Abstract
T cell activation is a complex process that requires multiple cell signaling pathways, including a primary recognition signal and additional costimulatory signals. TCR signaling in the absence of costimulatory signals can lead to an abortive attempt at activation and subsequent anergy. One of the best-characterized costimulatory pathways includes the Ig superfamily members CD28 and CTLA-4 and their ligands CD80 and CD86. The development of the fusion protein CTLA-4–Ig as an experimental and subsequent therapeutic tool is one of the major success stories in modern immunology. Abatacept and belatacept are clinically approved agents for the treatment of rheumatoid arthritis and renal transplantation, respectively. Future interventions may include selective CD28 blockade to block the costimulatory potential of CD28 while exploiting the coinhibitory effects of CTLA-4.
The concept of T cell costimulation is one of the most important advances to emerge in the field of immunology in the past few decades. Unlike the discovery and characterization of Ig as the putative BCR, elucidation of the TCR occurred relatively recently. It was not until the early 1980s that the TCR was identified as the protein that provided specificity to T cell responses (1, 2). Although characterization of the TCR helped to elucidate the mechanisms of T cell specificity, it was also apparent that Ag recognition alone was insufficient for successful T cell activation. Earlier studies by Lafferty and Woolnough (3) suggested that additional costimulatory signals are required to orchestrate an effective immune response. In fact, Jenkins and Schwartz demonstrated that Ag recognition (signal 1) in the absence of costimulation, or signal 2, leads to the induction of an active process of non-responsiveness, termed anergy (4). This characteristic of the immune system is now thought to be one of the major mechanisms in tolerance to self-antigens in the periphery, crucial in the prevention of autoimmunity. Although these concepts were evolving in generalities, there were no specific molecules yet identified. Subsequent work was fueled by an intense interest to better define which pathways were essential for T cell–dependent immune responses. In contrast to what is now a common practice in pharmaceutical research, early investigations surrounding costimulatory pathways began as a desire to better define the molecular mechanisms involved in T cell activation rather than an aspiration to develop a predefined biologic product. In the end, it was the expectation that a heightened understanding of basic immunology might subsequently drive the emergence of a tool with therapeutic usefulness.
Identifying costimulatory molecules
In the early 1980s there was an active effort to identify and characterize immune cell surface molecules using mAbs. By the end of the decade, dozens of lymphocyte cell surface molecules had been identified but, for the most part, little was known about their function. The search began to determine whether any of these newly discovered proteins functioned as costimulatory factors. A common technique to probe the functions of the newly identified cell surface molecules was to evaluate the impact of specific mAbs in cellular assays. Experiments of this sort revealed that anti-CD28mAbs had a profound impact on T cell proliferation (5). Intact Abs generally stimulated proliferation, whereas Fab fragments were inhibitory. Further characterization of CD28 revealed that its extracellular domain was homologous to the Ig V region domains and identified it as an Ig superfamily member. Given its structural similarity and the results with anti-CD28 Ab treatment, the supposition was that CD28 on T cells served as a receptor to a yet-uncharacterized ligand or ligands. Indeed, it was subsequently shown that CD28 is constitutively expressed on all naive T cells in mice, as well as almost all CD4+ T cells and the majority of CD8+ T cells in humans. Additional studies demonstrated that the percentage of CD28− CD4+ and CD8+ T cells increases with aging and with states of chronic inflammation or infection, suggesting that, unlike naive T cells, memory/Ag-experienced T cells down-regulate CD28 on their cell surface, reflective of a diminished reliance on costimulatory signals (6, 7).
