Abstract
The posterior face of the cornea consists of the corneal endothelium, a monolayer of cuboidal cells that secrete and attach to Descemet’s membrane, an exaggerated basement membrane. Dysfunction of the endothelium compromises the barrier and pump functions of this layer that maintain corneal deturgesence. A large number of corneal endothelial dystrophies feature irregularities in Descemet’s membrane, suggesting that cells create and respond to the biophysical signals offered by their underlying matrix. This review provides an overview of the bidirectional relationship between Descemet’s membrane and the corneal endothelium. Several experimental methods have characterized a richly topographic and compliant biophysical microenvironment presented by the posterior surface of Descemet’s membrane, as well as the ultrastructure and composition of the membrane as it builds during a lifetime. We highlight the signaling pathways involved in the mechanotransduction of biophysical cues that influence cell behavior. We present the specific example of Fuchs’ Corneal Endothelial Dystrophy as a condition in which a dysregulated Descemet’s membrane may influence the progression of disease. Finally, we discuss some disease models and regenerative strategies that may facilitate improved treatments for corneal dystrophies.
Keywords: Endothelium, Descemet’s Membrane, Mechanotransduction, Extracellular Matrix, Topography, Modulus, Fuchs’ Corneal Endothelial Dystrophy
The endothelium is the most posterior layer of the cornea and plays a critical role in maintaining corneal transparency, regulating deturgescence by providing both barrier and transport functions1. A monolayer of corneal endothelial cells (CEnCs) maintains a barrier through tight junctions and adherens junctions1–4. Osmotic pressure drives water from the anterior segment into the corneal tissues, and the endothelial layer maintains deturgescence through active fluid transport, energetically maintained by Na+/K+-ATPases5–7. Corneal endothelial cells also produce a specialized basement membrane that forms Descemet’s membrane (DM)8. Anterior to DM is the stroma, which constitutes the bulk of the cornea9. Bowman’s layer10, a specialized acellular extracellular matrix (ECM), separates the stroma from the anterior corneal epithelium in humans, but is absent in all domestic animals11. A stratified squamous nonkeratinized epithelium makes up the anterior face of the cornea, with basal columnar cells are anchored to the underlying anterior basement membrane11..
It is well-documented that biophysical cues, such as substratum topography and stiffness, intrinsic attributes of all extracellular matrices, profoundly modulate a host of fundamental cell behaviors12–20. Corneal cells interact with a rich variety of in vivo biophysical stimuli: the stroma and basement membranes present them with a range of stiffnesses and complex topographies21. Our laboratory and others have documented that the biophysical attributes of matrices represent ubiquitous and potent cellular stimuli that modulate morphology22–26, adhesion27, motility28,29, proliferation30, gene expression and regulation31, and cell differentiation18,20,32 in a wide array of cell types. These cues also impact how cells respond to soluble signaling molecules and therapeutic agents17,18,20. Insights gleaned from research on biophysical stimuli in the cornea inform potential therapies for conditions including corneal wounds33–35, as well as to tissue-engineered corneal constructs for transplantation36. In particular, topographical and mechanical stimuli have been introduced to CEnCs to increase proliferation26,30, maintain phenotype37, and produce cell sheets for transplantation38–40. Given the evidence supporting the role of mechanical signals in the function of CEnCs, it is surprising that the roles of these signals in homeostasis and pathogenesis have not been explored more thoroughly.
In this review, we present evidence that biophysical interactions must be considered when developing a complete model of the corneal endothelium in health and disease. We begin by describing experimental methods used to explore the biophysical microenvironment of corneal cells, particularly the stiffnesses of the tissues with which these cells interact. Next, we describe the ultrastructure of DM and the variations in the ECM at different stages of an organism’s lifespan. We then highlight the signaling pathways that are likely to be involved in transducing mechanical signals from the ECM to the nucleus, thereby influencing cell behavior. To illustrate the reciprocal relationship between ECM and CEnCs, we describe a proposed interaction between DM and endothelial cells, engaging biophysical stimuli in Fuchs’ Corneal Endothelial Dystrophy (FCED), a corneal endothelial disease marked by characteristic abnormalities of DM. We then highlight the existing literature on in vitro studies of ECM biomolecules produced by CEnCs. We conclude by discussing corneal endothelial regeneration, an active area of research in which a deeper understanding of mechanical cues could have a beneficial impact.
1. Methods for characterizing the biophysical properties of corneal tissues
To investigate biophysical cues and their impact, it is necessary to characterize the mechanical microenvironment that cells experience in vivo, and to represent these cues in vitro. Tissues are mechanically quantified by measuring the elastic or Young’s modulus41, a property that defines the sample’s stiffness or its ability to resist deformation under an applied stress41. The elastic moduli of many biological tissues including the cornea have been reported and, interestingly, the reported values for a single tissue type can span several orders of magnitude, largely depending on the method of sample preparation and/or measurement42 (Table 2). Tensile measurements tend to be of higher magnitude than indentation-based measurements, such as those acquired through atomic force microscopy (AFM) or mechanical interferometry imaging, as the former measures bulk deformations in the tissue (with contributions from the ECM, cells, fibrillar and network-like proteins, and constrained water42) whereas the latter methods measure localized deformations on small length scales42,43.
Table 2.
Elastic moduli of corneal layers as measured by atomic force microscopy (AFM) in humans and rabbits.
| Corneal layer | Elastic Modulus | |
|---|---|---|
| Humans | Rabbits | |
| Anterior Basement Membrane | 7.5 ± 4.2 kPa45 | 4.5 ± 1.2 kPa47 |
| Bowman’s Layer | 109.8 ± 13.2 kPa46 | Absent |
| Stroma | 33.1 ± 6.1 kPa 46 | 1.1 ± 0.6 kPa (anterior face) 0.38 ± 0.22 kPa (posterior face)47 |
| Descemet’s membrane | 50 ± 17.8 kPa45 1.8 ± 0.8 MPa (hydrated) and 4.8 ± 1.2 GPa (dehydrated)163 |
11.7 ± 7.4 kPa47 |
| Endothelium | 4.1 ± 1.7 kPa47 | |
In thin, heterogeneous tissue samples such as the endothelium or DM, AFM is ideal for measuring the micron-scale deformations that cells and their local ECM environments experience42,44. Our lab has extensively used AFM to characterize the stiffness of the distinct layers of the human and rabbit cornea45–47 (detailed in Table 1), as well as the normal human trabecular meshwork (4.0 ± 2.2 kPa48). The properties of the ECM can vary considerably between species47,49,50, and each layer in the rabbit eye is consistently softer than the corresponding structure in the human eye47. For further information, we direct the reader to these reviews on the nuances of stiffness measurements in ocular tissues42,44 and comprehensive summaries of biomechanical measurements of ocular tissue51–55.
Table 1.
A comparison of measurements of the stiffness of Descemet’s membrane in various species using different several analytical methods. In the mechanical interferometry imaging data set, the “short term” value refers to an instantaneous measurement whereas the “long term” values refers to a time dependent measurement including non-linear effects in response to constant stress over a 200 sec interval. Atomic Force Microscopy measurements were instantaneous. Measured values are highly dependent on analytical methods, making it necessary to consider the strengths and limitations of each method during experimental design and data interpretation.
