Abstract
The purposes of this study were to assess the prevalence, malignancy rate, and characteristics of incidental thyroid nodules (ITNs), and to identify factors that contribute the additional workup by ultrasound.
The medical records and imaging features of ITNs reported via thoracic computed tomography (CT) were retrospectively reviewed to determine the size, multiplicity, attenuation, shape, and presence of calcification. To identify the factors associated with additional workup, we compared the workup and non-workup groups in terms of nodule characteristics, indications, and CT slices. We identified factors that could distinguish malignant ITNs from non-malignant nodules.
A total of 60,921 thoracic CT scans met the inclusion criteria, and ITNs were reported using formal radiology in 2733 patients (4.5%). Among all patients with reported ITNs, 546 (20.0%) underwent further workup. Of these patients, 62 (2.3%, 62/2773) were diagnosed with malignant nodules. Multivariable analysis identified multiple factors associated with additional workup, including female sex, younger age, larger nodule size, calcification, anteroposterior to transverse dimension ratio >1, heterogeneous attenuation in the nodule, and scanning indications such as infection or screening. However, only calcification was associated with malignant nodules (odds ratio [OR] = 2.313; 95% confidence interval [CI], 1.301–4.113).
We observed discordance between the numbers of reported ITNs and case with additional workup and identified multiple factors associated with additional workup. We have, therefore, demonstrated a need for reliable subsequent evaluation guidelines and note that the appearance of calcification in an ITN on imaging may be a factor indicating the need for additional workup.
Keywords: multidetector computed tomography, thorax, thyroid neoplasms, thyroid nodule
1. Introduction
The incidental thyroid nodule (ITN), defined as a thyroid lesion identified during an imaging study for non-thyroid-related reasons, has been proposed as a significant contributor to the more frequent detection of thyroid cancers.[1,2] Specifically, the incidence of thyroid cancer nearly tripled in the United States between 1975 and 2009.[3] The reasons underlying this increasing incidence of thyroid cancer are unclear. Some previous studies have suggested that this rise occurred because of increased detection of ITNs consequent to improvements in imaging modalities and technology.[1,2] In particular, advancements in multi-slice computed tomographic (CT) scanner technology have yielded significant improvements, including improved resolution, and thus have increased the detection of small thyroid nodules.[4] In addition, the increased use of diagnostic CT scans has led to the increased detection of ITNs.[1] Several studies have discussed ITNs on neck-only CT scans or scans comprising combinations of regions such as the neck, cervical spine, and chest.[1,5,7] The reported prevalence of ITNs on CT ranges from 16% to 25%, and varies with the target body part and CT detector slice type.[5–8] ITNs are commonly identified during thoracic CT scans.[1,9] However, information regarding the significance of ITNs on thoracic-only CT scans in the adult population is less well studied.
Previous studies of ITN detection via CT have reported data obtained through dedicated radiology reviews, in which radiologists reviewed images specifically to detect thyroid nodules.[5–8,10] However, radiologists’ reporting practices vary widely.[9,11] Accordingly, these results may overestimate the prevalence of ITNs reported in clinical practice.
The management of ITNs presently remains unclear. Although the American Thyroid Association (ATA) recommends that all patients with ITNs undergo further ultrasound evaluation,[12] the American College of Radiology (ACR) proposed a recommendation for management of ITNs identified on CT.[13] Although many ITNs are reported and recommended for further evaluation by radiologists, only a few patients with ITNs actually undergo an additional workup.[7,10,14] However, few reports have discussed the factors that influence decisions regarding additional workup.[14]
The primary purposes of this study were to investigate the prevalence of reported ITNs and the malignancy rates and imaging features of these nodules, and to compare these findings with a dedicated review of thoracic contrast-enhanced multi-slice CT data and observation of the clinical outcomes of these nodules. The secondary purpose was to identify factors that contribute to the performance of an additional workup in clinical practice.
2. Materials and methods
2.1. Study population
The institutional review board at our institution approved the protocol for this retrospective study; informed consent was waived. We retrospectively searched patients who underwent thoracic contrast-enhanced CT (CECT) scans at our hospital between January 1, 2005 and December 31, 2014. Patients with ITNs were identified through a radiologic report search, by using the search terms “thyroid nodule” and/or “thyroid lesion.” A patient was excluded if he or she had a history of any thyroid disease, such as “thyroid cancer,” “thyroid carcinoma,” “papillary carcinoma,” “medullary carcinoma,” or “follicular carcinoma” or had undergone a prior thyroid evaluation via ultrasound or fine-needle aspiration (FNA).
2.2. CT imaging technique
All thoracic CECT studies were performed on one of the following CT scanners: a single-detector row spiral CT scanner (Genesis Hi-speed RP; GE Healthcare, Little Chalfont, UK), 16-slice CT scanner (SOMATOM Sensation 16; Siemens AG, Munich, Germany), or a 64-slice CT scanner (LightSpeed VCT or Optima 660; GE Healthcare, Milwaukee, WI). The area of coverage extended from the lower neck to the adrenal glands. Acquisition parameters included detector row configurations of 0.625, 1.25, and 5 mm and section thicknesses of 1.25 to 5 mm. All scanning was performed after the intravenous administration of non-ionic water-soluble contrast material.