Subsequent studies set out to identify the natural ligand(s) for CD28 through a series of interesting experiments. Aruffo and Seed (8) cloned CD28 and overexpressed it in a transfected cell line as a tool to decipher which cell types and, ultimately which cell surface protein(s), served as ligand(s) to CD28. Further studies narrowed the search to those cells capable of Ag presentation and identified the cell surface protein B7/BB-1 or B7-1 (named after the Ab clones that were used to identify it but later renamed as CD80) as the purported ligand of CD28 (9, 10) (Fig. 1A). Immediate efforts were made to evaluate the biologic effect of anti–B7-1 Abs (11). Intriguingly, in several systems Ab therapy directed at CD80 alone was ineffective, prompting further studies that eventually identified a second ligand, B7-2 (CD86), as an alternative receptor to CD28 (12, 13). Interestingly, unlike CD80, the expression of which is inducible upon activation of APCs (dendritic cells, activated B cells, monocytes/macrophages) and some T cells following exposure to inflammatory signals (e.g., IFN-γ, TNF, pathogen- or danger- associated molecular patterns, alarmins), CD86 is constitutively expressed on resting cells, including interdigitating and peripheral blood dendritic cells/monocytes, Langerhans cells, and memory/germinal center B cells. CD86 expression is further upregulated in the context of inflammation. Thus, CD86 seemed likely to play a more critical role in providing early and sustained costimulatory signals, whereas CD80-derived signals modulate the fate of the T cell response rather than its initiation. Finally, a pairing of cell surface molecules was identified, providing legitimacy to the theory of costimulation, which had been described almost a decade earlier. It was the start of an expanding area of research that continues today.
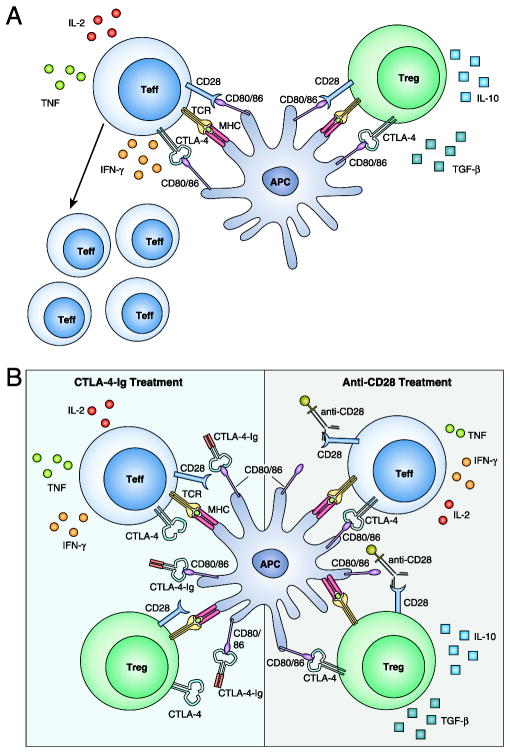
FIGURE 1.
Impact of CTLA-4 Ig versus anti-CD28 domain Abs on alloreactive T cell responses. (A) Alloreactive T cells become activated and differentiate into cytokine-secreting effector T cells (Teffs) upon TCR recognition of alloantigen. Tregs can dampen this response by CTLA-4–mediated competition for CD28 ligands (CD80 and CD86) and by secretion of antiinflammatory cytokines IL-10 and TGF-β. (B) CTLA-4–Ig inhibits Teff function but also impairs Treg survival and suppressive capacity, whereas selective CD28 blockade better inhibits Teff accumulation and function and better maintains Treg suppressor function by preserving CTLA-4–mediated coinhibitory signals.
CTLA-4 and therapeutic potential
With the pathway identified, it was only a matter of time before molecules with therapeutic potential were developed and tested in various experimental models of disease. The technology for generating Ig-fusion proteins using recombinant engineering techniques had been recently perfected, providing a method to readily generate therapeutics with prolonged half-lives. Peach and colleagues (14) developed a soluble CD28 fusion protein, CD28-Ig, presuming that it would block the costimulatory interactions between CD28 and its ligands, CD80 and CD86. Although studies with it and B7-Ig molecules confirmed the costimulatory properties of this pathway, further studies described a relatively weak affinity of CD28 for its ligands, suggesting that an unreasonable concentration of fusion protein would be required in vivo to achieve biologic activity. Fortuitously in the midst of this work, Linsley and colleagues made a serendipitous discovery.
Several years earlier, another Ig superfamily member had been cloned as a part of a series of experiments designed to describe the cell surface molecules upregulated during T cell activation. CTL Ag 4 (CTLA-4), also designated CD152, was shown to resemble CD28, specifically a shared series of cysteine residues in the extracellular domain (15–17). Further investigations into the function of CTLA-4 were stymied, primarily as a result of the lack of reagents to probe its function. Following the same approach used with CD28, Linsley’s group generated a fusion protein, CTLA-4–Ig, and tested its ability to bind the B7 molecules. To their surprise, CTLA-4-Ig not only bound to the B7 proteins, but it did so much more effectively than CD28 (18). Given the higher affinity of CTLA-4 for the B7 molecules, >20-fold higher than CD28-Ig, it was recognized that CTLA-4–Ig could be an effective therapeutic to block CD28–B7 molecular interactions and, thus, inhibit T cell costimulation and resultant activation and effector function (Fig. 1B, left panel). Although the function of CTLA-4 itself was not clearly defined at that time, future studies went on to define a coinhibitory role (19).