| Species | Modulus | Measurement technique |
|---|---|---|
| Rat | 2·81 ± 0·51 MPa50 | Tensile testing |
| Cow | 6·14 ± 0·41 MPa50 | Tensile testing |
| Pig | 4·29 ± 0·35 MPa50 | Tensile testing |
| Human | 2·57 ± 0·37 MPa50 50 ± 17.8 kPa45 339.2 ± 22.2 kPa (short term) and 20.2 ± 0.71 kPa (long term)43 1.8 ± 0.8 MPa (hydrated) and 4.8 ± 1.2 GPa (dehydrated)163 |
Tensile testing Atomic Force Microscopy Mechanical Interferometry Imaging |
| Rabbit | 11.7 ± 7.4 kPa47 | Atomic Force Microscopy |
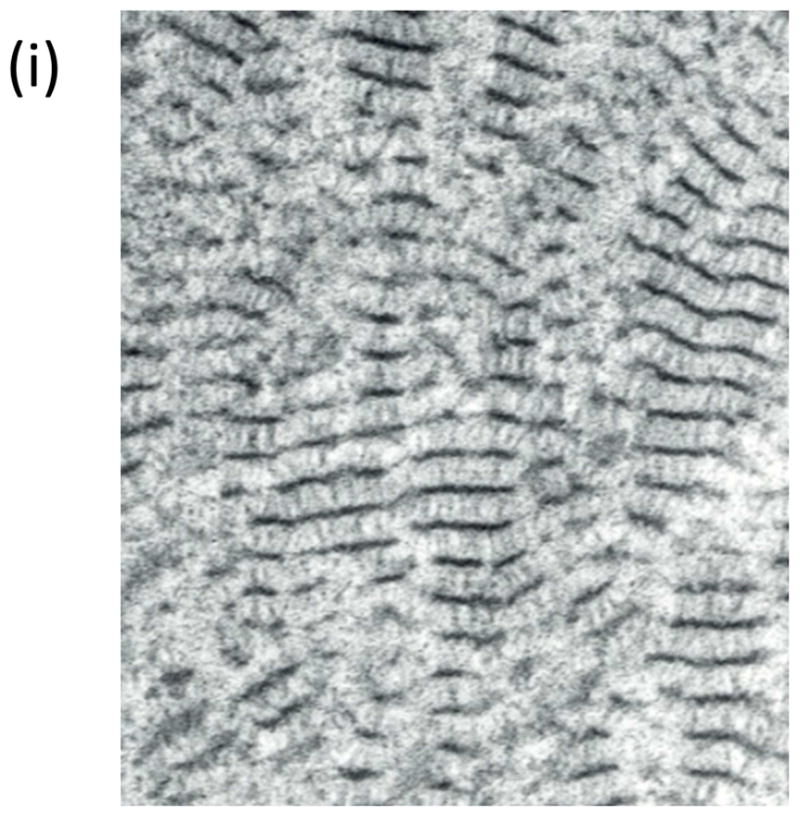
Topographical characterization of human21 and canine56 DMs using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and AFM has documented DM to possess a rich felt-like surface of intertwining fibers, interspersed with elevations and pores. These features, shown in Figure 156,57, have sizes on the order of tens of nanometers and fractal dimension of ~2.221. In comparison to the anterior corneal epithelial basement membrane, DM has smaller and more tightly organized features21,56,58,59. The more compact organization of DM is reflected in its increased stiffness relative to the anterior corneal epithelial basement membrane45,47. Cellular basement membrane assembly is influenced by cell-matrix interactions60 and the biophysical attributes of ECM represent important inputs.
Figure 1. The basements membranes of the canine cornea.
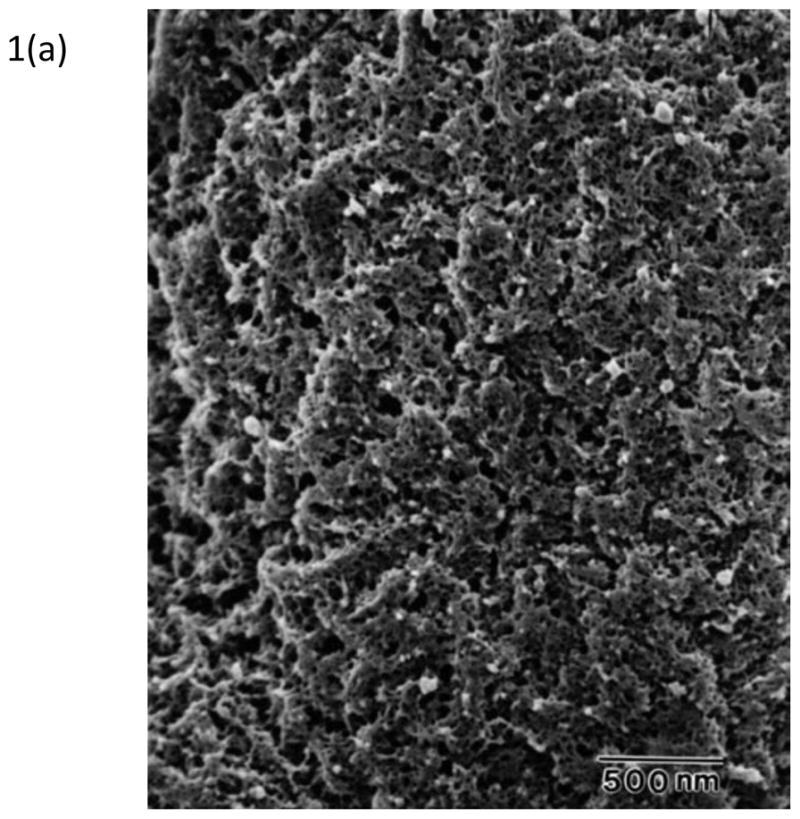
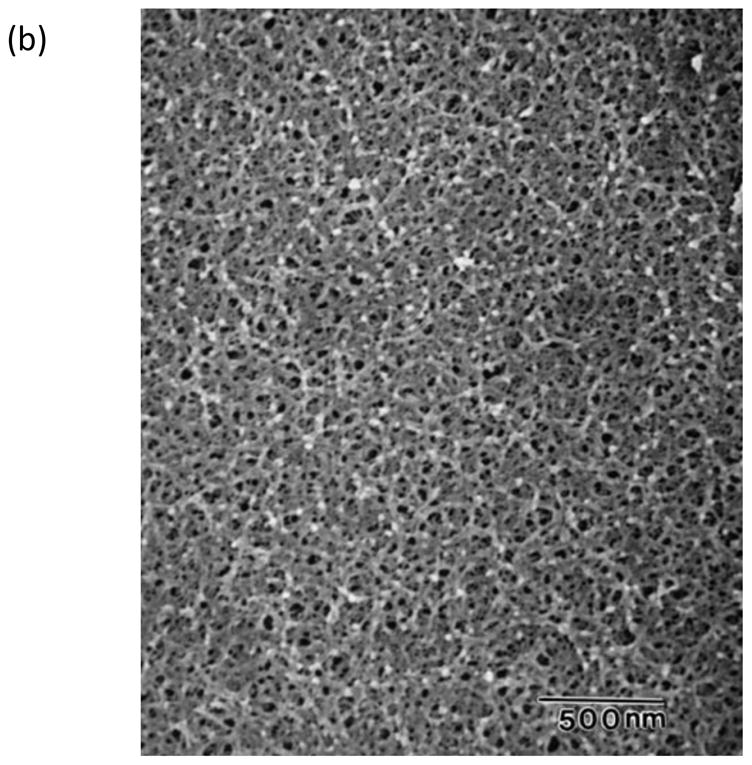
(a) Scanning electron micrograph of the canine corneal epithelial basement membrane. The basement membrane has an intricate surface topography consisting of a meshwork of fibers and pores. (Reprinted with permission from Bentley et al., 2001. ©Association for Research in Vision and Ophthalmology) (b) Scanning electron micrograph of Descemet’s membrane of the canine cornea. The complex topography of intertwined fibers and pores of varying sizes is similar to the topography found in the epithelial basement membrane. (Reprinted with permission from Abrams et al., 2002.)
2. The development and ultrastructure of Descemet’s Membrane
Like all basement membranes61, DM is a compact sheet of ECM biomolecules, including proteins and glycosaminoglycans. The composition and ultrastructure of the deposited components vary by developmental stage and pathological state62, forming distinct layers as they accumulate over a lifetime63. Since the mechanical properties of ECM are related to its quantity, its constituent biomolecules, and its degree of crosslinking (through enzymes such as lysyl oxidase and transglutaminases)64, we hypothesize that these variations would be reflected in differences in the biophysical cues experienced by endothelial cells on DM, as such influences have been observed in other cell types65. Since there is no significant remodeling of DM after deposition, the transverse view serves as an interpretable historical record of development and pathology63,66.
The thickness of DM ranges from ~3 μm at birth to >10 μm in old age63. The anterior region is deposited prenatally, from ~12 weeks after conception, to birth63. By 16 weeks after conception, a striated pattern appears in transverse sections63 and is known as the anterior banded layer (ABL), composed primarily of collagen IV and collagen VIII67,68. Ultrastructurally, in the ABL collagen VIII forms several layers of stacked hexagonal lattices62,68, shown in Figure 269–71. The lattice pattern arises from the molecular structure of collagen VIII as two types of short polypeptide chains, α1 and α2, assemble into homotrimers, then into a tetrahedral intermediate structure, and finally into hexagonal lattices72 which form characteristic bands (with 100 nm spacing) when stacked62,73. In normal human corneas, the two types of collagen VIII polypeptide chains are co-localized as bands within the ABL74. This characteristic banded pattern of the ABL is entirely absent in knock-out mutant mice that lack genes for both the α1 and α2 polypeptide chains75. These mice have thinner DMs and a lower endothelial cell count suggesting the ABL to be critical to endothelial cell proliferation during development75.
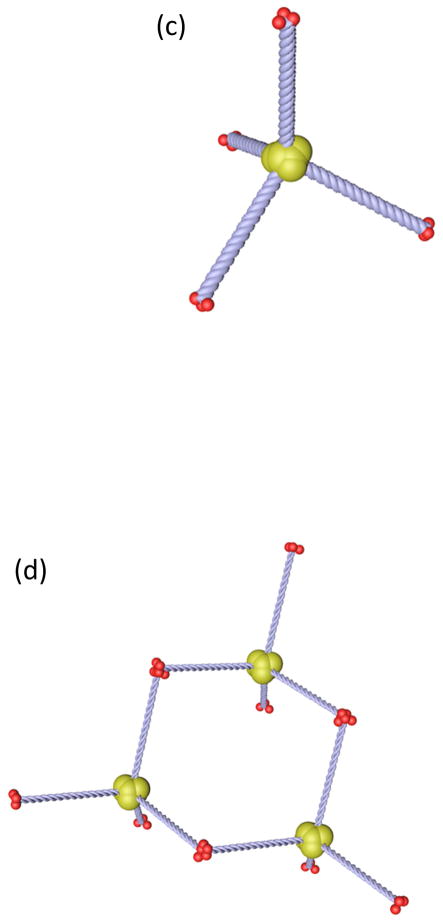
Figure 2. The assembly of Collagen VIII, an extracellular matrix protein critical in the assembly of Descemet’s membrane.