2.3. Chart review and image analysis
Medical records were reviewed to obtain demographic patient information, indications for imaging, previous medical and surgical histories, and pathologic reports.
All thoracic CECT scans related to ITN reports were retrospectively reviewed by two board-certified radiologists in the subspecialty of thoracic imaging and were correlated with formal radiologic reports. ITNs on CT scans were reviewed with respect to size, multiplicity, attenuation, shape, and presence of calcification. Nodule size was measured in millimeters using an electronic caliper tool. Regarding multiplicity, cases were classified as one or more than one nodule. Nodule density was categorized into hyper-, hypo-, iso-, or heterogeneous attenuation relative to the thyroid parenchyma. Nodule shape was classified according to whether the ratio of the anteroposterior to transverse dimension (AP/T) did or did not exceed 1. Through a chart review, patients with ITNs on CT scans were subdivided into those who underwent further ultrasonographic workup within 2 years and those who did not undergo further evaluation. Patients who underwent further evaluation >2 years after the initial ITN diagnosis were categorized into the latter group. For patients who underwent an additional workup, further information was obtained regarding whether FNA and/or surgical resection had been performed and the existence of a final pathologic diagnosis, which was defined as a malignant lesion (suspicious for carcinoma or carcinoma) detected via FNA. Cases without cytologically definite malignant lesions were categorized as non-malignant lesions. However, for patients who underwent surgery, the final pathologic diagnosis was defined via surgical pathology.
A dedicated blinded review of 890 randomly selected thoracic CECT scans was performed by same radiologists mentioned above. The incidence of ITNs from radiologic reports recorded during daily practice was compared with the results of this dedicated, experienced radiologic review of random samples.
2.4. Statistical analysis
The Wilson method was used to calculate 95% confidence intervals (CIs) around the prevalence of ITN on thoracic CECT.[15] The number of thoracic CT studies to be sampled was calculated to estimate the proportion of ITN on thoracic CT with 95% confidence and 6% absolute error, for an expected ITN incidence of 25% and loss to follow-up are expected may be 10%.
A linear regression analysis was used to fit the annual trends of performed CT studies, ITNs, additional ultrasound workups, and malignant nodules, and correlations between the reported ITNs and related annual parameters such as the CT volume, additional ultrasound volume, and malignant nodules were determined using the Spearman correlation coefficient (rho). Student t test was used to determine differences in continuous variables such as age and nodule size. Fisher exact test or the chi-squared test was used for analyses to determine correlations of CT features with non-malignant or malignant pathology and to identify differences in categorical valuables between patients with ITNs that did and did not undergo an additional workup. A multivariable logistic regression analysis was performed to determine the factors that conduct additional workup and clinical and imaging factors between non-malignant and malignant pathology. Odds ratios of tendency for subsequent evaluation for nodule attenuation on CT images and indication for CT scanning were obtained by using hyper-attenuation and malignancy as the reference group, respectively. The statistical analysis was conducted by the SPSS statistical software package (version 22.0; IBM Corp, Somers, New York). A P value <0.05 were considered statistically significant.
3. Results
3.1. Patient information
A total of 61,827 patients who underwent thoracic CECT scans during the above-mentioned 10-year period were identified. Of these, 906 patients were excluded because of known thyroid disease (520 patients) or prior ultrasound, FNA, or surgical diagnosis of thyroid disease (386 patients). Among the 60,921 remaining patients, 2733 patients with radiology reports that included the term “thyroid nodule” or “thyroid lesion” were identified. These 2733 patients with ITNs were subdivided into 546 patients who underwent additional ultrasound workups within 2 years and 2187 patients who either did not undergo further workups or underwent further evaluations >2 years after the initial diagnosis of ITN (Fig. 1).
Figure 1.