It soon became apparent that CTLA-4 was a negative regulator of CD28-mediated T cell costimulation (20). Ligation of constitutively expressed CD28 by the B7 molecules results in a positive costimulatory signal leading to activation, cytokine production, proliferation, and effector function (Fig. 1A). CTLA-4, in contrast, is upregulated on activated CD4+ and CD8+ T cells and, as a result of its higher affinity for the B7 molecules, outcompetes CD28 for CD80 and CD86 ligation. This results in an effective local blockade of positive signaling via CD28, and CTLA-4 itself also delivers a negative signal, inhibiting TCR- and CD28-mediated signal transduction and tempering the overall immune response. Internalization of cell surface CTLA-4 is rapid, with >80% of surface CTLA-4 being internalized within 5 min (21). Interestingly, more recent data support a role for trans-endocytosis of CD80 and CD86 from APCs as a means to effectively remove the ligands for CD28 and reduce costimulatory capacity (22). Thus, CTLA-4 seems to function in both a cell-intrinsic and a cell-extrinsic manner to regulate T cell responses (23).
In contrast to conventional T cells, CD4+CD25+FOXP3+ regulatory T cells (Tregs) constitutively express CTLA-4, and the activation of these cells results in further upregulation of CTLA-4 (24, 25). It is now clear that the presence of CTLA-4 and Tregs is critical for control of normal immune responses, because mice genetically engineered to lack CTLA-4 develop lymphoproliferative disorders and die early of autoimmune disease (26, 27). Additional studies associated various polymorphisms of the CTLA-4 gene in humans with several common autoimmune conditions (28–30). In addition to the critical role of CTLA-4 in modulating Treg suppressive function, CD28 plays an essential role in the thymic development and peripheral homeostasis of Foxp3+ Tregs. Tang et al. (31) showed that CD28 signaling is required for the maintenance of a stable pool of Tregs in the periphery by enhancing their survival and promoting their self-renewal in an IL-2–dependent fashion. In particular, CD28 engagement functions to increase IL-2 production by conventional T cells, as well as the expression of CD25 on Tregs. Work from Turka and colleagues (32) clarified the cell-intrinsic role of CD28 in Treg function by demonstrating that Treg-specific CD28 conditional-knockout animals possessed a highly nonfunctional Treg compartment and developed severe autoimmunity. These mechanistic findings are corroborated by data showing that CTLA-4–Ig treatment of mice and humans results in a decrease in peripheral Treg frequencies (33, 34). The impact of reduced Treg number and functionality on the efficacy of CD28 costimulation blockers is an area of active investigation.
Early investigations had already shown that interactions between CD28 and B7-1 provided costimulation that drove allogeneic proliferation and cytotoxicity of human and murine lymphocytes in vitro (35–38). In the same issue of Science, Linsley et al. (39) and Bluestone and colleagues (40) were the first to report the impact of CTLA-4–Ig treatment in vivo. Not only did CTLA-4–Ig effectively attenuate T-cell–dependent Ab responses, it inhibited rejection of transplanted xenogeneic islets and seemed to promote long-term donor-specific tolerance. Subsequent studies tempered the claim that CTLA-4–Ig can induce lasting tolerance, because results have been quite mixed (41–43). However, it was shown quite clearly that administration of CTLA-4–Ig can be an effective tool to modulate CD28 costimulation, resulting in prolonged allograft survival in a myriad of transplant models using various organ/tissue types.