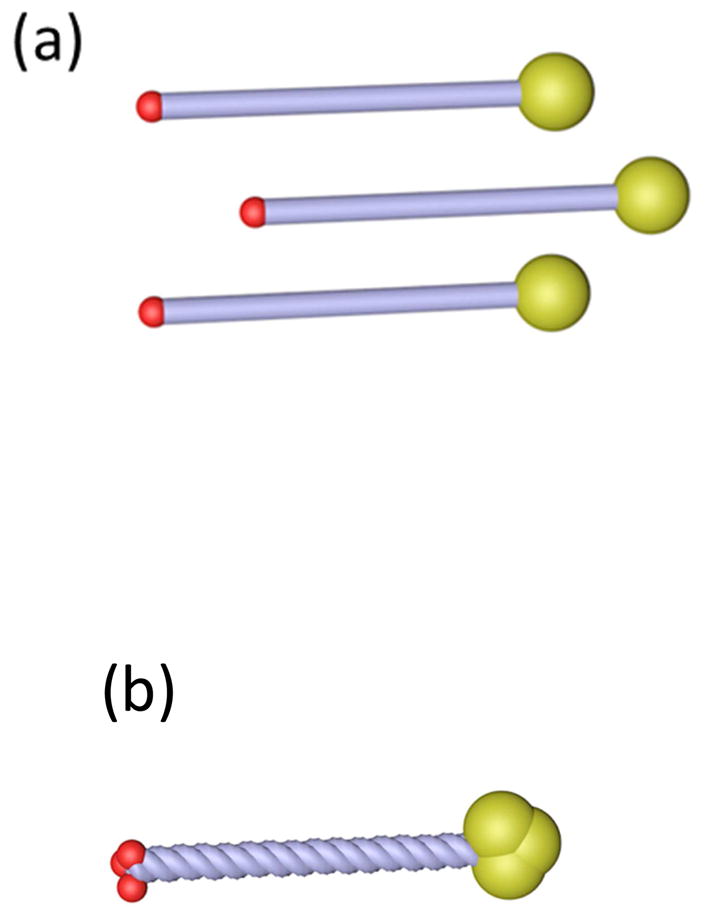
It comprises two types of polypeptide chains, α1(VIII) and α2(VIII) consisting of (a) two globular domains, the larger on the C-terminal side, connected by a rod-like triple-helical domain. (b) These polypeptides can form homotrimers. (c) Four of these homotrimers may associate through their C-terminal domains to form tetrahedral assemblies. These tetrahedra may assemble further via (d) N- to N-terminal interactions or (e) N- to C-terminal interactions. (f) Extended networks of Collagen VIII assemblies may form a hexagonal network that (g) stacks vertically, shown here based on N- to N-terminal interactions. This theorized assembly pattern of Collagen VIII corresponds to the ultrastructure of Collagen VIII, imaged through transmission electron microscopy in Descemet’s membrane. (h) In the en face section, Collagen VIII appears as a hexagonal network, whereas (i) in the transverse section it appears as parallel bands. The imaged ultrastructure supports the theorized assembly of Collagen VIII. ((h) reprinted with permission from Sawada 1982. (i) reprinted with permission from Klintworth, 2009, licensed under the Creative Commons Attribution 2.0 Generic License)
Near birth, endothelial cell secretion of collagen VIII diminishes as the cells transition from a proliferative to non-proliferative state, but collagen IV secretion continues8,75,76. The ECM deposited thereafter lacks the banded pattern and is known as the posterior non-banded layer (PNBL)62. The PNBL can be interspersed with small inclusions of fibrillar or banded ECM thought to arise from endothelial cells that transiently lose their postnatal differentiated state63. Such anomalous deposits are often found within guttae and Hassel-Henle warts that form in aging corneas63, suggesting that these abnormal structures form when endothelial cells undergo changes in ECM expression patterns over a lifetime. At this time, to the authors’ knowledge, possible differences in the biophysical attributes of the PBL and PNBL remain unexplored. We feel it is likely that both compliance and/or topographic features would differ between these distinct regions of DM and that if such differences are present that they would influence the CEnC phenotype.
3. Biophysical signaling in the corneal endothelium
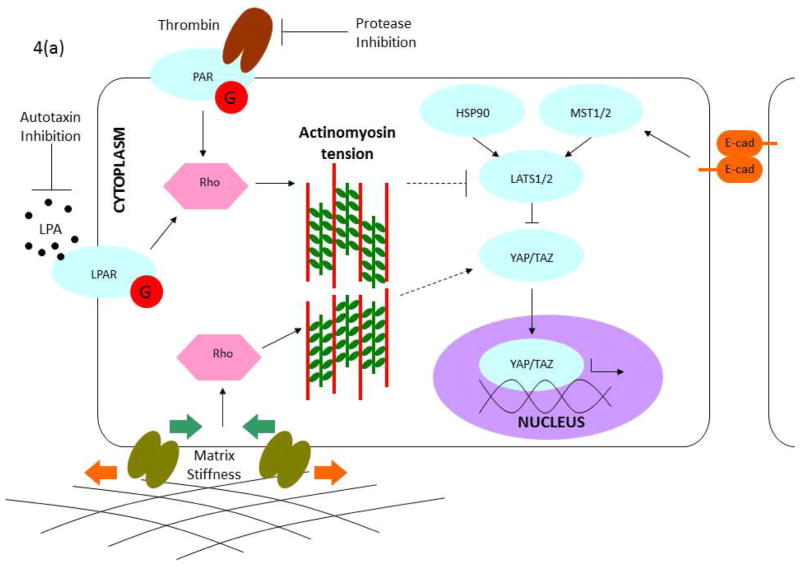
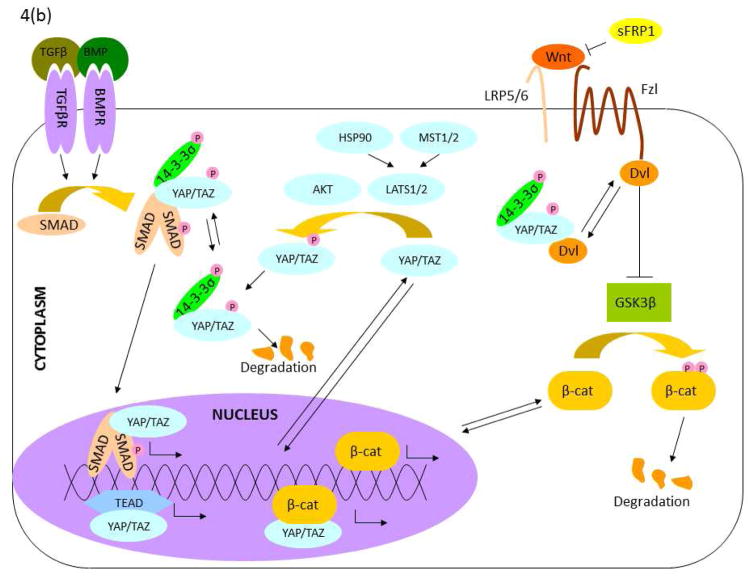
Since DM is the major source of biophysical stimuli for CEnCs, and since the cells themselves produce the ECM comprising DM, there is a bidirectional relationship between endothelial cells and the ECM they deposit66,77 (Figure 3). Such a relationship, known as “dynamic reciprocity”78–80, has been observed in many physiological systems, including the corneal stroma34. A detailed understanding of the signaling pathways that underlie these bidirectional relationships may reveal new targets for therapeutic intervention by interrupting the positive feedback loop of dysfunctional ECM – produced under pathological conditions – inducing further pathological cell behavior. The primary pathway impacted by the biophysical attributes of DM is the mechanotransduction pathway, which intersects downstream with the Hippo81, transforming growth factor β (TGF-β)82, Wingless/int (Wnt)83,84, and other signaling pathways85 that control gene expression. The intersection of critical signaling pathways is presented in Figure 486.
