by comparing various medical interventions with placebo. Thus, the optimal selection of drugs still remains controversial because it was unlikely to acquire comparisons between each available treatment options.
Given the lack of head-to-head RCTs between antiresorptive and bone-anabolic drugs for low BMD in CD patients, we conducted a systematic review with network meta-analysis, which permits the integration of direct and indirect comparisons and allows us to simultaneously compare multiple treatments.[29,30] In order to enhance statistical power and improve estimates of effect size, we analyzed and combined results of previous RCTs to assess the effectiveness and safety of antiresorptive and bone-anabolic drugs for low BMD in CD patients.
2. Methods
2.1. Search strategy
Electronic databases of PUBMED, MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials were searched from inception to 7 April 2016 for RCTs. Medical subject heading (MeSH) terms and keywords as follows: “inflammatory bowel disease,” “IBD,” “Crohn disease,” “CD,” “bone mineral density,” “bone density,” “osteoporosis,” “osteopenia,” “osteoporotic,” “OP,” and “bone loss” were used in the search strategy without language or date restrictions. We also scanned previous systematic reviews and meta-analysis to identify potential relevant studies. Titles and abstracts were carefully screened. Any disagreement between the two authors was resolved by further discussion. If there was no consensus, a third reviewer (corresponding author) was consulted. All analyses were based on previous published studies, thus ethical approval and patient consent were not required.
2.2. Selection criteria
We included (RCTs) comparing three interventions categories: antiresorptive drugs, bone-anabolic drugs, or placebo. Participants should be CD patients with osteoporosis or osteopenia. The primary outcome should include the change of lumbar spine bone mineral density (LSBMD), as measured by dual energy x-ray absorptiometry (DXA). We excluded (i) non-RCTs; (ii) review and meeting abstract; (iii) non-medicine intervention and mixed with ulcerative colitis (UC), or indeterminate colitis (IC) patients. There was no restriction on language, age, or sex.
2.3. Data extraction
Two authors independently reviewed abstracts and full texts of all eligible studies. We extracted information from all eligible studies on author names, publication year, number of patients, mean age, interventions, follow-up time. Any discrepancies in data extraction were resolved by the corresponding author.
2.4. Outcome measures
Primary outcome was improving LSBMD at 12 months (if the definite BMD value at 12 months was not available, the last time-point assessment in the trial would be taken); secondary outcome was the change of total hip BMD (THBMD) at 12 months (if the direct results of THBMD was not available, the value of total hip components would be taken, such as femoral neck, femoral trochanter) and incidence of adverse events (AEs).
2.5. Statistical analysis
We used WinBUGS (version 1.4.3, MRC Biostatistics Unit, Cambridge, UK) with a random effects model proposed by Chaimani (obtained from www.mtm.uoi.gr) within a Bayesian framework to perform network meta-analyses. The posterior parameters were calculated by Markov chain Monte Carlo methods in the network meta-analysis.[31] Non-informative uniform and normal prior distributions were performed and an automatically generated starting value was utilized to fit the model.[32] After an initial burn-in of 50,000, we conducted another 250,000 iterations. For dichotomous variables and continuous variables, treatment effects were summarized as odds ratio (OR) and standardized mean difference (SMD) with their corresponding 95% confidence interval (CI) or credible interval (CrI) (95% CI for direct evidences and CrI for indirect evidences), respectively. To make the ranking of the treatments for each outcomes, we calculated the surface under the cumulative ranking area (SUCRA).[33] For the primary outcomes, lower SUCRA value indicated better rank of the treatment, whereas it was opposite regarding the secondary outcomes.
We compared the pooled OR and SMD from network meta-analysis with the corresponding OR or SMD from traditional pair-wise meta-analysis to evaluate whether there was inconsistency between direct and indirect evidences. The traditional pair-wise meta-analysis was conducted for trials that directly compared different interventions using Stata (version 13.0, StataCorp, College Station, TX). To account for heterogeneity, the method of DerSimonian and Laird random effects model was used.[34] The heterogeneity between eligible studies was explored by Chi-square based Q-test in traditional pair-wise meta-analysis and the presence of heterogeneity was considered significant if P < 0.1.[35] Nevertheless, inconsistency was also quantitatively assessed by calculating inconsistency factors and their 95% CI in closed loops. The goodness-of-fit of the model was examined by calculating the posterior mean residual deviance, and the model was considered to fit the data well when the posterior mean residual deviance approximated the number of data points in the present study.[36]
To detect the small study effects of the data, we conducted comparison-adjusted funnel plots.[37] Sensitivity analysis was performed to authenticate the stability of the results according to the quality of study (excluding studies with a high risk of bias) and follow-up time (excluding studies with a short-term follow-up less than 12 months). This study was conducted and reported in accordance with the PRISMA guidelines.[38]
2.6. Assessment of risk of bias
We independently assessed the methodologic quality of included trials using the Cochrane Collaboration tool,[39] which included the following items: (i) random sequence generation; (ii) allocation concealment; (iii) blinding of patients and personnel; (iv) blinding of outcome assessment; (v) incomplete outcome data; (vi) selective reporting; and (vii) other bias.
2.7. Quality of evidence
A four-step approach was used to rate the quality of therapeutic effect estimates from network meta-analysis based on the grading of recommendation, assessment, development, and evaluation (GRADE).[40] Evidence evaluation includes direct, indirect as well as network estimates and it is rated as high, moderate, low and very low quality. At the beginning of the assessment, the quality of direct evidence was considered high and it may be rated down for the following reasons: risk of bias; inconsistency; indirectness; imprecision; publication bias. The rating for indirect evidence from the lower rating of the quality of direct evidence would be further rated down due to imprecision and indirectness. We used the higher rating of direct and indirect evidence as the quality rating for the network estimates.