Additional studies sought to observe the impact of CTLA-4–Ig on various autoimmune conditions. In a mouse model of lupus, CTLA-4–Ig was able to halt disease progression and reduce the severity of established disease (44). Treatment completely suppressed the development of anti-dsDNA Abs and prolonged survival, even when therapy was delayed until the most advanced stage of clinical disease. In contrast to the results seen in the lupus model, the results achieved with CTLA-4–Ig in animal models of multiple sclerosis have varied. In a mouse model of experimental allergic encephalitis, a dose of CTLA-4–Ig repressed the first occurrence of disease but not subsequent relapses (45). Unexpectedly, multiple treatments with CTLA-4–Ig prior to disease onset resulted in worsening of disease compared with control animals. Additional experiments revealed that blocking CD80 resulted in protection from disease, whereas selective blockade of CD86 resulted in disease exacerbation. This called into question the impact of targeting not only CD28 costimulatory activity, but also the coinhibitory effects of CTLA-4, suggesting that certain disease processes were differentially dependent on CD28 and CTLA-4 signaling and perhaps not all T cell subsets were equally reliant on CD28 signaling, a point that we address later in this review.
Further studies in a rat model of collagen-induced arthritis demonstrated that prophylactic treatment (CTLA-4–Ig treatment prior to immunization with bovine type II collagen) effectively prevented the onset of disease (46). Joints from treated animals showed no abnormalities in contrast to control animals in which there was complete erosion of the articular cartilage and bone. Subsequent studies confirmed that blockade of both B7 molecules was required for the clinical effect because treatment with anti-CD80 or anti-CD86 alone failed to prevent disease (47). The promise shown in these experimental models spurred the clinical development of CTLA-4–Ig in human trials of autoimmunity and, eventually, transplant.
Clinical trials in autoimmunity and transplant
Approximately 5%of the population of the United States has an autoimmune disease. The majority of these diseases (psoriasis, rheumatoid arthritis [RA], multiple sclerosis, lupus) are chronic and, as such, require lifelong medication after diagnosis; thus, the potential market for disease-modifying medications is significant. By far, the most significant effort to evaluate the clinical efficacy of CTLA-4–Ig has been in patients with RA. RA is an immune-mediated disease that is characterized by pain, swelling, and destruction of joints, with resultant disability. The disease is driven, in part, by the development of autoantigen-specific T cells that mediate pathologic effects. Within the inflamed joint there are lymphoid aggregates with clonally expanded T cells expressing phenotypic markers of activation, including CD28 and CTLA-4; thus, it seemed reasonable to examine the impact of CTLA-4–Ig in these patients (48). The clinical benefits of costimulation modulation in RA were confirmed through an extensive clinical trial program, with >10 y of follow-up. CTLA-4–Ig was assessed in several double-blinded, randomized, placebo-controlled phase II and phase III trials. There were statistically significant and clinically meaningful improvements in signs and symptoms of disease, physical function, and health-related quality of life in those patients treated with CTLA-4–Ig compared with placebo (49, 50). Subsequent trials revealed that CTLA-4–Ig provided additional benefit in patients whose disease was not adequately controlled with other treatments, such as methotrexate or anti-TNF therapies (51–53). As a result of the benefit documented in these trials, CTLA-4–Ig (abatacept [ORENCIA]) was approved by the U.S. Food and Drug Administration in December 2005 for use in patients with RA, becoming the first selective costimulation modulator to be introduced into clinical practice.
CTLA-4–Ig was tested in several other autoimmune conditions with mixed results. The clinical efficacy of CTLA-4–Ig in autoimmunity was actually first assessed in patients with the inflammatory skin disorder psoriasis vulgaris (54). In a small phase I dose-escalation study, nearly half of treated patients achieved a >50% sustained improvement in clinical disease activity, with greater response rates in patients receiving the highest doses. The study documented a dose-dependent reduction in the number of infiltrating T cells when comparing pre- and posttreatment skin biopsies, as well as normalization of keratinocyte proliferation and maturation, and reduced expression of CD80, CD86, CD40, MHC class II, and CD83 on lesional dendritic cells (55). Treatment with CTLA-4–Ig was also assessed in patients with multiple sclerosis in a phase I single-dose trial with limited follow-up. Although the treatment appeared safe, and patients who received CTLA-4–Ig showed a reduction in myelin basic protein–specific T cell responses, there was no significant change in clinical parameters (magnetic resonance imaging or neurologic symptoms) (56). Abatacept was also found to slow, but not eliminate, progression of disease in patients with the recent onset of type I diabetes mellitus (57). Additional trials were conducted in patients with asthma, lupus, and ulcerative colitis, but none of these revealed a significant improvement in disease burden when patients were treated with CTLA-4–Ig (58–60). Given these results, clinical development in autoimmunity has been largely confined to patients with RA. In addition to the significant interest in autoimmunity, there continued to be a drive toward evaluating CTLA-4–Ig treatment in the field of transplantation.