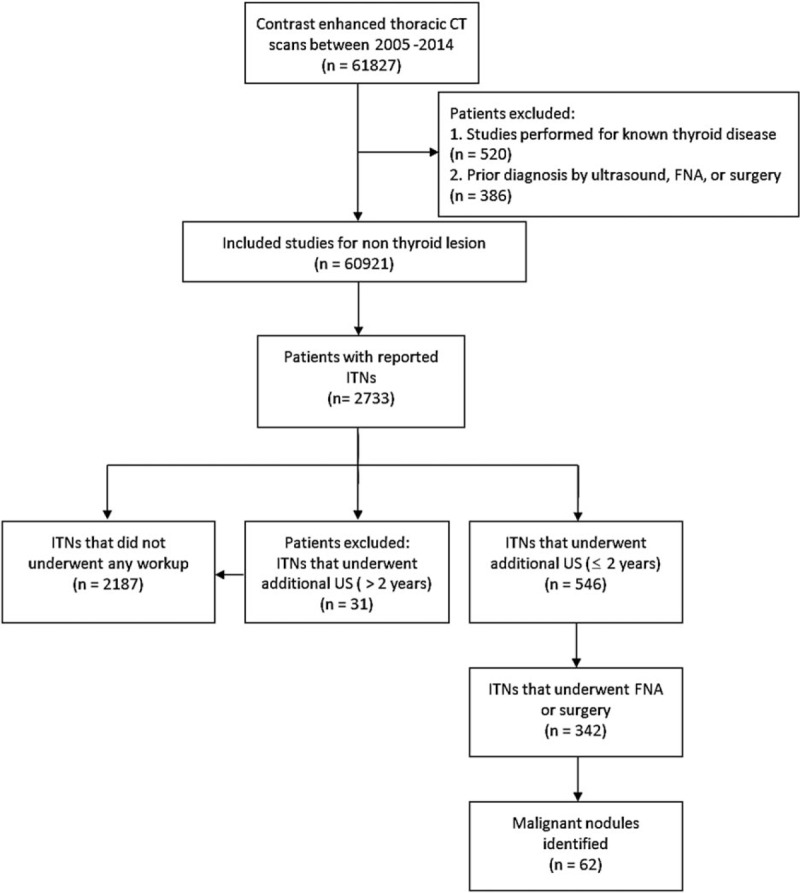
Flow chart of study inclusion and exclusion criteria used to subdivide patients into workup and non-workup groups. FNA = fine-needle aspiration, ITN = incidental thyroid nodule, US = ultrasound.
3.2. ITN imaging features, prevalence, and malignancy rate
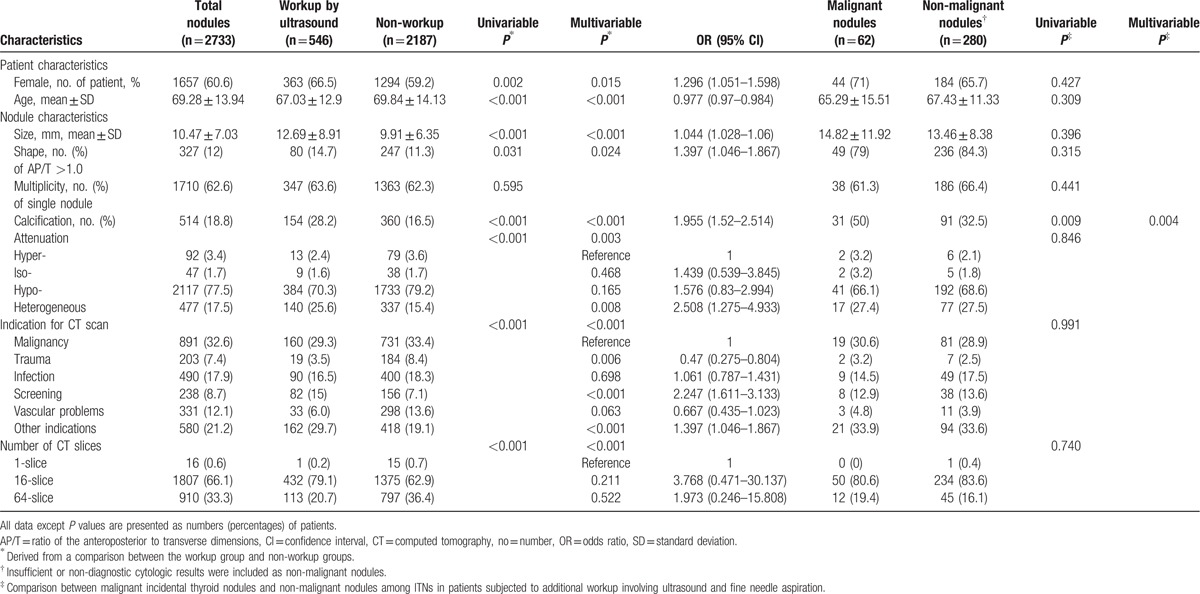
The prevalence of ITNs reported on thoracic CECT in the study population was 4.5% (2733 of 60,921 studies; 95% CI, 4.3–4.7%). Among these studies, 1657 (60.6%) involved women and 1710 (62.6%) identified a single thyroid nodule. The most common indication for a thoracic CT scan was malignancy (891/2733, 32.6%), followed by non-specific complaints (580, 21.2%). On CT, the average size of the dominant nodule in the axial plane was 10.47 ± 7.03 mm, and the majority of nodules measured <10 mm (1533/2733, 56.1%). In addition, 514 nodules (18.9%) exhibited visible calcification on CT, and 327 ITNs (12.0%) had an AP/T ratio >1.0. Regarding the density of the dominant nodule, the majority of nodules exhibited hypo-attenuation (2117/2733, 77.5%; Table 1).
Table 1.
Comparison of ITNs with workup by ultrasound and no workup and characteristics of malignant incidental thyroid nodule.