3. Results
3.1. Characteristics of included studies
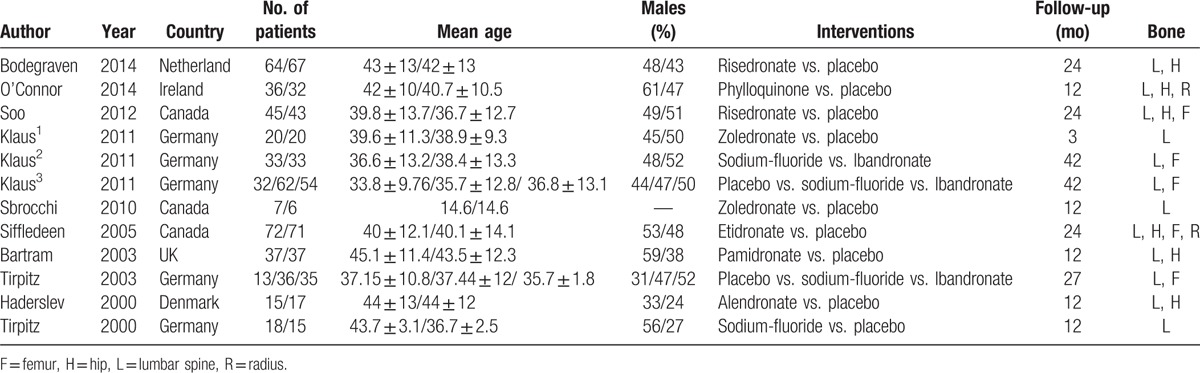
The flow diagram of study selection is provided in Fig. 1. First, 1682 literature citations were identified. After duplicates’ removal, a total of 1314 citations were yielded. After reading titles and abstracts, 1277 ineligible records were excluded, leaving 37 articles. Furthermore, 25 studies were excluded for the following reasons: non-RCTs, review article, meeting abstract, non-medicine intervention, and mixed with UC or IC. Finally, 12 eligible studies were included with a total of 920 subjects who received 1 of the 8 agents or placebo in this network meta-analysis. These studies come from different countries, 5[19–21,24,26] were reported in Germany, 3[18,22,23] from Canada, and 4[16,17,25,27] from Netherlands, Ireland, Danmark, and UK, respectively. The mean age of participants in trials ranged from 14.6 to 45.1 years.
Figure 1.
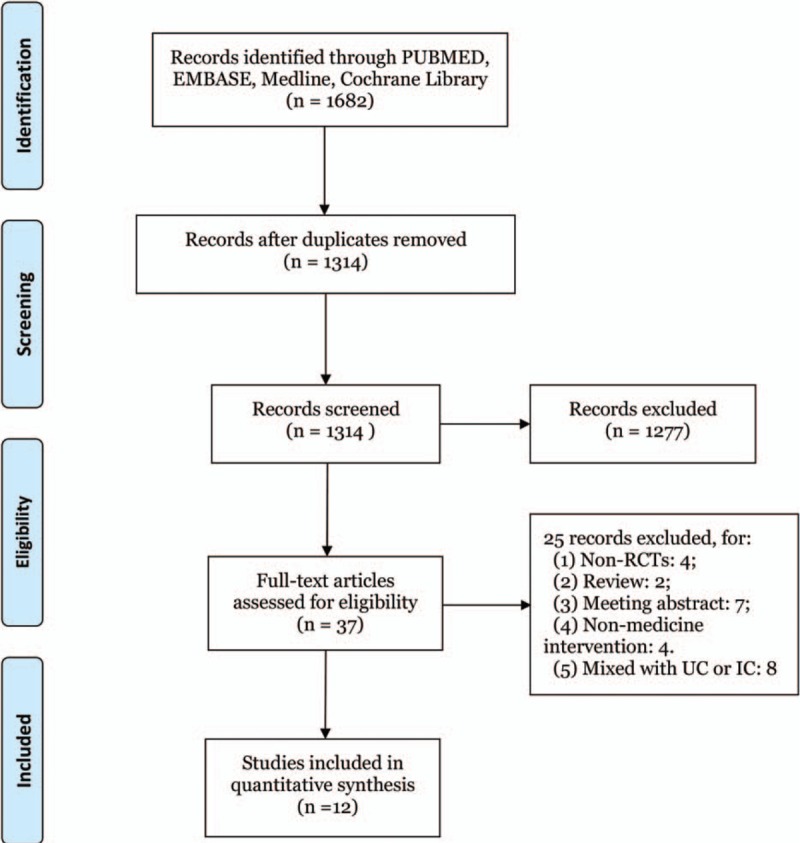
Flow diagram of study selection. A total of 12 studies were included in this network meta-analysis after literature search and selection.
Among these 12 eligible studies, there were 2-arm (n = 10) or 3-arm (n = 2) RCTs. Four studies compared ibandronate and sodium-fluoride with each other or against placebo (head to head trials: n = 3). Two studies compared risedronate with placebo with same dosage schedule. Likewise, 2 studies compared zoledronate with placebo. The remaining 4 articles were compared etidronate, pamidronate, alendronate, phylloquinone with placebo, respectively (Table 1).
Table 1.
Characteristics of included studies.
3.2. Risk of bias in included studies
The risk of bias in all included studies is illustrated in Supplementary Figure S1. Nine studies provide sufficient details of randomization. Three studies revealed inadequate in terms of allocation concealment. Two studies did not blind to participants or study personnel and 1 trial had a high risk of bias due to unblinding of outcome assessment. Considering incomplete outcome data domain, 9 studies were cited as a low risk of bias. Eight studies had a low risk of reporting bias.