T cells play a key role in the rejection of transplanted organs; thus, it seemed like an ideal indication to test the efficacy of costimulation blockade. Numerous studies in murine models had shown significant promise (40, 41, 61). Surprisingly, when preclinical studies were performed in nonhuman primates to assess the effects of CTLA-4–Ig on transplant rejection, the results were quite disappointing (62, 63). These data suggested that more effective blockade of CD28 signaling might be required in the more rigorous nonhuman primate model. Given that the affinity of CTLA-4–Ig is ~100-fold higher for CD80 compared with CD86, Peach et al. (14) reasoned that a molecule engineered with a higher affinity to CD86 might be more efficacious. Subsequent efforts resulted in the development of a mutant version of CTLA-4–Ig, designated LEA29Y for the two amino acid substitutions that significantly increased the binding avidity to CD80 and CD86. This increase in binding avidity resulted in increased in vitro activity and improved efficacy in vivo, because LEA29Y treatment significantly prolonged allograft survival compared with CTLA-4–Ig (62, 64). In addition, when LEA29Y was combined with conventional immunosuppressants in a multidrug regimen, there was significant synergy resulting in enhanced protection from rejection. These studies formed the basis for a clinical trial in renal transplant recipients. The improved form of CTLA-4–Ig, now termed belatacept (NULOJIX), was evaluated in a phase II clinical trial involving ~200 patients that compared belatacept with the calcineurin inhibitor cyclosporine. The results were encouraging; belatacept was equally effective at preventing rejection and demonstrated substantially improved renal function, as well as reduced chronic allograft nephropathy, at 1 y, suggesting likely improvement in long-term outcomes (65). A subsequent phase III trial, the Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial (BENEFIT), confirmed the improvements in renal function and, in additional follow-up studies, documented reduced metabolic and cardiovascular toxicities (66, 67). The most recent 7-y follow-up data from this trial indicate that those patients treated with belatacept enjoy a survival advantage, as well as a 43% risk reduction for the combined end point of death and graft loss (68). Intriguingly, despite improved renal function, the groups treated with belatacept had higher rates and grades of rejection compared with patients treated with calcineurin inhibitor–based immunosuppression. In addition, there was a significantly higher incidence of posttransplant lymphoproliferative disorder in EBV-seronegative recipients, prompting a warning to avoid its use in these individuals (69). Based on the earlier results, belatacept was given U.S. Food and Drug Administration approval in June 2011 for use in kidney transplant recipients and became the first new immunosuppressant approved for use in transplantation in over a decade.
Conclusions
We now know that T cell activation is a much more complex and dynamic event than first described, with signal strength, cytokine microenvironment, and various costimulatory and coinhibitory molecules contributing to the ultimate path of T cell activation and differentiation. The CD28/CTLA-4 pathway was proved to be one of the most critical events in this process. The discovery and description of T cell costimulation and the subsequent work to develop fusion proteins that specifically block these signals represents one of the most important advances in modern immunology. Although we have reviewed the beneficial effects of abatacept and belatacept in autoimmunity and transplant, there remain a significant number of questions relating to their mechanisms of action. It is now apparent that certain T cell subsets, such as CD8+ memory T cells or Th17 cells, may downregulate CD28 and/or are less dependent on signaling through this pathway and, therefore, are less susceptible to treatment with CTLA-4–Ig (70, 71). These Ag-experienced or memory T cells and specific T cell subsets (e.g., Th17) likely play an important role in the breakthrough rejection responses seen in the belatacept-treated kidney transplant patients or in the patients with autoimmune diseases in whom CTLA-4–Ig treatment is ineffective. Other disease processes are highly dependent on the coinhibitory signals transmitted by CTLA-4 and, thus, treatment with CTLA-4–Ig may actually exacerbate disease. This may be due, in part, to Tregs, which have the ability to suppress auto- and alloimmune responses and may be dependent on cell surface expression of CTLA-4 for their mechanism of action, suggesting that treatment with CTLA-4–Ig or belatacept may inhibit Treg function. Indeed, coinhibitory signals were shown to be critical in antitumor T cell responses where blocking Abs directed against coinhibitory receptors, such as CTLA-4, demonstrated extreme promise in patients with advanced stage melanoma and lung cancer; however, a significant number of these patients, not unexpectedly, developed autoimmune-like syndromes while receiving anti–CTLA-4 Ab therapy, highlighting the importance of this molecule in normal immune homeostasis (72, 73).