Among all patients with ITNs, 546 (20.0%) underwent follow-up thyroid ultrasound workups within 2 years of the initial ITN diagnosis. Of these 546 patients, 342 underwent FNA or proceeded to surgery, and 63 were diagnosed with malignant nodules. Among 63 malignant nodules, 57 nodules were papillary carcinoma and six other malignant nodules were diagnosed as suspicious metastatic thyroid nodules subjected to FNA without surgical resection, and therefore those were not considered final pathologic diagnoses. Thirty-six patients were treated surgically, and all underwent preoperative FNA. Thirty-three malignancies were identified in the final pathology reports of patients who underwent surgery; 2 patients had received benign pathologic results from preoperative FNA. The remaining 3 patients with suspected malignant cytology according to preoperative FNA results were found to have benign disease according to the final surgical pathologic analysis. The remaining 277 patients who had undergone subsequent workups were found to have benign nodular hyperplasia or an insufficient cytologic diagnosis. The prevalence of malignancy among ITNs on thoracic CECT was 2.3% (62/2773; 95% CI, 1.8–2.9%) and the prevalence among all studies was 0.1% (62/60921; 95% CI, 0.08–0.13%).
3.3. Dedicated radiologic review
A board-certified radiologist conducted a dedicated, blinded radiologic review of 890 thoracic CECT scans with a particular focus on thyroid nodule detection. This review identified 209 incidental thyroid nodules (23.5%). There were no descriptions of these ITNs in the radiologic clinical reports. The mean nodule size was 6.00 mm ± 3.28 mm (range, 2–25 mm). The mean size of clinically reported ITNs was significantly larger than that of nodules detected through a dedicated blinded review (P < 0.001).
3.4. Trends in ITNs, additional ultrasound workup, malignant nodules, and influence of multi-slice detector
The total number of studies performed annually increased throughout the study period, except for the number of CT studies performed in 2006. The number of ITNs also increased annually in a linear manner during the study period (P < 0.001). However, additional ultrasound workups and malignant nodule detection did not increase annually in a linear manner (P = 0.088 and 0.393, respectively; Fig. 2). Furthermore, a very strong linear relationship (P < 0.001, rho = 0.952) was observed between the numbers of ITNs and thoracic CECT studies per year, and non-significant linear relationships were observed between the numbers of ITNs and additional ultrasound workups or between the numbers of ITNs and malignant nodules (P = 0.108 and 0.411, respectively). Strong linear relationships were also observed between the annual numbers of additional ultrasound workups and detected malignant nodules (P = 0.02, rho = 0.717).
Figure 2.
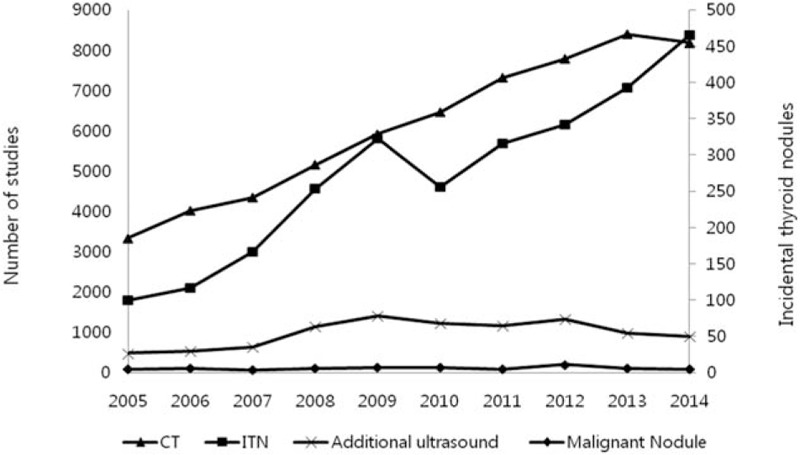
Annual trends in the total number of thoracic CECT, number of ITNs, number of additional ultrasound evaluations, and number of malignant nodules at our institution during the study period. The annual number of ITNs increased in a linear manner as a result of an increase in the total number of studies (P < 0.001). The numbers of additional ultrasounds and malignant ITNs, however, did not exhibit linear annual increases. CECT = contrast-enhanced computed tomography, ITN = incidental thyroid nodule.
The ITN prevalence differed significantly according to the CT scanner slice number. In other words, an increased CT slice number corresponded with an increase in the ITN prevalence. For single-slice CT scans, the ITN prevalence was 1.16% (16/1380), whereas these rates increase to 4.40% (1807/41110) with 16-slice scans and 4.94% (910/18431) with 64-slice scans (P < 0.001). The malignancy rates according to CT slice number were 0% (0/16) with single-slice scans, 2.8% (50/1807) with 16-slice scans, and 1.3% (12/910) with 64-slice scans (P = 0.047).
3.5. Factors affecting additional workups
Patients who underwent further evaluation had a mean nodule size of 12.69 ± 8.91 mm, which was larger than the mean nodule size of patients who did not undergo further workups (9.91 ± 6.35 mm, P < 0.001). An increased workup frequency (28.2%) was also observed for cases involving ITNs with calcification relative to those without calcification (16.5%, P < 0.001). Patients with ITNs with an AP/T ratio >1.0 were more likely to have undergone subsequent evaluation (14.7%) than were those with an AP/T ratio ≤1.0 (11.3%, P = 0.031). The workup rate differed significantly according to attenuation (P < 0.001), as ITNs with heterogeneous attenuation were more frequently associated with further workup. Patients who did and did not undergo further workup also differed significantly with respect to the indication for CT evaluation. Specifically, patients who underwent scanning to evaluate a known malignancy or vascular problem were less likely to undergo a further workup. However, patients who participated in a CT study for screening or other reasons were more likely to undergo an additional workup (Table 1).