3.3. Outcomes
3.3.1. Efficacy: The improvement of LSBMD
The comparisons on changes of LSBMD with various medical therapies in CD patients by network meta-analysis are shown in Supplementary Figure S2 (A). The 12 RCTs were included in our study enrolled 920 patients (Table 1), Of these patients, 353 participants were assigned to placebo therapy,[16–19,21–27] 149 to sodium-fluoride therapy,[19,20,24,26] 122 to ibandronate therapy,[19,20,24] 109 to risedronate therapy,[16,18] 72 to etidronate therapy,[23] 37 to pamidronate therapy,[25] 36 to phylloquinone therapy,[17] 27 to zoledronate therapy,[21,22] 15 to alendronate therapy.[27]
Results of comparisons on the change of LSBMD in various treatment strategies in our network meta-analysis are shown in Fig. 2(A). Compared with placebo, zoledronate (SMDs 2.74, 95% CrI 1.36–4.11) reached statistical significance in improving LSBMD of CD patients, followed by sodium-fluoride (SMDs 1.23, 95% CrI 0.19–2.26). Similarly, zoledronate (SMDs 2.49, 95% CrI 0.21–4.78 and SMDs 2.68, 95% CrI 0.42–4.96) showed significant difference with pamidronate and etidronate, respectively. Additionally, alendronate (SMDs 1.23, 95% CrI −0.66 to 3.14), risedronate (SMDs 0.15, 95% CrI −1.13 to 1.44), ibandronate (SMDs 0.79, 95% CrI −0.37 to 1.95), etidronate (SMDs 0.05, 95% CrI −1.77 to 1.87), and pamidronate (SMDs 0.24, 95% CrI –1.59 to 2.07) except phylloquinone (SMDs −0.16, 95% CrI −1.99 to 1.67) all had a tendency to improve LSBMD in CD patients compared with the placebo group, but there were no statistical significance. In addition, zoledronate was significantly superior to risedronate (SMDs −2.59, 95% CrI −4.45 to −0.72) and phylloquinone (SMDs −2.89, 95% CrI −5.17 to −0.60) in improving LSBMD of CD patients.
Figure 2.
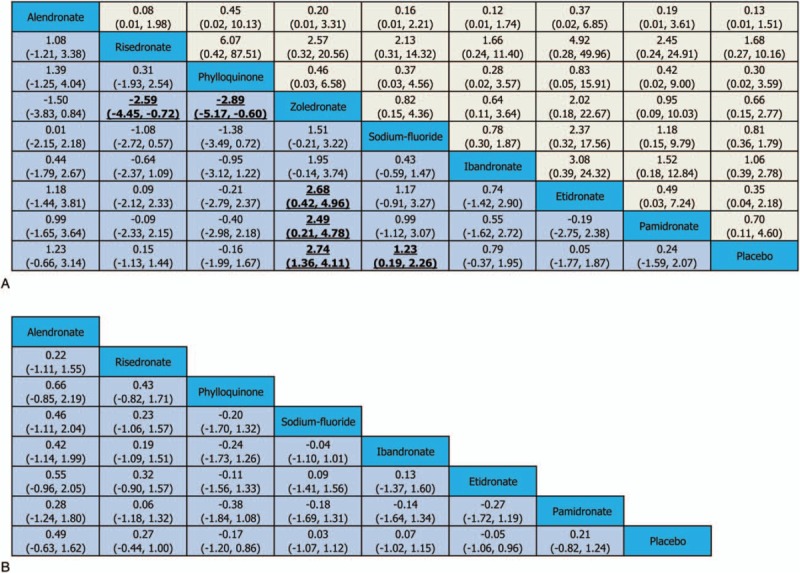
Efficacy and safety of agents in Crohn disease patients with low bone mineral density (BMD): the efficacy was estimated in 2 lower triangle comparing column-defining with row-defining treatments (A, the change of lumbar spine BMD (LSBMD); B, the change of total hip BMD [THBMD]). The efficacy was estimated in upper triangle (A) comparing row-defining with column-defining treatments. For efficacy and safety assessment, treatment effects were summarized as standardized mean difference (SMD) and odds ratio (OR) with their corresponding 95% credible intervals, respectively. For change of BMD, SMD lower than 0 favor the row-defining treatment while for adverse effects, ORs lower than 1 favor the column-defining treatment.
SUCRA expressed as percentages (Fig. 3) were: 2.5% for zoledronate; 27% for sodium-fluoride; 32% for alendronate; 44% for ibandronate; 62% for pamidronate; 66% for risedronate; 68% for etidronate; 74% for phylloquinone; 75% for placebo. According to the synthetical data from SUCRA values, zoledronate (SUCRA = 2.5%) might have the highest probability to be the best treatment for improving LSBMD in CD patients and phylloquinone seemed to be the worst one within all available interventions (74%).
Figure 3.
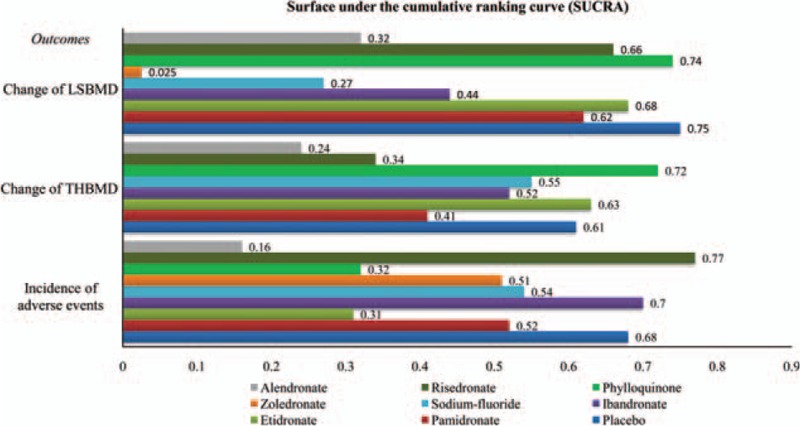
Surface under the cumulative ranking curve expressed as percentages ranking therapeutic effects of treatments for Crohn disease patients with low bone mineral density (BMD). The surface under the cumulative ranking area of each bar represents the probability size of each treatment. The color of the bar represents the specific therapeutic schedule. The abscissa represents the therapeutic probability of various drugs and the ordinate denotes the outcomes about the change of BMD and the incidence of adverse events. For the change of BMD, the pharmacological agent with the longest bar means the most efficacious treatment while agent with the shortest bar indicates the worest one. Oppositely, for the incidence of adverse events, the pharmacological agent with the shortest bar means the most safety treatment while agent with the longest bar indicates the worest one. LSBMD = lumbar spine bone mineral density, THBMD = total hip bone mineral density.