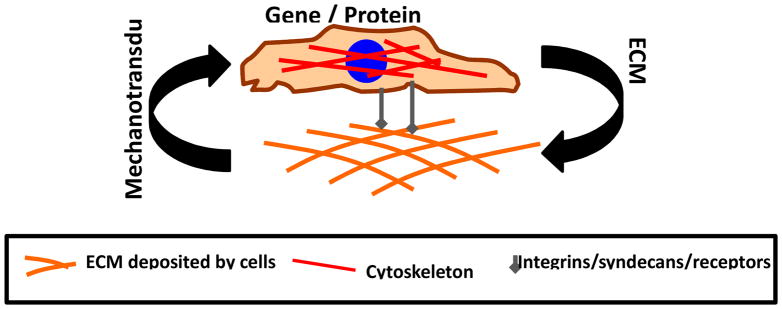
Figure 3. Dynamic Reciprocity.
Cells synthesize ECM proteins and deposit them into the extracellular space. The proteins assemble the matrix, presenting a rich set of topography and modulus cues to the cells. Integrins, syndecans, and other receptors mechanotransduce biophysical cues into the cells. Signaling molecules and the cytoskeleton convey these signals to the nucleus, where they influence cell behavior in many ways, including through changes in the expression of ECM genes and proteins.
Figure 4. Signaling pathways linked to mechanotransduction.
(a) Mechanical regulation of the Hippo pathway. Hippo is regulated by multiple signals generated by the physical (matrix stiffness) and biochemical (e.g. LPA, thrombin) environment. Importantly, many of these signals are modulated by tension in the actinomyosin cytoskeleton. Modulation of cytoskeletal mechanics through G-protein coupled receptors and matrix biophysics can likewise inhibit YAP/TAZ directly at the nuclear translocation stage or through activation of Hippo components. Note: This schematic is simplified to clarify the major components in the mechanical regulation of YAP/TAZ signaling. (b) Crosstalk between YAP/TAZ and TGF-β, and between YAP/TAZ and Wnt. TGF-β superfamily signaling is initiated by the binding of an extracellular ligand (e.g. TGF-β and BMP), which leads to the phosphorylation of SMADs and the formation of a complex with a Co-SMAD and 14-3-3s. After translocation to the nucleus, these complexes initiate the TGF-β/BMP transcriptional program. The SMAD complex also interacts with YAP/TAZ to initiate different transcriptional programs in the nucleus. Canonical Wnt is initiated by the binding of a Wnt ligand to the Fzd/LRP receptor complex. This induces the inhibitory behavior of Dvl on the Axin/APC/GSK3b complex, freeing β-catenin to translocate to the nucleus and initiate the Wnt transcriptional program. YAP/TAZ can inhibit Wnt signaling through inhibition of Dvl in the cytoplasm (TAZ) or in the nucleus (YAP) or cytoplasmic sequestration of β-catenin (YAP). Alternatively, YAP can encourage the transcriptional activity of β-catenin. Note: This schematic is simplified to clarify the intersections of YAP/TAZ and Wnt signaling. (Adapted with permission from Morgan, 2013)
Mechanotransduction
Mechanotransduction is the process by which cells sense extracellular mechanical cues, including ECM stiffness, topography, and spatial constraints, and convert them into intracellular biochemical signals leading to cellular responses14,15. Transmembrane mechanoreceptors such as integrins and syndecans bind to ECM molecules and detect stresses in the extracellular environment87. On the cytoplasmic side, they are linked to the actin cytoskeleton which is critical in generating and transmitting tension throughout the cell, controlling cell morphology and activating downstream signaling pathways88. The cell can probe the stiffness of its surroundings by contracting its actin cytoskeleton – a compliant substrate offers less resistance to deformation and induces less stress in the contractile machinery than a stiff one88. Mechanical stresses transmitted through mechanoreceptors to the cytoskeleton can alter the assembly dynamics of actin filaments, inducing conformational changes in actin-binding proteins that play regulatory roles further downstream89. The cytoskeleton also links to structures in the nucleus, coupling integrin activation directly to nuclear conformational changes and gene transcription activity90. It thus plays a central role in enabling extracellular biophysical cues to regulate a variety of cell behaviors, including survival, proliferation, differentiation, migration, adhesion, and polarity88,91–93. Integrins additionally regulate the actin cytoskeleton94 by activating the Rho/ROCK pathway through Src-family kinases and Rho-family GTPases95 and influence migration, proliferation, and apoptosis96. Rho-associated protein kinase (ROCK) has been linked to proliferation97–101, wound healing processes33,98,102–104 and the barrier function1,105–107 in CEnCs100.
YAP/TAZ and Hippo
The Hippo pathway, critical for management of organ size during development, has been recently shown to intersect with the mechanotransduction pathway. Two transcriptional regulators and key players in the Hippo pathway, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), were identified by Dupont and colleagues as a necessary relay for conveying mechanotransduction signals to the nucleus for gene regulation81,108. In cells adherent to soft substrates or confined to a small area, YAP/TAZ accumulate in the cytoplasm and eventually degrade, whereas when cells are spread or attached to stiff surfaces, YAP/TAZ relocate to the nucleus and induce gene expression81,109. The mechanisms behind these localization patterns are unclear, although the regulation of the actin cytoskeleton by the mechanotransduction pathway appears to play key roles81,108. For instance, G-protein coupled receptors acting through the actin cytoskeleton inhibit YAP localization in the nucleus by acting on LATS 1/2110. The specific roles of YAP/TAZ in the corneal endothelium are currently under investigation: YAP expression in the nucleus and cytoplasm increases in CEnCs at the periphery of the endothelium, coinciding with higher proliferative potential111. Additionally, cultured CEnCs have been induced to proliferate by increasing YAP expression112. Because YAP and TAZ are targets and effectors of numerous cell-signaling pathways, including Hippo, TGF-β, Akt, and both canonical and non-canonical Wnt pathways86,113,114, they can orchestrate the influence of mechanical cues on a wide range of cell behaviors, including epithelial-mesenchymal transition115.
TGF-β
Members of the TGF-β family are cytokines that are generally inactive in the ECM until released upon injury or mechanical stress, and then bind to membrane receptors116. This initiates downstream signaling, activating several types of Smad proteins that form a complex, translocate to the nucleus, and promote transcription.116 In CEnCs and other cell types, they induce the synthesis, remodeling, and degradation of ECM proteins117, and trigger the transdifferentiation activity known as epithelial-mesenchymal transition (EMT)118–120, which is a feature of FECD121–124. Changes in matrix protein expression are likely to alter the biophysical properties of the secreted ECM, invoking a mechanotransduction response within the cell. Additionally, the Smad protein complex interacts with YAP/TAZ in some cells to evoke an enhanced fibrotic or metastatic response116,125. Through these mechanisms, the TGF-β pathway may impact the dynamic reciprocity between CEnCs and the ECM.
AKT
The Akt pathway promotes survival and growth in response to extracellular signals, including cytokines, hormones, and ECM interactions. It is initiated when the cell surface receptor phosphatidylinositol 3-kinase (PI3K) is activated. Akt, a serine/threonine kinase, is then activated and initiates downstream processes126. In CEnCs, fibroblast growth factor 2 (FGF-2) initiates the Akt pathway and promotes proliferation127. Furthermore, Akt-induced proliferation in CEnCs is inhibited by TGF-β2 via the latter’s transcription products127. The Akt pathway also intersects with the downstream relays of the mechanotransduction pathway: Akt interacts with YAP/TAZ and localizes them in the cytoplasm while limiting their degradation128. Through these interactions, the Akt pathway has multiple impacts on dynamic reciprocity.
Wnt
The Wnt pathway plays key roles in development, homeostasis, disease, and mechanotransduction. In the canonical pathway, Wnt ligands bind to membrane receptors, initiating a downstream signaling cascade that stabilizes intracellular β-catenin, saving it from degradation and allowing it to regulate transcription in the nucleus116. If sufficient Wnt antagonists are present, the pathway is inhibited and cytoplasmic β-catenin is marked for degradation, limiting its transcriptional activity116. However, β-catenin can be rescued from degradation by association with YAP/TAZ. The Wnt pathway is thereby influenced by downstream relays of mechanotransduction. A few more interactions are worth noting: In the corneal endothelium, matrix metalloproteinase inhibitors can reverse EMT through inhibiting the Wnt pathway, thereby linking pathological cell behavior to ECM remodelling129. A non-canonical (β-catenin independent) Wnt pathway regulates the cytoskeleton via Rho GTPases130. Rho GTPases activated through PI3K are linked to increased EMT in CEnCs131. This EMT increase occurs when CEnCs are induced to proliferation through disruption of their junctions and the addition of FGF-2131. However, Wnt activation can be bypassed by silencing nuclear p150 catenin to activate the Rho pathway132. All these interactions provide points at which the Wnt pathway can have an influence on the dynamic reciprocity between the endothelium and DM.
The signaling pathways described above converge on two cellular responses in particular: proliferation and EMT-induced ECM expression. Changes in the ECM, in turn, influence the cell mechanically, creating a closed loop of influence in which abnormal matrix can trigger the expression of more abnormal matrix. Both cellular responses are relevant to the corneal endothelium in health and disease.