The results of the univariable and multivariable analyses were similar. Female and younger patients, patients with larger ITNs (>10 mm), presence of calcification, AP/T ratio >1.0, heterogeneous attenuation versus hyper-attenuation, and scanning for the evaluation of infection, screening, and other reasons were associated with an increased likelihood of further workup for ITNs versus patients with malignancy. However, patients who underwent CT evaluations for trauma or vascular abnormalities were less likely to undergo further evaluation, compared with those who had underlying malignancy (Table 1 and Fig. 3). The CT slice number was an influencing factor, although 16- and 64-slice CT scanners did not differ significantly with respect to ORs calculated using single-slice CT as the reference.
Figure 3.
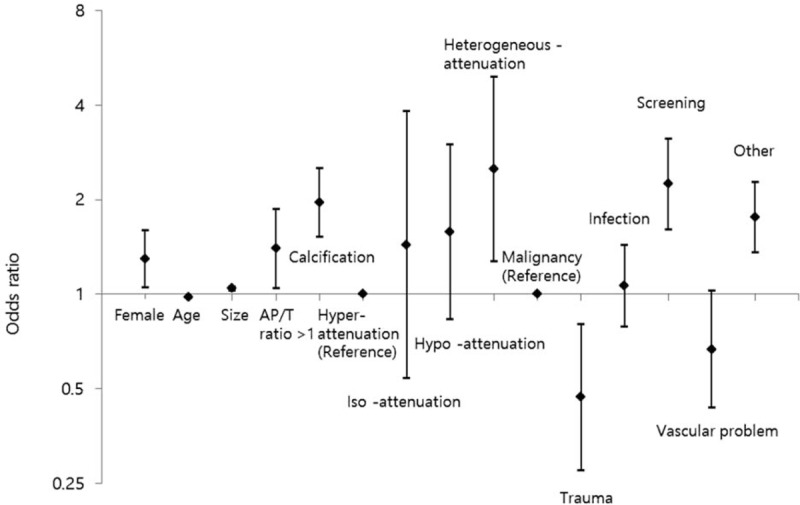
Odds ratios for trends in the performance of additional workups and comparisons of each nodule characteristic, such as an AP/T ratio >1; calcification; and nodule iso-, or hypo-, or heterogeneous attenuation (reference: hyper-attenuation), with each indication for a CT study, including trauma, infection, screening, vascular problem, and other (reference: malignancy). AP/T ratio = ratio of the anteroposterior to transverse dimensions, CT = computed tomography.
3.6. Differences between non-malignant and malignant nodules
A few characteristics were associated with malignant ITNs detected via thoracic CECT in patients who underwent additional workups. The average malignant ITN size was 14.82 ± 11.92 mm; that of non-malignant ITNs was 13.46 ± 8.38 mm (P = 0.309). In a total of 62 malignant ITNs, 31 exhibited calcification on thoracic CECT (50%); among the 280 non-malignant ITNs, 91 exhibited calcification (32.5%) on both univariable and multivariable analyses (P < 0.05). A significant association was observed between malignant nodules and the imaging feature of calcification (OR = 2.313; 95% CI, 1.301–4.113). However, there were no significant differences in terms of demographic factors, other nodule characteristics, indications for CT scanning, and CT slice number (P > 0.05) in the univariable and multivariable analyses (Table 1).
4. Discussion
Our study was the first and largest to examine the clinical reporting, workup, and outcomes of reported ITNs detected via thoracic CECT. Several published studies have described the use of multiple imaging modalities, including CT, positron emission tomography-CT, magnetic resonance (MR), and/or ultrasonography, to determine the prevalence of ITNs.[7,16–18] Of these modalities, CT is the most commonly used for identifying ITNs.[9,16–18] Radiologists who interpret CT scans including thyroid scans, in daily practice encounter ITNs and therefore face the issue of how best to report and manage these lesions. In addition, few previous studies were based only on CT images, and these almost exclusively comprise studies of the neck only or a combination of regions, including the neck, cervical spine, and/or chest.[1,5,6] Thoracic CT scans are used broadly for evaluations in the context of various medical conditions, and are a common method of ITN detection.[1,9] Unlike neck or cervical CT, thoracic CT does not focus on the thyroid. Therefore, it is difficult to characterize ITNs, which are typically small in size, on thoracic CT images.[4] In addition, beam-hardening artifacts resulting from the positions of the patient's arm and clavicle and/or a high density of intravenous contrast can obscure lesions or cause pseudolesions.[4] However, only two published studies that focused on ITNs detected via thoracic CECT in adults and children have been reported.[8,19] Accordingly, data of ITNs on thoracic CT could help both clinicians and radiologists to understand the clinical significance and appropriate management of these lesions.