3.3.2. Efficacy: The change of THBMD
There were 7 trials introduced the improvement of THBMD as an assessment of drug efficacy. The network of included medical therapies comparisons is shown in Supplementary Figure S2 (B). A total of 280 CD patients with low BMD were assigned to placebo group,[16–18,23–25,27] 15 to alendronate group,[27] 109 to Risedronate group,[16,18] 36 to phylloquinone group,[17] 36 to sodium-fluoride group,[24] 35 to ibandronate,[24] 72 to etidronate group,[23] 37 to pamidronate group[25] (Table 1).
Network results regarding the change of THBMD are shown in Fig. 2(B). Most of the included drugs revealed an increase of THBMD when compared with placebo, unfortunately, no obviously significance could be identified (alendronate: SMD 0.49, 95%CrI −0.63 to 1.62; risedronate: SMD 0.27, 95%CrI −0.44 to 1.00; sodium-fluoride: SMD 0.03, 95%CrI −1.07 to 1.12; ibandronate: SMD 0.07, 95%CrI −1.02 to 1.15; pamidronate: SMD 0.21, 95%CrI −0.82 to 1.24).
The value of SUCRA as shown in Fig. 3 was partially consistent with the results of primary outcome, and alendronate (SUCRA = 24%) indicated the highest probability of being the most efficacious agent for improving THBMD, nevertheless, phylloquinone seemed to be the worst one (SUCRA = 72%).
3.3.3. Safety: Adverse events (AEs)
The comparisons on the AEs in our network are shown in Supplementary Figure S2 (C). Nine 2-arm and 2 3-arm studies comparing sodium-fluoride (n = 4), ibandronate (n = 3), etidronate (n = 1), risedronate (n = 1), pamidronate (n = 1), phylloquinone (n = 1), zoledronate (n = 2), or alendronate (n = 1) with each other or against placebo were included in the network meta-analysis. A total of 262 CD patients with low BMD were assigned to placebo group,[17–19,21–27] 134 to sodium-fluoride group,[19,20,24,26] 117 to ibandronate group,[19,20,24] 49 to etidronate group,[23] 45 to risedronate group,[18] 37 to pamidronate group,[25] 36 to phylloquinone group,[17] 27 to zoledronate group[21,22], and 15 to alendronate group[27] (Table 1).
Results of comparisons on the incidence of AEs in various treatment strategies in this study are shown in Fig. 2(A). Although the occurrence of AEs in the RCTs demonstrated no statistical difference between agents and placebo, the incidence of AEs in alendronate (OR 0.13, 95% CrI 0.01–1.51), risedronate (OR 1.68, 95% CrI 0.27–10.16), phylloquinone (OR 0.30, 95% CrI 0.02–3.59), zoledronate (OR 0.66, 95%CrI 0.15–2.77), sodium-fluoride (OR 0.81, 95% CrI 0.36–1.79), ibandronate (OR 1.06, 95% CrI 0.39–2.78), etidronate (OR 0.35, 95% CrI 0.04–2.18), and pamidronate (OR 0.70, 95% CrI 0.11–4.60) compared with placebo, which had a certain trend to be higher than placebo.
Simultaneously, the corresponding SUCRA values (provided the optium treatment decision for clinicians) is depicted in Fig. 3. The values were 77% for risedronate, 70% for ibandronate, 68% for placebo, 54% for sodium-fluoride, 52% for pamidronate, 51% for zoledronate, 32% for phylloquinone, 31% for etidronate, 16% for alendronate, respectively. According to the synthetical evaluation of SUCRA values, risedronate (SUCRA = 77%) had the highest probability of being the safest medicine for increasing LSBMD of CD patients and alendronate ranked the worst (16%).
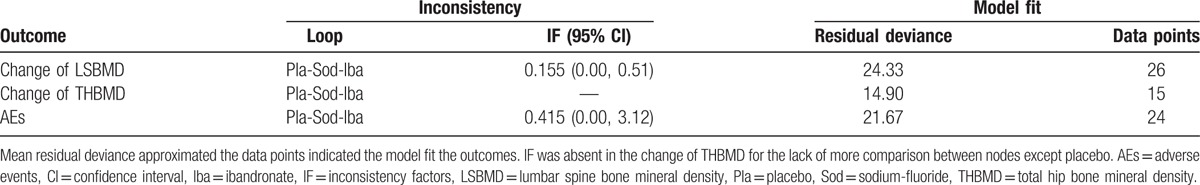
3.4. Evaluation of inconsistency and fit of the models
The results of the pair-wise and corresponding Bayesian network meta-analysis are shown in Fig. 4, Supplementary Figure S3 and Figure S4. The effect size and relevant CI or CrI delivered no obvious discrepancy between the 2 different types of comparisons, indicating that there were no inconsistencies. Moreover, the consistency was also confirmed by the quantitative assessment in closed loops (Table 2). The result of the model test indicated that the posterior mean residual deviance approximated the data points in both primary and secondary outcomes (Table 2), namely, the present model fit the data well.
Figure 4.