Given the importance of intact CTLA-4 signaling in the control of normal immune responses, more recent efforts have directed the focus toward CD28-specific therapy. Conceptually selective CD28 blockade would interrupt the costimulatory signals transmitted through CD28 while preserving the inhibitory impact of intact CTLA-4 ligation (Fig. 1B). More than a decade ago, the CD28-specific mAb TGN1412 was evaluated for safety in a phase I trial (74). Unfortunately, there were disastrous results; all six healthy trial participants developed life-threatening complications from massive T cell activation and cytokine storm (75). Despite reassuring preclinical data, the intact Ab cross-linked CD28 molecules and led to nonspecific, polyclonal T cell activation. Results from this trial likely tempered any additional efforts to develop CD28-directed therapies at that time. More recently, there has been a renewed interest in developing CD28-specific therapies. Two groups, Effimune and Bristol-Myers Squibb, are moving forward with clinical trials of Abs engineered to selectively target CD28 in patients with autoimmune conditions. Both reagents are single-chain anti-CD28–specific molecules: a pegylated humanized Fab′ fragment in the case of Effimune’s FR104 or Bristol-Myers Squibb’s pegylated anti-CD28 domain Ab lulizumab (Fig. 1). Both compounds seem to be devoid of the activating properties seen with TGN1412 and are highly effective in preclinical models of transplantation and autoimmunity, presumably because they preserve coinhibitory signals transmitted through CTLA-4 (76–79). Importantly, selective CD28 blockade better preserves Treg functionality and better inhibits pathogenic memory T cell responses in transplantation relative to CTLA-4–Ig (76, 80– 82). Although there have been other promising compounds that target various costimulatory pathways (e.g., anti-CD154, anti-CD40, anti–LFA-1, LFA-3–Ig, anti-OX40L), none are used in patients. For now, the CD28 pathway remains the only costimulatory pathway for which there are approved immunomodulatory medications to treat patients with autoimmune conditions or to prevent rejection in those undergoing kidney transplant. The development of T cell costimulatory blockade is a prime example of researchers Translating Immunology into clinical results.
Acknowledgments
This work was supported by National Institutes of Health Grants R37 AI040519 (to C.P.L.), U19 AI051731 (to C.P.L. and A.B.A.), and R01 AI104699 and R01 AI073707 (to M.L.F.).
Abbreviations used in this article
- CTLA-4
CTL Ag 4
- RA
rheumatoid arthritis
- Teff
effector T cell
- Treg
regulatory T cell
Footnotes
Disclosures
A.B.A. and M.L.F. have received research funding from Bristol-Myers Squibb. C.P.L. has no financial conflicts of interest.
References
- 1.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty KJ, Woolnough J. The origin and mechanism of the allograft reaction. Immunol Rev. 1977;35:231–262. doi: 10.1111/j.1600-065x.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 6.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10:14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143:2714–2722. [PubMed] [Google Scholar]
- 11.Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA. 1991;88:6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 13.Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsten T, Lee KP, Harris ES, Petryniak B, Craighead N, Reynolds PJ, Lombard DB, Freeman GJ, Nadler LM, Gray GS, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 16.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 17.Harper K, Balzano C, Rouvier E, Mattéi MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 18.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 20.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iida T, Ohno H, Nakaseko C, Sakuma M, Takeda-Ezaki M, Arase H, Kominami E, Fujisawa T, Saito T. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165:5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, Abbas AK, Sharpe AH, Sansom DM, Walker LS. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 25.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 26.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 27.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 28.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 29.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 30.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 31.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, Chandraker A. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 2012;12:846–855. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 35.Koulova L, Clark EA, Shu G, Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173:759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176:1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 40.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 41.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei RQ, Gibson ML, Zheng XG, Myrdal S, Gordon D, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 43.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 45.Racke MK, Scott DE, Quigley L, Gray GS, Abe R, June CH, Perrin PJ. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96:987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26:2320–2328. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 48.Verwilghen J, Lovis R, De Boer M, Linsley PS, Haines GK, Koch AE, Pope RM. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J Immunol. 1994;153:1378–1385. [PubMed] [Google Scholar]