In our study, the prevalence of ITNs on thoracic CECT was 4.5% (95% CI, 4.3–4.7%), according to radiologic reports. Earlier reviews of CT scans conducted for non-thyroid reason revealed ITN prevalence rates ranging from 16% to 25%.[6,8] In particular, a study of thoracic CT reported an ITN prevalence of 25.1% on thoracic CECT scans.[8] However, those studies were based on dedicated reviews, in which radiologists reviewed images with a specifically focus on thyroid nodules. Such a study design might overestimate the prevalence of ITNs reported in actual clinical practice. The results of our study, which was based on clinical reports, yielded lower ITN prevalence on thoracic CECT than was previously reported for an adult population; however, our result from a dedicated review was 23.5%, similar to that of the previous report. Furthermore, our findings demonstrated that the prevalence of clinically reported ITN was significantly lower than the prevalence in a dedicated radiology review, suggesting a tendency to report large-sized ITNs in clinical practice. This underreporting of ITNs in clinical radiology reports, compared with dedicated radiology reviews, is consistent with the findings of an earlier study.[16] This result indicates that the prevalence of clinically reported ITNs depend on the rate at which radiologists miss ITNs, as well as the reporting style, and suggest that a number of ITNs are not routinely reported to clinicians in actual practice. Several studies have demonstrated high variability in radiologists’ reporting practices.[9,11] These results support the need for reliable guidelines for reporting ITNs detected on CT.
According to our results, the numbers of additional ultrasound evaluations and malignant nodules did not significantly increase during the study period (P > 0.05), unlike the volume of CT scans and number of ITNs during the same period (P < 0.001). In addition, linear correlations between the number of ITNs and the numbers of other parameters (e.g., additional ultrasound evaluations and malignant nodules), were non-significant, although a very strong linear correlation (P < 0.001) was identified between the annual numbers of ITNs and thoracic CT studies. In addition, the ITN prevalence gradually increased (P < 0.001) as the number of CT slices increased. However, 16-slice CT scanners had a significantly higher prevalence, compared with 64-slice CT scanners (P < 0.05). Although the number of ITNs on CT increased along with increases in the performed CT volume and advancements in CT scanner technology, a substantial number of newly identified ITNs on thoracic CECT might actually be non-malignant nodules. In another respect, the ITN malignancy rate depends on the diagnostic technique and clinician's workup rate.[13] In our study, the malignancy rate among reported ITNs was 2.3% (95% CI, 1.8–2.9%). Several studies have reported various malignancy rates among ITNs identified using CT or magnetic resonance imaging and used FNA, core needle biopsy, or surgery, as the technique to diagnose malignancy.[5–7,10] In an earlier meta-analysis of nodules suspected of malignancy according to FNA results, a quarter of the nodules were later identified as benign.[20] In our study, 2 surgical patients with suspected malignancies according to cytological results were found to have benign disease. In addition, subsequent workup for reported ITNs has not been routine in previous studies, with reported workup rates of 16% to 37%.[6,7,10,14] Similarly, our study observed a workup rate of 20%. Low workup rates could be explained by several factors. First, radiologists have a low threshold, and advances in multi-slice detector CT scanner technology and an increased CT volume might lead to an excess of reported ITNs. Consequently, clinicians might grow weary of this gradual increase in radiologic reporting and implement a high threshold for subsequent ITN evaluations. Although the number of ITNs increased continuously in 2013 and 2014, the number of additional ultrasounds decreased during the same period in our study. In addition, the ITN prevalence with 64-slice CT was significantly higher than that with 16-slice CT. However, the workup rates according to CT slice number were 23.9% (432/1807) for 16-slice and 12.4% (113/910) for 64-slice; accordingly, the subsequent workup rate was statistically lower at a higher slice number (P < 0.001). Second, a previous observational trial reported that in the absence of surgery, a large number of microcarcinomas either remained stable or decreased in size[21]; according to other reports, the mortality rate associated with thyroid cancer remained stable despite a dramatic increase in the disease incidence.[3,22] This factor could also influence clinicians’ decisions to perform additional workups.