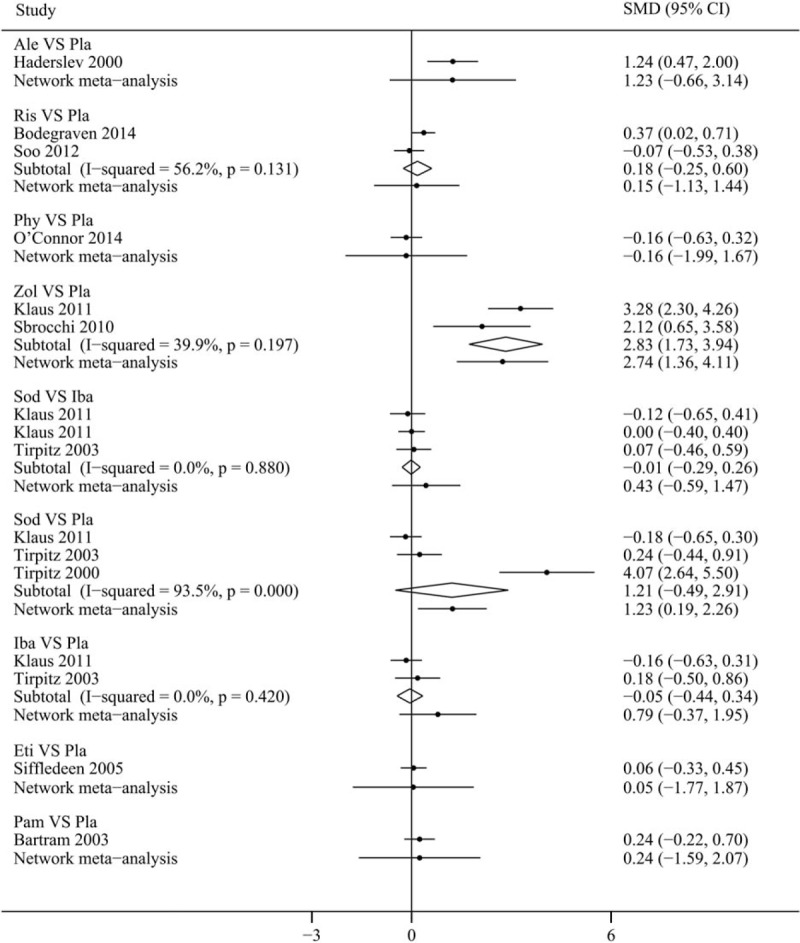
Forest plot with 95% confidence interval (CI) or credible intervals (CrI) (95% CI for direct evidences and CrI for indirect evidences) for continuous variable (change of bone mineral density in lumbar spine) by traditional meta-analysis and Bayesian network meta-analysis. Ale = alendronate, Ris = risedronate, Phy = phylloquinone, Zol = zoledronate, Sod = sodium-fluoride, Iba = ibandronate, Eti = etidronate, Pam = pamidronate, Pla = placebo.
Table 2.
Heterogeneity and evaluation of model fit in included.
3.5. Quality of evidence
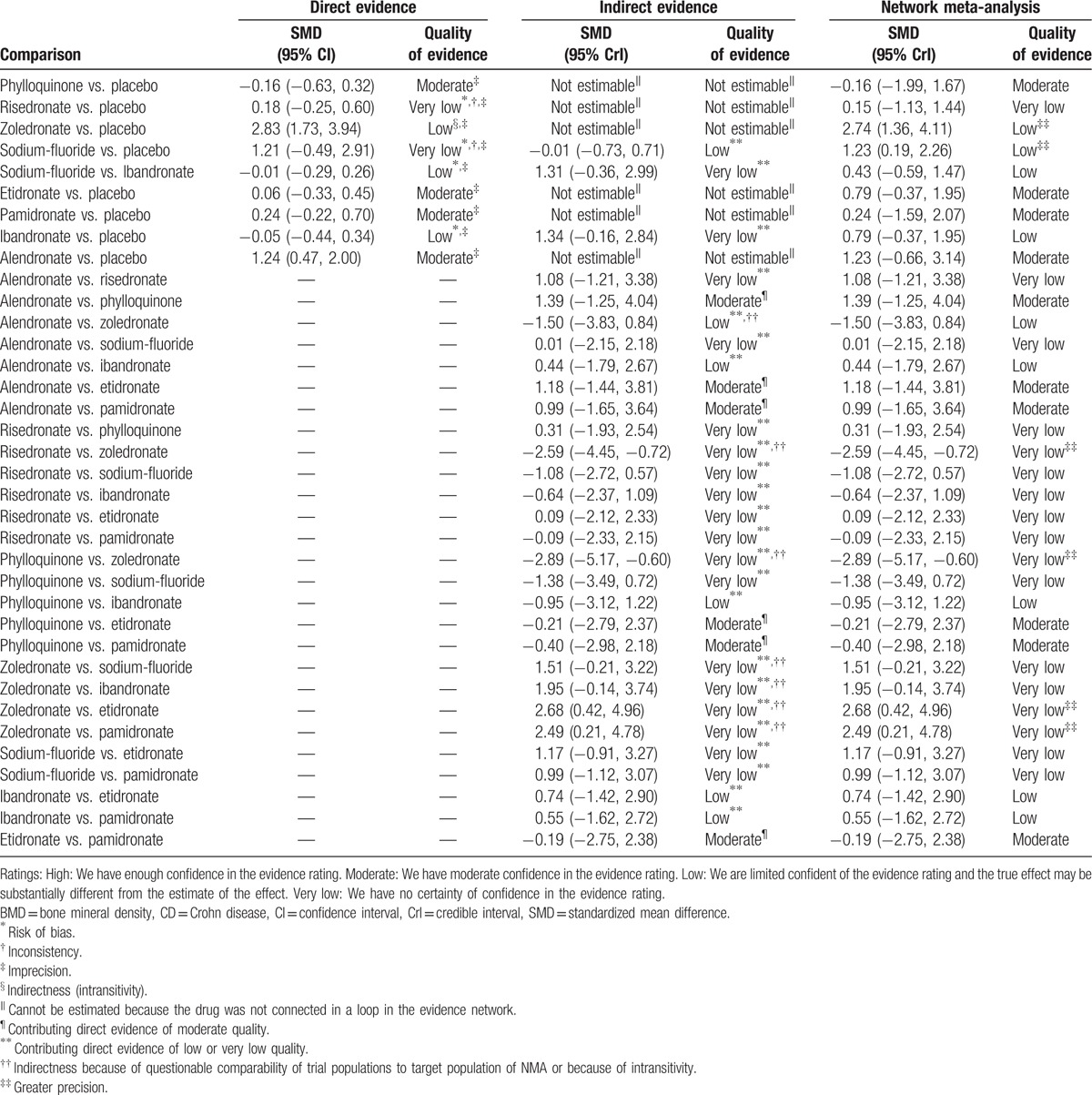
The GRADE approach was applied to the primary outcomes about the change of BMD in lumbar spine (LS) at 12 months (Table 3). The quality of direct and indirect evidence was very low, low, or moderate for all comparisons. Likewise, the quality for network meta-analysis was also very low, low, or moderate.