- 49.Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I, Becker JC. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46:1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- 50.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 51.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 52.Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, Steinfeld S, Tindall E, Becker JC, Li T, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:2263–2271. doi: 10.1002/art.21201. [DOI] [PubMed] [Google Scholar]
- 53.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, Westhovens R. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 54.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, Lebwohl MG, Guzzo CA, Jegasothy BV, Linsley PS, Krueger JG. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–694. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viglietta V, Bourcier K, Buckle GJ, Healy B, Weiner HL, Hafler DA, Egorova S, Guttmann CR, Rusche JR, Khoury SJ. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71:917–924. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 57.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parulekar AD, Boomer JS, Patterson BM, Yin-Declue H, Deppong CM, Wilson BS, Jarjour NN, Castro M, Green JM. A randomized controlled trial to evaluate inhibition of T-cell costimulation in allergen-induced airway inflammation. Am J Respir Crit Care Med. 2013;187:494–501. doi: 10.1164/rccm.201207-1205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace DJ, Bae SC, Sigal L, Becker JC, Kelly S, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 60.Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143(1):62–69.e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 62.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 63.Levisetti MG, Padrid PA, Szot GL, Mittal N, Meehan SM, Wardrip CL, Gray GS, Bruce DS, Thistlethwaite JR, Jr, Bluestone JA. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–5191. [PubMed] [Google Scholar]
- 64.Adams AB, Shirasugi N, Durham MM, Strobert E, Anderson D, Rees P, Cowan S, Xu H, Blinder Y, Cheung M, et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002;51:265–270. doi: 10.2337/diabetes.51.2.265. [DOI] [PubMed] [Google Scholar]
- 65.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, et al. Belatacept Study Group. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 66.Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S, Gang S, Gaite LE, Moal MC, Mondragon-Ramirez GA, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13(11):2875–2883. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 67.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyó J, Neumayer HH, Lang P, Larsen CP, Mancilla-Urrea E, Pestana JM, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 68.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 69.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 70.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krummey SM, Floyd TL, Liu D, Wagener ME, Song M, Ford ML. Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade. J Immunol. 2014;192:2495–2504. doi: 10.4049/jimmunol.1301332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyi C, Carvajal RD, Wolchok JD, Postow MA. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer. 2014;2:35. doi: 10.1186/s40425-014-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 74.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 75.Hanke T. Lessons from TGN1412. Lancet. 2006;368:1569–1570. doi: 10.1016/S0140-6736(06)69651-7. author reply 1570. [DOI] [PubMed] [Google Scholar]
- 76.Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, Tillou X, Wu G, Reneaudin K, Hervouet J, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2010;2(17):17ra0. doi: 10.1126/scitranslmed.3000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suchard SJ, Davis PM, Kansal S, Stetsko DK, Brosius R, Tamura J, Schneeweis L, Bryson J, Salcedo T, Wang H, et al. A monovalent anti-human CD28 domain antibody antagonist: preclinical efficacy and safety. J Immunol. 2013;191:4599–4610. doi: 10.4049/jimmunol.1300470. [DOI] [PubMed] [Google Scholar]
- 78.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, Vanhove B. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab′ antibody. Am J Transplant. 2012;12(10):2630–2640. doi: 10.1111/j.1600-6143.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- 80.Ville S, Poirier N, Blancho G, Vanhove B. Co-stimulatory blockade of the CD28/CD80-86/CTLA-4 balance in transplantation: impact on memory T cells? Front Immunol. 2015;6:411. doi: 10.3389/fimmu.2015.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poirier N, Chevalier M, Mary C, Hervouet J, Minault D, Baker P, Ville S, Le Bas-Bernardet S, Dilek N, Belarif L, et al. Selective CD28 antagonist blunts memory immune responses and promotes long-term control of skin inflammation in nonhuman primates. J Immunol. 2016;196:274–283. doi: 10.4049/jimmunol.1501810. [DOI] [PubMed] [Google Scholar]
- 82.Ville S, Poirier N, Branchereau J, Charpy V, Pengam S, Nerriere-Daguin V, Le Bas-Bernardet S, Coulon F, Mary C, Chenouard A, et al. Anti-CD28 antibody and belatacept exert differential effects on mechanisms of renal allograft rejection. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015070774. [DOI] [PMC free article] [PubMed] [Google Scholar]