In our study, we observed a strong linear relationship of the volume of additional ultrasounds with the number of malignant nodules (P = 0.02), but not with the number of ITNs (P > 0.05). Our results indicate that clinicians’ decisions to perform additional workups might be more important for the detection of malignant nodules following the reporting of ITNs on CT. However, the management guidelines for ITNs identified on CT remain controversial. The ATA recommends that all ITNs identified on CT be subjected to subsequent ultrasonographic evaluation, but does not directly specify the management of ITNs.[12] It must be noted, however, that attempts to conduct routine workups of ITNs based on nodule size alone have introduced issues related to cost-effectiveness.[7] However, ACR recommendation was based on a categorical method known as the 3-tiered system. In 3 retrospective studies, this system was found to reduce the need for additional workup.[10,14,23] In an ACR white paper recommendation, the patient's age and clinical condition (e.g., life expectancy, comorbidities) and the size of ITNs identified via imaging are included as formal criteria for the management of ITNs. Tanpitukpongse et al[14] revealed that the only two factors that should affect decisions regarding further workup are a younger patient age and larger nodule size. In addition to these factors, our study demonstrated through a multivariable analysis that decisions regarding additional workups for ITNs were influenced by multiple factors, including demographic factors (e.g., female sex, younger patient age), imaging features of ITNs (larger nodule size, AP/T ratio >1.0, presence of calcification, and heterogeneous attenuation vs. hyper-attenuation as a reference), and indications for thoracic CT (e.g., screening or evaluation for non-specific complaint vs. underlying malignancy as a reference). The multiple factors identified in our study also included the previously identified factors of a younger age and larger nodule size. Three particular factors—patient age, nodule size, and indication—were also included in the ACR white paper recommendation, and indications indirectly reflected patients’ clinical condition. Patient age is not considered as a factor in ultrasound guidelines, but recent studies have found that younger patients were significantly more likely to have malignant nodules than were older patients,[5,24] and also had a slightly higher risk of tumor progression.[25] Nodule size is used as a cutoff when determining whether to perform additional ultrasound evaluations.[12] Patients with the indications of a screening evaluation or other cause were more likely to undergo an additional workup, compared with patients who underwent evaluation of a known malignancy, trauma, or vascular problem. A previous study also suggested that patients with the indication of a known malignancy or trauma were less likely to undergo a subsequent evaluation.[14] Because the clinical prognosis of thyroid cancer is relatively good, clinicians might be less likely to conduct additional workups for patients with relatively critical comorbidities or limited life expectancies. However, our study identified other factors, including demographic factors (e.g., female sex) and nodule characteristics, which were not included in the ACR recommendation. Female sex was found to influence decisions regarding additional workups. Thyroid cancer is approximately 3-fold more common in women than in men, and a nearly 4-fold increase in the incidence was observed in women.[3] Among various factors evaluated in our study, imaging features such as calcification, shape, and attenuation of the ITN were important contributing factors with relatively high odds ratios, suggesting significant associations of these factors with the performance of additional workups. In particular, the ultrasonographic features of calcification and shape (AP/T ratio >1) were suggestive of malignancy.[26] Although we did not review the existence of recommendations for subsequent ultrasound workups on radiology reports, radiologists might be more likely to report ITNs and recommend further workups.
Two previous studies have evaluated the use of CT imaging to differentiate benign from malignant thyroid nodules. One study suggested that distinguishing features were not detectable on CT.[5] However, the other study reported that calcification, an AP/T ratio >1, and relatively high attenuation were more frequent in malignant than in benign nodules, and suggested that nodule characteristics might be important factors in the differentiation of benign and malignant nodules.[6] These results were contradictory. The results of our study also demonstrated that malignant nodules were more likely to exhibit calcification on CT than were non-malignant nodules (P = 0.004). Among the multiple factors that affected decisions about additional workups, calcification was only factor that suggested the presence of a malignant nodule in both the univariable and multivariable analyses. Both the ATA guideline and the ACR white paper recommendation included nodule size as a formal criterion[12,13]; however, our results suggested that imaging features, such as the calcification of ITNs on CT, are likely to be potential factors that lead to recommendations for additional workup, and therefore further investigation is needed to determine the imaging features that differentiate non-malignant and malignant ITNs on CT scans. Although patients with malignant nodules were more frequently younger and had a larger nodule size, those differences were not statistically significant (P > 0.05).
This study had several limitations. First, this was a retrospective study at a single institution. The ITN reporting style was specific to the radiologists at our institution. Second, the text report search underestimated the number of ITNs. We used the search terms “thyroid nodule” and “thyroid lesion,” but did not include other related terms such as “mass.” Third, some patients with ITNs might have been lost to follow-up, leading to underestimation of the number of malignant nodules. Fourth, some nodules with insufficient or unsatisfactory cytologic results were categorized as non-malignant nodules. Although these nodules comprised a minority (17/280, 6.1%) of non-malignant nodules, they might have affected our results. Fifth, all patients with ITNs who underwent ultrasound workups did not receive confirmed pathologic diagnoses. In addition, not all final diagnoses of ITNs were based on surgical pathology; some were based only on cytology. Furthermore, the final pathologic diagnoses of some nodules changed after surgery. However, it is not a current standard practice to surgically confirm the diagnoses of ITNs with benign cytological results. In addition, although many clinicians are interested in imaging feature for indetermidiate stage such as atypia of undetermined significance or follicular lesion of undetermined significance, unfortunately, analyzing the borderline cytologic data cannot perform because there were a few cases. Therefore, further evaluation with correlation between variable pathologic type and CT feature will be needed. Finally, our study only considered imaging characteristics of malignant ITNs without attention for specific histological or cytological data. However, there was not enough case that correlation between malignant imaging feature, such as calcification, and histological type. Actually, our result shows total 63 malignant nodules almost papillary carcinoma, except for six metastatic nodules and only papillary carcinoma has calcification. Although we did not compared with histological type and imaging feature, the fact that calcification were found in only papillary carcinoma on CT scan, may be meaningful result.