Table 3.
Estimates of effects and quality ratings for comparison of drugs for low BMD in CD patients.
3.6. Publication bias and sensitivity analysis
The result of the comparison-adjusted funnel plots did not uncover any evidence of apparent asymmetry (Supplementary Figure S5). Sensitivity analysis regarding the quality of study and follow-up time did not significantly alter the results of 3 outcomes (Supplementary Table S1 and Table S2).
4. Discussion
In this network meta-analysis, we included 12 RCTs comparing the efficacy and safety of 8 agents for the management of low BMD in CD patients: 3 studies used ibandronate, 2 studies used zoledronate, 2 studies used risedronate, 4 studies used sodium-fluoride, the rest 4 studies used etidronate, pamidronate, alendronate, and phylloquinone, respectively. The efficacy and safety of these agents were analyzed as the primary and secondary outcomes, respectively. We provided hierarchies for clinicians in the treatment process. Our results suggested that zoledronate might have the highest probability to be the best treatment for increasing LSBMD in CD patients among all included agents. The comprehensive analysis of the secondary efficacy assessment concerning the change of THBMD revealed no obvious significance between all agents and placebo, and alendronate yielded a trend to be the best agent for improving THBMD. For the safety assessment, risedronate showed the greatest power to decrease the risk of AEs while alendronate performed the worst, but all of them were not statistically sound, possibly due to small sample size.
Previous studies indicated that bisphosphonates had beneficial effect for low BMD among postmenopausal women or patients without CD who were undertaken glucocorticoid administration.[41–46] Stokkers et al[47] reported that 49 patients with IBD (44 CD patients) received injection of pamidronate, the BMD of lumbar spine was increased. Similarly, Bartram et al[25] also confirmed that intravenous pamidronate had a therapeutic effect in CD patients with low BMD. The study by Sbrocchi et al[22] demonstrated that zoledronate lead to a significant increase in LSBMD at 6 and 12 months with a well-tolerated infusion. Although the above studies showed that several drugs were effective in CD patients with low BMD, there still lacks study to show which agent specifically targets on bone health of CD patients. Therefore, we performed this network meta-analysis to evaluate the efficacy and safety of different agents of antiresorptive drugs (bisphosphonates), bone-anabolic drugs (sodium-fluoride and phylloquinone) for treatment of low BMD in CD patients.
We found that a single dose of intravenous zoledronate might have the highest probability to be the most beneficial strategy for increasing LSBMD of CD patients, followed by sodium-fluoride and both reached statistical significance. The outcome was consistent with the confirmed beneficial effect of a single dose zoledronate infusion in a study conducted by Reid et al.[48] Actually, it is generally believed that oral bisphosphonate is associated with low absorption and poor patients’ compliance due to its side effects in an empty stomach in CD patients with low BMD whose digestive tracts have been compromised,[49,50] and our results were consistent with the statement that zoledronate was better than other bisphosphonate. Interestingly, only zoledronate had statistical beneficial effect of therapy for lumbar spine bone density in this network meta-analysis while other types of bisphosphonate rendered no significant difference when compared with placebo. Several possible reasons to explain this result as follows. First, small cohorts in our study may not have enough powerful effect size to show a statistical difference between the treatment and placebo group. Second, few articles were published to demonstrate the efficacy of etidronate, pamidronate, alendronate, and phylloquinone. Third, patients in different RCTs have different severity of low bone density.
The results obtained by the network meta-analysis demonstrated that sodium-fluoride had a beneficial effect in increasing bone density of CD patients, which was consistent with previous studies on postmenopausal women reported in a traditional meta-analysis.[51] This traditional meta-analysis confirmed that the incidence of new nonvertebral fractures and gastrointestinal adverse events were higher than those in sodium-fluoride group especially with high doses and in a non-slow-release form than placebo group at the forth year and reached statistical difference, thus, the use of sodium-fluoride was not recommended because the higher dose of sodium-fluoride, the higher incidence of nonvertebral fractures, and gastrointestinal tract side effects. Also, it had no effects on the reduction of the incidence of vertebral fracture. In view of our results, the long-term use of sodium-fluoride in clinical practice should be with a bit more cautions.