4. Dysfunction of the corneal endothelium
Primary corneal diseases are widespread and represent the third leading cause of blindness70,133. Diseases of the corneal endothelium are especially burdensome. While the CEnCs of some species, such as rabbits and cows, retain some proliferative capabilities throughout life134, human CEnCs are especially notable for their low proliferation rate, which is insufficient to replace cells lost through age, trauma, or disease135. The remaining cells migrate and enlarge to maintain the intact monolayer136 leading to subsequent cellular polymegathism and pleomorphism in the endothelium137. If the endothelial cells cannot maintain an intact monolayer, their barrier as well as pump functions are disrupted leading to stromal edema and a progressive decrease in visual acuity70. The endothelium can degenerate because of primary corneal dystrophies, or secondary to other disturbances to the eye such as glaucoma138, uveitis139, or intraocular surgery140. The likelihood of secondary changes such as bullous keratopathy are increased if the endothelium has pre-existing signs of vulnerability, and candidates for anterior segment surgery should be screened for low endothelial cell counts141–143. The health of CEnCs is closely linked to the state of DM to which they adhere. Many dystrophies are associated with the production of abnormal ECM or amyloid deposits that are likely to change the topography and modulus of the membrane144,145, suggesting that biophysical interactions with cells may have an impact on a wide range of corneal diseases. Here, we provide a brief introduction of the biophysical properties associated with corneal endothelial dystrophies.
4.1 Fuchs’ Corneal Endothelial Dystrophy
Fuchs’ Corneal Endothelial Dystrophy (FCED) is the most common corneal endothelial dystrophy in the United States70. Clinically, FCED manifests as a thickened and multi-layered DM, with the presence of corneal guttae, corneal edema and deterioration of vision74.
It occurs in two forms, a late-onset variant and a rarer early-onset variant146. In addition to their clinical presentation of thickened DM and gutta formation, the two forms of FCED involve Collagen VIII in distinct ways. Fuchs’ dystrophy has been linked to mutations in genes with a variety of functions, begging the question of how a diverse set of genes lead to the common phenotype of abnormal ECM production. Here, we discuss the alterations of ultrastructure and composition of DM in FCED, discuss the associated genetic anomalies that may alter the ECM composition and organization, and consider how these changes may impact intrinsic biophysical attributes, cell function and survival.
Ultrastructural studies of DM in FCED patients reveal a characteristic pattern. Descemet’s membrane of FCED patients’ corneas is thicker than that of age-matched normal corneas, although the PNBL is thinner. In late-onset FCED, the ABL is slightly thicker than normal. There is a gradual transition from the PNBL to a posterior collagenous layer (PCL) interspersed with banded patterns termed “widespaced collagens” similar to that of the ABL62,66,147. Some corneas, particularly those with pronounced edema, also have a loose “fibrillar layer” between the banded PCL and endothelial cells147. The arrangement of wide-spaced, amorphous, and fibrillar ECM is increasingly irregular and distorted toward the posterior of the cornea147. The guttae that appear on the posterior DM in FCED are also composed of ECM similar to the PCL147. In early-onset FCED, the ABL is notably thicker than normal74. It is followed by a PNBL similar to that in late-onset FCED, and an internal collagenous layer (ICL) reminiscent of the PCL in late-onset FCED74. Posterior to this is a very thick, collagen VIII-rich posterior striated layer, which does not occur in late-onset FCED74. Guttae are smaller than in late-onset FCED and buried within other deposited ECM material148.
Corneal endothelial cells have the latent ability to synthesize a larger set of ECM protein types than they normally produce, and diseases may arise when regulation of protein expression is dysfunctional149. In late-onset FCED, there is increased deposition of collagen IV, laminin, fibronectin, and several other ECM components in the posterior of DM74,150.
While Collagen VIII is normally found only in the ABL of DM, appearing as arrayed domains of the α1 and α2 chains74, this arrangement of Collagen VIII is disrupted in FCED. Collagen VIII are also expressed in the PCL, indicating that its production is resumed after cessation in infancy62. Interestingly, Collagen VIII plays a different but central role in early-onset FCED also. This condition is associated with mutations in COL8A2. A few familial pedigrees and sporadic individuals have been identified that carry missense mutations in COL8A2 (either Q455K, Q455V, or L450W148,151). In patients with the L450W mutation, intracellular and extracellular collagen VIII accumulation correlated with the severity of the disease152. Corneas of patients with early-onset FCED, as well as corneas in mouse models with COL8A2 knock-in mutations, have a thickened DM, guttae, changes in endothelial cell morphology, and cell loss, supporting the idea that mutations in Collagen VIII can produce signs of FCED independently of other mutations153,154.
It is as yet unclear what mechanisms trigger changes from one type of ECM composition to another, and why the various layers in normal and pathogenic DMs appear as they do. However, we can speculate on how these ECM alterations modify the biophysical environment experienced by cells. For instance, since point mutations can affect protein folding, it is likely that mutant COL8A2 in early-onset FCED leads to the expression of collagen VIII chains that do not assemble into the same macromolecular structures as normal chains do155. This could alter the biophysical properties of DM, thereby triggering expression changes within the cell.
In addition to the COL8A2 mutations in early-onset FCED, several genetic mutations have been associated with FCED, and these are comprehensively reviewed in the literature70,156. The affected genes TCF4 and TCF8 express the transcription factors E2-2 and ZEB1 respectively, which are known to promote EMT157,158. TCF8 also alters the expression of Collagen IV, which is abundant in DM159. Changes in these key functions of ZEB1 may contribute to abnormal ECM deposition. Several mutations, in COL8A2152, SLC4A11160, and LOXHD1161, cause cytoplasmic accumulation of proteins. These may induce gutta formation through a mechanism involving the accumulation of proteins in the endoplasmic reticulum, and subsequent unfolded protein response, that has been proposed by Son, et al162.
In FCED, the PCL is less stiff than the PNBL of the normal DM, and this may be due to alterations in the composition, assembly patterns, and hydration content of the matrix163,164. The composition of the ECM can greatly influence the modulus165. The heterogeneity of the PCL ultrastructure may interrupt the packing structure of ECM biomolecules, changing the PCL density and modulus relative to the normal PNBL166,167. As the DM is normally defined by the network structure of collagen IV, the presence of a fibrillar layer is also likely to change the surface attributes with which endothelial cells interact168. Wide-spaced collagens also alter the topography of the DM surface, as revealed by AFM imaging163.
As guttae are physical structures growing on DM, they exert particular biophysical cues on CECs. The guttae appear as round, flat-topped growths that are 5 to 50 μm in diameter163 and up to 20 μm in height169. The height of topographic features has been shown to impact cell behaviors though at scales significantly smaller than guttae170. A preliminary report suggests that the micron-scale dimensions of guttae can impact CEnC behaviors including migratory behavior and monolayer formation in vitro 152. In histopathological sections of FCED corneas, the cells exhibit a spread morphology over guttae with cell bodies displaced laterally to the guttae stems but with sections of cell membrane intact over gutta apices171. This displacement is sufficient to distort the cells and apply tensile forces to their actin cytoskeletons. Such mechanical stretch has been associated with EMT in other cell types172, and this distortion of CEnCs may induce or contribute in part to the EMT-like behavior seen in FCED. With limited space for organelles in the stretched areas and for Na+/K+-ATPase on the diminished lateral membranes, the pump function of the corneal endothelium may be quite diminished171. However the barrier function of the endothelium is retained as cells remain adherent to one another until late in disease progression171.
For a complete understanding of the role of biophysical cues in pathogenesis of FCED, we need to fully characterize the biophysical cues presented by DM in FCED, understand how the signaling pathways initiated by mechanotransduction determine the downstream impact on cell health, gutta formation and ECM protein expression. While there are several animal models for FCED, including transgenic mice153,154 and Boston terriers173, the biophysical attributes of DM and endothelium in these species remains uncharacterized. Given the challenges in observing the early development of FCED, in vitro cell models of the disease could help us fill these knowledge gaps.
4.2 Other endothelial disorders
Posterior Polymorphous Corneal Dystrophy is a rare, slowly progressive or non-progressive condition that develops during the first decade of life but is often asymptomatic.174 It is characterized by a multilamellar DM with vesicle- and band-like lesions and focal nodular excrescences70,175. Congenital hereditary endothelial corneal dystrophy presents as a cloudiness or opacification and edema of the cornea that is present at birth or develops in infancy70. Disruption of collagen fibrils occurs in the stroma as well as the production of a fibrous collagen layer posterior to DM70. Subepithelial amyloid deposits have been reported in several cases156,176–179. X-linked corneal endothelial dystrophy was recently discovered in 60 members of a single family180. The corneas developed opacifications including congenital cloudiness, subepithelial band keratopathy, and endothelial pits180. Examination of one cornea revealed an irregularly thickened DM, consisting of an abnormal ABL, an abnormal posterior banded zone of varying ultrastructure and thickness, and an absent PNBL180. Iridocorneal endothelial syndrome (ICE) is a group of related corneal disorders in which endothelial cells migrate onto the iris and block the drainage angle, leading to iris atrophy and glaucoma181. In a study of 27 ICE affected corneas, the majority possessed DMs with a posterior layer of microfibrils embedded in an amorphous matrix. In several others, there were no banded collagenous layers either anterior or posterior to the non-banded layer182. The commonality in features across several corneas suggests these patterns are specific to the mechanisms of ICE182.