5. Conclusion
Our study identified the clinical and imaging characteristics of ITNs and suggested the clinical significance of reporting ITNs detected via thoracic CECT in a large cohort of patients. We identified a discrepancy between the rates of reported ITNs and subsequent workup, and found that multiple factors were associated with the decision to perform an additional workup. Accordingly, we demonstrated the need for reliable guidelines for subsequent evaluation and conclude that the ITN imaging feature of calcification is a potential factor that indicates the need for additional workup.
Footnotes
Abbreviations: ACR = American College of Radiology, AP/T = ratio of anteroposterior to transverse dimension, ATA = American Thyroid Association, CECT = contrast-enhanced computed tomography, CI = confidence interval, FNA = fine-needle aspiration, ITN = incidental thyroid nodule.
This work was supported by Inha University Research Grant.
The study sponsor had no involvement in the conduct of the study or in the writing of the article.
An appropriate institutional review board approved the study (for studies involving human subjects or animals).
The authors declare that they have no conflicts of interest to disclose.
References
- [1].Hoang JK, Choudhury KR, Eastwood JD, et al. An exponential growth in incidence of thyroid cancer: trends and impact of CT imaging. AJNR Am J Neuroradiol 2014;35:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164–7. [DOI] [PubMed] [Google Scholar]
- [3].Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317–22. [DOI] [PubMed] [Google Scholar]
- [4].Ahmed S, Horton KM, Jeffrey RB, et al. Incidental thyroid nodules on chest CT: Review of the literature and management suggestions. AJR Am J Roentgenol 2010;195:1066–71. [DOI] [PubMed] [Google Scholar]
- [5].Shetty SK, Maher MM, Hahn PF, et al. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol 2006;187:1349–56. [DOI] [PubMed] [Google Scholar]
- [6].Yoon DY, Chang SK, Choi CS, et al. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr 2008;32:810–5. [DOI] [PubMed] [Google Scholar]
- [7].Youserm DM, Huang T, Loevner LA, et al. Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol 1997;18:1423–8. [PMC free article] [PubMed] [Google Scholar]
- [8].Ahmed S, Johnson PT, Horton KM, et al. Prevalence of unsuspected thyroid nodules in adults on contrast enhanced 16- and 64-MDCT of the chest. World J Radiol 2012;4:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoang JK, Riofrio A, Bashir MR, et al. High variability in radiologists’ reporting practices for incidental thyroid nodules detected on CT and MRI. AJNR Am J Neuroradiol 2014;35:1190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen XV, Choudhury KR, Eastwood JD, et al. Incidental thyroid nodules on CT: Evaluation of 2 risk-categorization methods for work-up of nodules. AJNR Am J Neuroradiol 2013;34:1812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grady AT, Sosa JA, Tanpitukpongse TP, et al. Radiology reports for incidental thyroid nodules on CT and MRI: high variability across subspecialties. AJNR Am J Neuroradiol 2015;36:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised american thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214. [DOI] [PubMed] [Google Scholar]
- [13].Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 2015;12:143–50. [DOI] [PubMed] [Google Scholar]
- [14].Tanpitukpongse TP, Grady AT, Sosa JA, et al. Incidental thyroid nodules on CT or MRI: Discordance between what we report and what receives workup. AJR Am J Roentgenol 2015;205:1281–7. [DOI] [PubMed] [Google Scholar]
- [15].Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–72. [DOI] [PubMed] [Google Scholar]
- [16].Uppal A, White MG, Nagar S, et al. Benign and malignant thyroid incidentalomas are rare in routine clinical practice: A review of 97,908 imaging studies. Cancer Epidemiol Biomark Prev 2015;24:1327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chaikhoutdinov I, Mitzner R, Goldenberg D. Incidental thyroid nodules: Incidence, evaluation, and outcome. Otolaryngol Head Neck Surg 2014;150:939–42. [DOI] [PubMed] [Google Scholar]
- [18].Bahl M, Sosa JA, Nelson RC, et al. Imaging-Detected incidental thyroid nodules that undergo surgery: A single-center experience over 1 year. AJNR Am J Neuroradiol 2014;35:2176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baez JC, Zurakowski D, Vargas SO, et al. Incidental thyroid nodules detected on thoracic contrast-enhanced CT in the pediatric population: Prevalence and outcomes. AJR Am J Roentgenol 2015;205:W360–5. [DOI] [PubMed] [Google Scholar]
- [20].Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 2012;56:333–9. [DOI] [PubMed] [Google Scholar]
- [21].Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28–35. [DOI] [PubMed] [Google Scholar]
- [22].Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 2014;371:1765–7. [DOI] [PubMed] [Google Scholar]
- [23].Hobbs HA, Bahl M, Nelson RC, et al. Journal Club: incidental thyroid nodules detected at imaging: can diagnostic workup be reduced by use of the Society of Radiologists in ultrasound recommendations and the three-tiered system? AJR Am J Roentgenol 2014;202:18–24. [DOI] [PubMed] [Google Scholar]
- [24].Rago T, Fiore E, Scutari M, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol 2010;162:763–70. [DOI] [PubMed] [Google Scholar]
- [25].Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Coquia SF, Chu LC, Hamper UM. The role of sonography in thyroid cancer. Radiol Clin North Am 2014;52:1283–94. [DOI] [PubMed] [Google Scholar]