Besides the spine, a markedly increased rates of hip fracture could also be observed among patients with CD or other types of IBD.[18] Thus, additionally, we induced an assessment of BMD change within total hip to further detect the effect of included treatments. However, the final results were unconspicuous. To determine the potential role of etidronate in the improvement of BMD, Siffledeen et al[23] conducted a RCTs recruiting 154 CD patients with a follow-up of 24 months. They proposed an interesting result that both the etidronate and the non-etidronate treated groups showed a statistical trend of BMD improvement compared with the baseline state at all involved bones, except the total hip. Although the improvement was probably attributed to the utilization of supplemental calcium and vitamin D, the total hip might render an insensitive to these exogenous agents and other supplements. This could be partially interpreted by the difference of surface-to-volume ratio and vascularity between trabecular bone and cortical bone.[52]
The incidence of AEs in bisphosphonates, sodium-fluoride, and phylloquinone concomitant has a trend to be higher than placebo, but no significant differences were identified. Several factors could interpret this result: first, the overall 12 RCTs in our network meta-analysis was not powerful enough to demonstrate statistical significance difference between intervention and placebo group; second, few articles were published to demonstrate the safety of etidronate, pamidronate, alendronate, and phylloquinone.
Some strengths in our article: (i) it is the first and the most comprehensive network meta-analysis to investigate different intervention in treating low BMD in patients with CD; (ii) design and outcome assessments were consistent in all trials included in our study; (iii) the latest guideline of GRADE approach was applied to rate the quality of evidence of eight agents for network meta-analysis. However, our article also has limitations: (i) due to the lack of data to yield outcomes (such as fracture rate) in most trails included in our article, we could only extract the change of LSBMD value to evaluate the efficiency of various treatment options; (ii) due to the small sample sizes in our study, most network comparisons were in low quality by GRADE assessment.
In conclusion, our network meta-analysis suggests that zoledronate might have the highest probability to be the best treatment for increasing LSBMD in CD patients among all other agents. For the safety assessment, risedronate showed the greatest power to decrease the risk of AEs while alendronate performed the worst, but all of them were not statistically sound. Future, more high quality RCTs (larger number of CD patients with low BMD and longer follow-up time) are needed to make head-to-head comparisons of treatments.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AEs = adverse events, BMD = bone mineral density, CD = Crohn disease, CI = confidence interval, CrI = credible interval, DXA = dual energy x-ray absorptiometry, GRADE = grading of recommendation, assessment, development, and evaluation, IBD = inflammatory bowel disease, IC = indeterminate colitis, LSBMD = lumbar spine bone mineral density, MeSH = medical subject heading, OR = odds ratio, RCTs = randomized controlled trials, SMD = standardized mean difference, SUCRA = surface under the cumulative ranking area, THBMD = total hip bone mineral density, UC = ulcerative colitis.
Treatment for low bone mineral density in Crohn disease.
XJZ, CCZ, and HC have contributed equally to this study.
Grant Support: This work was supported by grants from the National Natural Science Foundation of China (No. 81470827 and No. 81270469).
Author Contributions: Conceived and designed the experiments: XJZ, CCZ, and HC. Performed the experiments: XJZ, CCZ, HC, and JJM. Analyzed the data: CCZ. Contributed reagents/materials/analysis tools: YJZ, PXW, YZ, and HQM. Wrote the paper: XJZ and CCZ. Proofread and revised the paper: HC and HJZ.
All authors approved the submitted version of the manuscript.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007;44:653–74. [DOI] [PubMed] [Google Scholar]
- [2].Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- [3].Schulte C, Dignass AU, Mann K, et al. Reduced bone mineral density and unbalanced bone metabolism in patients with inflammatory bowel disease. Inflamm Bowel Dis 1998;4:268–75. [DOI] [PubMed] [Google Scholar]
- [4].Von Tirpitz C, Pischulti G, Klaus J, et al. Pathological bone density in chronic inflammatory bowel diseases—prevalence and risk factors. Z Gastroenterol 1999;37:5–12. [PubMed] [Google Scholar]
- [5].Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut 1987;28:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghosh S, Cowen S, Hannan WJ, et al. Low bone mineral density in Crohn's disease, but not in ulcerative colitis, at diagnosis. Gastroenterology 1994;107:1031–9. [DOI] [PubMed] [Google Scholar]
- [8].Jahnsen J, Falch JA, Aadland E, et al. Bone mineral density is reduced in patients with Crohn's disease but not in patients with ulcerative colitis: a population based study. Gut 1997;40:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol 2011;300:G191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bernstein CN, Seeger LL, Sayre JW, et al. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Miner Res 1995;10:250–6. [DOI] [PubMed] [Google Scholar]
- [11].Staun M, Tjellesen L, Thale M, et al. Bone mineral content in patients with Crohn's disease. A longitudinal study in patients with bowel resections. Scand J Gastroenterol 1997;32:226–32. [DOI] [PubMed] [Google Scholar]
- [12].Driscoll RH, Jr, Meredith SC, Sitrin M, et al. Vitamin D deficiency and bone disease in patients with Crohn's disease. Gastroenterology 1982;8:1252–8. [PubMed] [Google Scholar]
- [13].Von Tirpitz C, Epp S, Klaus J, et al. Effect of systemic glucocorticoid therapy on bone metabolism and the osteoprotegerin system in patients with active Crohn's disease. Eur J Gastroenterol Hepatol 2003;15:1165–70. [DOI] [PubMed] [Google Scholar]