Although the mechanical, topographical, and biochemical properties of DM in these diseases have not been well characterized, we note a recurring incidence of abnormal ultrastructure and the appearance of lesions, excrescences and craters in DM70,175,180,181,183. These features very likely offer abnormal topographical and mechanical cues to endothelial cells growing on and interacting with the membrane, which could trigger pathological processes such as EMT184 or apoptosis185. While it is yet unclear whether these biophysical abnormalities in DM are responsible for, or merely coincide with, endothelial cell dysfunction and premature degeneration, there are other instances in the biomedical literature of dysregulated extracellular matrix causing pathological behavior including fibrosis, atherosclerosis and tumorigenesis186,187. Furthermore, our lab has demonstrated that changes to substratum compliance and topography can promote the transformation of corneal fibroblasts to myofibroblasts18,20, leading us to hypothesize that the biophysical characteristics of DM impact endothelial cell morphology and function.
5. In vitro studies of ECM in corneal endothelial cells
To delineate the mechanisms of corneal endothelial dystrophies and identify efficacious treatments, it is critical to establish in vitro disease models using cell culture. A complete description of mechanism needs to capture the bidirectional interactions between cells and ECM, and characterize ECM expression changes in the presence of mutations, stress, EMT, and disease states. While some studies of CECs have measured ECM gene and protein expression changes, the resulting matrix has rarely been characterized.
The major protein component of the ECM expressed by CEnCs is collagen188, predominantly collagens IV, VIII, XII, and XIII150. The production of a matrix, similar to DM in appearance and collagen content, by rabbit CEnCs was first reported in 1974189. Over the next two decades, studies showed that the content of the ECM produced by CEnCs could be modulated by treating the cell culture with growth factors, such as FGF-2190 and TGF-β117, that also play a role in EMT191. Cells cultured at suboptimal conditions, e.g. passaged repeatedly at subconfluence in the absence of FGF-2, eventually shift to a higher production of collagen I188. One study demonstrated that ECM production by CEnCs in response to TGF-β depends on the expression levels of the EMT-inducers Snail1 and ZEB1, which tend to be elevated in FCED-derived cells121. Immortalized cells derived from normal and FCED-affected endothelia were cultured to deposit ECM on polyester membranes, and transmission electron microscopy was used to quantify and compare the thicknesses of the deposited matrices. In the FCED-derived cells, expression of collagen I, collagen IV, and fibronectin was elevated, and the ultrastructure revealed more fibrillar ECM compared to in normal phenotype cells.
A key in vitro strategy to understanding the role of ECM in disease and mechanotransduction is to culture cells on ECM substrates with differing biophysical attributes and investigate their responses. An early study culturing normal CEnCs for a week on FCED-derived DM did not show abnormalities in morphology or monolayer formation, although the study was limited in time and did not investigate cell function192. Interestingly, cells cultured on pseudophakic bullous keratopathy-derived membranes adopted an abnormal non-confluent stellate form, indicating that this rapid-onset condition disrupts DM and modulates cell growth more acutely192. CEnCs have also been plated on ECM-coated tissue culture dishes193 and soft substrates37 to assess which surfaces best promote the normal corneal endothelial phenotype. Synthetic substrates that are biomimetic of DM stiffness have also been developed, particularly to enhance proliferation and monolayer stability in artificial corneal grafts37,194. Another approach to study cell response to ECM is to deposit a matrix from cells in a long-term culture, decellularize the matrix, and then use the matrix as a substrate for a de novo cell culture195 but this approach has not been applied to CEnCs to the authors’ knowledge.
The development of in vitro models that mimic both homeostatic and disease states would better enable us to recapitulate the abnormal ECM signals in corneal dystrophies, assess their impact on cell fate, and characterize the resultant expressed matrix biomolecules.
6. Impact of biophysical cues on regenerative treatments
The successful in vitro culture of CEnCs is a prerequisite for novel regenerative treatments. Presently, the most common treatment for corneal endothelial dystrophies is transplantation of the full-thickness cornea or DM with associated CenCs, but a global shortage of available donors has driven the development of cell replacement therapy approaches196 and synthetic corneal endothelium grafts196,197. Cell replacement therapy involves the culture and expansion of CEnCs in vitro, and subsequent transplantation into the eye without a carrier material. Successful cultures have been established by overcoming proliferative arrest of CECs through the disruption of junctions198, and treatment with small molecules (e.g. Rho-kinase inhibitors)104,112 and growth factors (e.g. FGF-2)188,199. Furthermore, corneal endothelial precursor cells from the peripheral cornea have a higher than average proliferative capacity and corneal endothelial stem cells are located in a sequestered niche at the limbus between the endothelium and trabecular meshwork200. Cultures established with the endothelial stem cells have been delivered into animal models of bullous keratopathy, where they adhered to DM and enhanced corneal clarity201.
For ease of delivery, it may be necessary to culture corneal endothelial cells on a carrier before implanting the construct as an artificial graft. Grafts have been prepared by culturing endothelial cells on native tissue such as decellularized DMs202, amniotic membranes203, and lens capsules204. Carriers of cultured cells have also been synthesized with biological materials such as collagen I205,206, silk fibroin207, and chitosan208. ECM-based coatings are frequently applied to the carriers to enhance cell function 193,209–211. Blended biomaterials combine the desired properties of their components to produce surfaces that have clarity, biocompatibility, and mechanical strength212,213. Another desired trait, the non-destructive detachment of intact endothelial monolayers, has been achieved through the development of thermoresponsive38,214,215 and biodegradable208,212,214,216 substrates.
These artificial grafts and carriers have not yet recapitulated the functionality of the native cornea to a clinically-relevant degree: cell density and quality do not match the native endothelium, the Na+/K+-ATPase pump function is 75–95% that of normal human donor corneas, and the corneas engrafted with cellularized matrices eventually lose their transparency197. Introducing optimized biophysical cues into matrix design parameters may alleviate many challenges in recapitulating corneal function in these artificial constructs by improving functional protein expression. Endothelial cell morphology is dramatically improved on substrates imitating the stiffness of normal DM in comparison to softer or stiffer substrates37. Similarly, cells grown on substrates presenting biomimetic topographic cues also exhibited differential protein expression. Immortalized human CEnCs grown on nanopillars, micropillars, and microwells displayed varied levels of the ZO-1 and Na+/K+-ATPase proteins, with the highest expression on substrates that minimized the cells’ surface area26.
The mechanical properties of carrier materials have been improved to produce substrates that are strong enough to survive cell culture and handling, but the provision of biophysical cues has not been adequately explored and this remains an understudied area. Incorporation of these features into the design of carrier substrates may be critical to developing grafts with long-term success.
Conclusion
CEnCs, and the matrix that they secrete to produce the DM, have a bidirectional relationship. Corneal endothelial health relies on a normal and intact DM, and in turn healthy endothelial cells are necessary for the production of a normal extracellular matrix. This relationship in endothelial health and disease has been understudied and it critical that we consider the role of biophysical signals provided by the ECM. Fortunately, there are many tools available to characterize the cells’ mechanical microenvironment and recapitulate it in vitro to provide a better model of what the corneal endothelium senses in vivo. The insights gleaned from investigating these interactions will expand our understanding of the pathogenesis of corneal endothelial dystrophies, and lead to improved outcomes for treatments.
Research Highlights.
Descemet’s membrane presents biophysical cues to endothelial cells
Mechanotransduction influences cell behavior such as matrix production
In dynamic reciprocity, biophysical cues and matrix production modulate each other
Understanding these relationships helps develop models for corneal dystrophies
Acknowledgments
The authors thank Dr. Paul Russell for his generous input during the preparation of this document and Dr. Chrisoula Skouritakis, Director of Media Lab Services of the Department of Surgical and Radiological Sciences, for her assistance in preparing figures.