- [14].Andreassen H, Hylander E, Rix M. Gender age, and body weight are the major predictive factors for bone mineral density in Crohn's disease: a case-control cross-sectional study of 113 patients. Am J Gastroenterol 1999;94:824–8. [DOI] [PubMed] [Google Scholar]
- [15].Klaus J, Armbrecht G, Steinkamp M, et al. High prevalence of osteoporotic vertebral fractures in patients with Crohn's disease. Gut 2002;51:654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Van Bodegraven AA, Bravenboer N, Witte BI, et al. Treatment of bone loss in osteopenic patients with Crohn's disease: A double-blind, randomised trial of oral risedronate 35 mg once weekly or placebo, concomitant with calcium and vitamin D supplementation. Gut 2014;63:1424–30. [DOI] [PubMed] [Google Scholar]
- [17].O’Connor EM, Grealy G, McCarthy J, et al. Effect of phylloquinone (vitamin K1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn's disease. Br J Nutr 2014;112:1163–74. [DOI] [PubMed] [Google Scholar]
- [18].Soo I, Siffledeen J, Siminoski K, et al. Risedronate improves bone mineral density in Crohn's disease: a two year randomized controlled clinical trial. J Crohns Colitis 2012;6:777–86. [DOI] [PubMed] [Google Scholar]
- [19].Klaus J, Reinshagen M, Herdt K, et al. Bones and Crohn's: No benefit of adding sodium fluoride or ibandronate to calcium and vitamin D. World J Gastroenterol 2011;7:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Klaus J, Reinshagen M, Herdt K, et al. Intravenous ibandronate or sodium-fluoride—a 3.5 years study on bone density and fractures in Crohn's disease patients with osteoporosis. J Gastrointestin Liver Dis 2011;20:141–8. [PubMed] [Google Scholar]
- [21].Klaus J, Haenle MM, Schröter C, et al. A single dose of intravenous zoledronate prevents glucocorticoid therapy-associated bone loss in acute flare of Crohn's disease, a randomized controlled trial. Am J Gastroenterol 2011;106:786–93. [DOI] [PubMed] [Google Scholar]
- [22].Sbrocchi AM, Forget S, Laforte D, et al. Zoledronic acid for the treatment of osteopenia in pediatric Crohn's disease. Pediatr Int 2010;52:754–61. [DOI] [PubMed] [Google Scholar]
- [23].Siffledeen JS, Fedorak RN, Siminoski K, et al. Randomized trial of etidronate plus calcium and vitamin D for treatment of low bone mineral density in Crohn's disease. Clin Gastroenterol Hepatol 2005;3:122–32. [DOI] [PubMed] [Google Scholar]
- [24].Von Tirpitz C, Klaus J, Steinkamp M, et al. Therapy of osteoporosis in patients with Crohn's disease: A randomized study comparing sodium fluoride and ibandronate. Aliment Pharmacol Therap 2003;17:807–16. [DOI] [PubMed] [Google Scholar]
- [25].Bartram SA, Peaston RT, Rawlings DJ, et al. A randomized controlled trial of calcium with vitamin D, alone or in combination with intravenous pamidronate, for the treatment of low bone mineral density associated with Crohn's disease. Aliment Pharmacol Therap 2003;18:1121–7. [DOI] [PubMed] [Google Scholar]
- [26].Tirpitz C, Klaus J, Brückel J, et al. Increase of bone mineral density with sodium fluoride in patients with Crohn's disease. Eur J Gastroenterol Hepatol 2000;12:19–24. [DOI] [PubMed] [Google Scholar]
- [27].Haderslev KV, Tjellesen L, Sorensen HA, et al. Alendronate increases lumbar spine bone mineral density in patients with Crohn's disease. Gastroenterology 2000;119:639–46. [DOI] [PubMed] [Google Scholar]
- [28].Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2014;12:32.e5–44.e5. [DOI] [PubMed] [Google Scholar]
- [29].Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1–9. [DOI] [PubMed] [Google Scholar]
- [31].Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sutton A, Ades AE, Cooper N, et al. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008;26:753–67. [DOI] [PubMed] [Google Scholar]
- [33].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [34].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [35].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med 2003;22:3687–709. [DOI] [PubMed] [Google Scholar]
- [37].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- [41].Wang C. Efficacy and safety of zoledronic acid for treatment of postmenopausal osteoporosis: a meta-analysis of randomized controlled trials. Am J Ther 2016;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [42].Gu HF, Gu LJ, Wu Y, et al. Efficacy and safety of denosumab in postmenopausal women with osteoporosis: a meta-analysis. Medicine (Baltimore) 2015;94:e1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gu JM, Wang L, Lin H, et al. The efficacy and safety of weekly 35-mg risedronate dosing regimen for Chinese postmenopausal women with osteoporosis or osteopenia: 1-year data. Acta Pharmacol Sin 2015;36:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Feng Z, Zeng S, Wang Y, et al. Bisphosphonates for the prevention and treatment of osteoporosis in patients with rheumatic diseases: a systematic review and meta-analysis. PLoS One 2013;8:e80890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Amiche MA, Albaum JM, Tadrous M, et al. Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporos Int 2016;27:1989–98. [DOI] [PubMed] [Google Scholar]
- [46].Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;1:CD001155. [DOI] [PubMed] [Google Scholar]
- [47].Stokkers PC, Deley M, Van Der Spek M, et al. Intravenous pamidronate in combination with calcium and vitamin D: highly effective in the treatment of low bone mineral density in inflammatory bowel disease. Scand J Gastroenterol 2006;41:200–4. [DOI] [PubMed] [Google Scholar]
- [48].Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 2002;346:653–61. [DOI] [PubMed] [Google Scholar]
- [49].Cosman F. Treatment of osteoporosis and prevention of new fractures: role of intravenously administered bisphosphonates. Endocr Pract 2009;15:483–93. [DOI] [PubMed] [Google Scholar]
- [50].Weycker D, Macarios D, Edelsberg J, et al. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int 2006;17:1645–52. [DOI] [PubMed] [Google Scholar]
- [51].Haguenauer D, Welch V, Shea B, et al. Fluoride for the treatment of postmenopausal osteoporotic fractures: a meta-analysis. Osteoporos Int 2000;11:727–38. [DOI] [PubMed] [Google Scholar]
- [52].Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999;25:97–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