Funding:
This study was funded by grants from the National Institute of Health K08 EY021142, R01 EY019970, R01 EY016134, and P30 EY12576
Abbreviations
- CEnC
corneal endothelial cells
- ECM
extracellular matrix
- DM
Descemet’s membrane
- FCED
Fuchs’ corneal endothelial dystrophy
- AFM
atomic force microscopy
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- ABL
anterior banded layer
- PNBL
posterior non-banded layer
- TGF-β
transforming growth factor β
- Wnt
Wingless/int
- YAP
Yes-associated protein
- TAZ
transcriptional coactivator with PDZ-binding motif
- EMT
epithelial-mesenchymal transition
- PI3K
Phosphoinositide 3-kinase
- FGF-2
fibroblastic growth factor-2
- PCL
posterior collagenous layer
- ICE
Iridocorneal endothelial syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Srinivas SP. Dynamic regulation of barrier integrity of the corneal endothelium. Optom Vis Sci. 2010;87:E239–254. doi: 10.1097/OPX.0b013e3181d39464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramachandran C, Srinivas SP. Formation and disassembly of adherens and tight junctions in the corneal endothelium: regulation by actomyosin contraction. Invest Ophthalmol Vis Sci. 2010;51:2139–2148. doi: 10.1167/iovs.09-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noske W, Fromm M, Levarlet B, Kreusel KM, Hirsch M. Tight junctions of the human corneal endothelium: morphological and electrophysiological features. Ger J Ophthalmol. 1994;3:253–257. [PubMed] [Google Scholar]
- 5.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95:2–7. doi: 10.1016/j.exer.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischbarg J. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J Exp Zool A Comp Exp Biol. 2003;300:30–40. doi: 10.1002/jez.a.10306. [DOI] [PubMed] [Google Scholar]
- 7.Hatou S, et al. The Effects of Dexamethasone on the Na,K-ATPase Activity and Pump Function of Corneal Endothelial Cells. Current Eye Research. 2009;34:347–354. doi: 10.1080/02713680902829624. Pii 910773411. [DOI] [PubMed] [Google Scholar]
- 8.Kabosova A, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007;48:4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Stromal thickness in the normal cornea: three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25:776–786. doi: 10.3928/1081597X-20090813-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MK, Foley JW, Birk DE, Fitch JM, Linsenmayer TF. Type V collagen and Bowman’s membrane. Quantitation of mRNA in corneal epithelium and stroma. J Biol Chem. 1994;269:24959–24966. [PubMed] [Google Scholar]
- 11.Adler FH, Hart WM. Adler’s physiology of the eye: clinical application. 9. Mosby Year Book; 1992. [Google Scholar]
- 12.Hay ED. Matrix-cytoskeletal interactions in the developing eye. J Cell Biochem. 1985;27:143–156. doi: 10.1002/jcb.240270208. [DOI] [PubMed] [Google Scholar]
- 13.Hao J, et al. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal. 2014;26:186–191. doi: 10.1016/j.cellsig.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 16.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomasy SM, Wood JA, Kass PH, Murphy CJ, Russell P. Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:952–958. doi: 10.1167/iovs.11-8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myrna KE, et al. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2012;53:811–816. doi: 10.1167/iovs.11-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghunathan VK, et al. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest Ophthalmol Vis Sci. 2013;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreier B, et al. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2013;54:5901–5907. doi: 10.1167/iovs.12-11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Petroll WM, Vishwanath M, Ma L. Corneal fibroblasts respond rapidly to changes in local mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:3466–3474. doi: 10.1167/iovs.04-0361. [DOI] [PubMed] [Google Scholar]
- 23.Karamichos D, Lakshman N, Petroll WM. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest Ophthalmol Vis Sci. 2007;48:5030–5037. doi: 10.1167/iovs.07-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee CT, Raghunathan VK, Nealey PF, Russell P, Murphy CJ. Topographic modulation of the orientation and shape of cell nuclei and their influence on the measured elastic modulus of epithelial cells. Biophys J. 2011;101:2139–2146. doi: 10.1016/j.bpj.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghunathan VK, et al. Nuclear and cellular alignment of primary corneal epithelial cells on topography. J Biomed Mater Res A. 2013;101:1069–1079. doi: 10.1002/jbm.a.34417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo S, Muhammad R, Peh GS, Mehta JS, Yim EK. Micro- and nanotopography with extracellular matrix coating modulate human corneal endothelial cell behavior. Acta Biomater. 2014;10:1975–1984. doi: 10.1016/j.actbio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Karuri NW, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreier B, Raghunathan VK, Russell P, Murphy CJ. Focal adhesion kinase knockdown modulates the response of human corneal epithelial cells to topographic cues. Acta Biomater. 2012;8:4285–4294. doi: 10.1016/j.actbio.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raghunathan V, et al. Influence of extracellular matrix proteins and substratum topography on corneal epithelial cell alignment and migration. Tissue Eng Part A. 2013;19:1713–1722. doi: 10.1089/ten.TEA.2012.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhammad R, et al. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta Biomater. 2015;19:138–148. doi: 10.1016/j.actbio.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Raghunathan VK, et al. Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells. PLoS One. 2014;9:e109811. doi: 10.1371/journal.pone.0109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petroll WM, Lakshman N. Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness. J Funct Biomater. 2015;6:222–240. doi: 10.3390/jfb6020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okumura N, et al. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Invest Ophthalmol Vis Sci. 2015;56:6067–6074. doi: 10.1167/iovs.15-17595. [DOI] [PubMed] [Google Scholar]
- 34.Petroll WM, Miron-Mendoza M. Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix. Exp Eye Res. 2015;133:49–57. doi: 10.1016/j.exer.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, et al. Biomimetic stochastic topography and electric fields synergistically enhance directional migration of corneal epithelial cells in a MMP-3-dependent manner. Acta Biomater. 2015;12:102–112. doi: 10.1016/j.actbio.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A, Brugnano J, Sun S, Vase A, Orwin E. The development of a tissue-engineered cornea: biomaterials and culture methods. Pediatr Res. 2008;63:535–544. doi: 10.1203/PDR.0b013e31816bdf54. [DOI] [PubMed] [Google Scholar]
- 37.Palchesko RN, Lathrop KL, Funderburgh JL, Feinberg AW. In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci Rep. 2015;5:7955. doi: 10.1038/srep07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teichmann J, et al. Human corneal endothelial cell sheets for transplantation: thermo-responsive cell culture carriers to meet cell-specific requirements. Acta Biomater. 2013;9:5031–5039. doi: 10.1016/j.actbio.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Niu G, et al. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials. 2014;35:4005–4014. doi: 10.1016/j.biomaterials.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Teo BK, Goh KJ, Ng ZJ, Koo S, Yim EK. Functional reconstruction of corneal endothelium using nanotopography for tissue-engineering applications. Acta Biomater. 2012;8:2941–2952. doi: 10.1016/j.actbio.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Askeland DR, Fulay PP, Wright WJ. The science and engineering of materials. 6. Cengage Learning; 2011. [Google Scholar]
- 42.McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng Part B Rev. 2011;17:155–164. doi: 10.1089/ten.TEB.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo L, Reed J, Gimzewski JK, Demer JL. Mechanical interferometry imaging for creep modeling of the cornea. Invest Ophthalmol Vis Sci. 2011;52:8420–8424. doi: 10.1167/iovs.11-7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Last JA, Russell P, Nealey PF, Murphy CJ. The applications of atomic force microscopy to vision science. Invest Ophthalmol Vis Sci. 2010;51:6083–6094. doi: 10.1167/iovs.10-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J Struct Biol. 2009;167:19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Last JA, Thomasy SM, Croasdale CR, Russell P, Murphy CJ. Compliance profile of the human cornea as measured by atomic force microscopy. Micron. 2012;43:1293–1298. doi: 10.1016/j.micron.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomasy SM, et al. Elastic modulus and collagen organization of the rabbit cornea: epithelium to endothelium. Acta Biomater. 2014;10:785–791. doi: 10.1016/j.actbio.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Last JA, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worthington KS, et al. Mechanical properties of murine and porcine ocular tissues in compression. Exp Eye Res. 2014;121:194–199. doi: 10.1016/j.exer.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danielsen CC. Tensile mechanical and creep properties of Descemet’s membrane and lens capsule. Exp Eye Res. 2004;79:343–350. doi: 10.1016/j.exer.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Hugar DL, Ivanisevic A. Materials characterization and mechanobiology of the eye. Mater Sci Eng C Mater Biol Appl. 2013;33:1867–1875. doi: 10.1016/j.msec.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Raghunathan VK, MJT, Russell P. In: Glaucoma Research and Clinical Advances: 2016 to 2018. Knepper PA, Samples JA, editors. Ch. 8. Kugler Publications; 2016. pp. 121–138. [Google Scholar]
- 53.Lombardo M, et al. Biomechanics of the anterior human corneal tissue investigated with atomic force microscopy. Invest Ophthalmol Vis Sci. 2012;53:1050–1057. doi: 10.1167/iovs.11-8720. [DOI] [PubMed] [Google Scholar]
- 54.Seifert J, et al. Distribution of Young’s modulus in porcine corneas after riboflavin/UVA-induced collagen cross-linking as measured by atomic force microscopy. PLoS One. 2014;9:e88186. doi: 10.1371/journal.pone.0088186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dias JM, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013;115:41–46. doi: 10.1016/j.exer.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abrams GA, Bentley E, Nealey PF, Murphy CJ. Electron microscopy of the canine corneal basement membranes. Cells Tissues Organs. 2002;170:251–257. doi: 10.1159/000047929. doi:47929. [DOI] [PubMed] [Google Scholar]
- 57.Bentley E, et al. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Invest Ophthalmol Vis Sci. 2001;42:2262–2269. [PubMed] [Google Scholar]
- 58.Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 60.Glentis A, Gurchenkov V, Matic Vignjevic D. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adh Migr. 2014;8:236–245. doi: 10.4161/cam.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood) 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 62.Levy SG, Moss J, Sawada H, Dopping-Hepenstal PJ, McCartney AC. The composition of wide-spaced collagen in normal and diseased Descemet’s membrane. Curr Eye Res. 1996;15:45–52. doi: 10.3109/02713689609017610. [DOI] [PubMed] [Google Scholar]
- 63.Murphy C, Alvarado J, Juster R. Prenatal and postnatal growth of the human Descemet’s membrane. Invest Ophthalmol Vis Sci. 1984;25:1402–1415. [PubMed] [Google Scholar]
- 64.Miller RT. Mechanical properties of basement membrane in health and disease. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhuri O, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 66.Waring GO., 3rd Posterior collagenous layer of the cornea. Ultrastructural classification of abnormal collagenous tissue posterior to Descemet’s membrane in 30 cases. Arch Ophthalmol. 1982;100:122–134. doi: 10.1001/archopht.1982.01030030124015. [DOI] [PubMed] [Google Scholar]
- 67.Ljubimov AV, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 68.Sawada H, Konomi H, Hirosawa K. Characterization of the collagen in the hexagonal lattice of Descemet’s membrane: its relation to type VIII collagen. J Cell Biol. 1990;110:219–227. doi: 10.1083/jcb.110.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fratzl P. Collagen: structure and mechanics. Springer; 2008. [Google Scholar]
- 70.Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawada H. The fine structure of the bovine Descemet’s membrane with special reference to biochemical nature. Cell Tissue Res. 1982;226:241–255. doi: 10.1007/BF00218356. [DOI] [PubMed] [Google Scholar]
- 72.Stephan S, Sherratt MJ, Hodson N, Shuttleworth CA, Kielty CM. Expression and supramolecular assembly of recombinant alpha1(viii) and alpha2(viii) collagen homotrimers. J Biol Chem. 2004;279:21469–21477. doi: 10.1074/jbc.M305805200. [DOI] [PubMed] [Google Scholar]
- 73.Jakus MA. Studies on the cornea. II. The fine structure of Descement’s membrane. J Biophys Biochem Cytol. 1956;2:243–252. doi: 10.1083/jcb.2.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gottsch JD, et al. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–4511. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
- 75.Hopfer U, et al. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005;19:1232–1244. doi: 10.1096/fj.04-3019com. [DOI] [PubMed] [Google Scholar]
- 76.Kapoor R, et al. Type VIII collagen has a restricted distribution in specialized extracellular matrices. J Cell Biol. 1988;107:721–730. doi: 10.1083/jcb.107.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zagorski Z, Naumann GO. Reactive production of extracellular matrix (ECM) by corneal endothelial cells. Acta Ophthalmol (Copenh) 1992;70:366–370. doi: 10.1111/j.1755-3768.1992.tb08581.x. [DOI] [PubMed] [Google Scholar]
- 78.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 79.Bornstein P, McPherson J, HS . In: Pathobiology of the Endothelial Cell. Nossel HL, Vogel HJ, editors. Academic Press; 1982. pp. 215–228. [Google Scholar]
- 80.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- 81.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 82.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akiyama T, Kawasaki Y. Wnt signalling and the actin cytoskeleton. Oncogene. 2006;25:7538–7544. doi: 10.1038/sj.onc.1210063. [DOI] [PubMed] [Google Scholar]
- 84.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 85.Jahed Z, Shams H, Mehrbod M, Mofrad MR. Mechanotransduction pathways linking the extracellular matrix to the nucleus. Int Rev Cell Mol Biol. 2014;310:171–220. doi: 10.1016/B978-0-12-800180-6.00005-0. [DOI] [PubMed] [Google Scholar]
- 86.Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFbeta have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Exp Eye Res. 2013;115:1–12. doi: 10.1016/j.exer.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 88.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci. 2012;125:3051–3060. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romet-Lemonne G, Jegou A. Mechanotransduction down to individual actin filaments. Eur J Cell Biol. 2013;92:333–338. doi: 10.1016/j.ejcb.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 92.Zimmermann P, David G. The syndecans, tuners of transmembrane signaling. FASEB J. 1999;13(Suppl):S91–S100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]
- 93.Ross TD, et al. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 95.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 96.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 97.Okumura N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 98.Okumura N, et al. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30(Suppl 1):S54–59. doi: 10.1097/ICO.0b013e3182281ee1. [DOI] [PubMed] [Google Scholar]
- 99.Okumura N, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181:268–277. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 100.Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95:60–67. doi: 10.1016/j.exer.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Okumura N, Kinoshita S, Koizumi N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea. 2014;33(Suppl 11):S37–41. doi: 10.1097/ico.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 102.Okumura N, et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011;95:1006–1009. doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- 103.Okumura N, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54:2493–2502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- 104.Okumura N, et al. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2014;55:318–329. doi: 10.1167/iovs.13-12225. [DOI] [PubMed] [Google Scholar]
- 105.Jalimarada SS, Shivanna M, Kini V, Mehta D, Srinivas SP. Microtubule disassembly breaks down the barrier integrity of corneal endothelium. Exp Eye Res. 2009;89:333–343. doi: 10.1016/j.exer.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srinivas SP. Cell signaling in regulation of the barrier integrity of the corneal endothelium. Exp Eye Res. 2012;95:8–15. doi: 10.1016/j.exer.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rajashekhar G, Shivanna M, Kompella UB, Wang Y, Srinivas SP. Role of MMP-9 in the breakdown of barrier integrity of the corneal endothelium in response to TNF-alpha. Exp Eye Res. 2014;122:77–85. doi: 10.1016/j.exer.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 109.Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 110.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J, Ho J, Sutu C, Afshari NA. Regulation of YAP activity in human corneal endothelial cells. Invest Ophth Vis Sci. 2015;56:4911–4911. [Google Scholar]
- 112.Hsueh YJ, et al. Lysophosphatidic acid induces YAP-promoted proliferation of human corneal endothelial cells via PI3K and ROCK pathways. Mol Ther Methods Clin Dev. 2015;2:15014. doi: 10.1038/mtm.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park HW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye X, Deng Y, Lai ZC. Akt is negatively regulated by Hippo signaling for growth inhibition in Drosophila. Dev Biol. 2012;369:115–123. doi: 10.1016/j.ydbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 115.Diepenbruck M, et al. Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. J Cell Sci. 2014;127:1523–1536. doi: 10.1242/jcs.139865. [DOI] [PubMed] [Google Scholar]
- 116.Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med (Lausanne) 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Usui T, et al. Extracellular matrix production regulation by TGF-beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 1998;39:1981–1989. [PubMed] [Google Scholar]
- 118.Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change in vitro. Invest Ophthalmol Vis Sci. 2015;56:1228–1237. doi: 10.1167/iovs.14-16166. [DOI] [PubMed] [Google Scholar]
- 119.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 120.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 121.Okumura N, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab Invest. 2015 doi: 10.1038/labinvest.2015.111. [DOI] [PubMed] [Google Scholar]
- 122.Okumura N, et al. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One. 2013;8:e58000. doi: 10.1371/journal.pone.0058000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matthaei M, et al. Endothelial cell microRNA expression in human late-onset Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2014;55:216–225. doi: 10.1167/iovs.13-12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matthaei M, et al. Epithelial-Mesenchymal Transition (EMT)-Related Cytokines in the Aqueous Humor of Phakic and Pseudophakic Fuchs’ Dystrophy Eyes. Invest Ophthalmol Vis Sci. 2015;56:2749–2754. doi: 10.1167/iovs.15-16395. [DOI] [PubMed] [Google Scholar]
- 125.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 127.Lu J, et al. TGF-beta2 inhibits AKT activation and FGF-2-induced corneal endothelial cell proliferation. Exp Cell Res. 2006;312:3631–3640. doi: 10.1016/j.yexcr.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 129.Ho WT, et al. Inhibition of matrix metalloproteinase activity reverses corneal endothelial-mesenchymal transition. Am J Pathol. 2015;185:2158–2167. doi: 10.1016/j.ajpath.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 130.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 131.Lee JG, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest Ophthalmol Vis Sci. 2006;47:2358–2368. doi: 10.1167/iovs.05-1490. [DOI] [PubMed] [Google Scholar]
- 132.Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J Cell Sci. 2012;125:3636–3648. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond) 2003;17:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 134.Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–1575. [PubMed] [Google Scholar]
- 135.Murphy C, Alvarado J, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984;25:312–322. [PubMed] [Google Scholar]
- 136.Hoppenreijs VP, Pels E, Vrensen GF, Oosting J, Treffers WF. Effects of human epidermal growth factor on endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1992;33:1946–1957. [PubMed] [Google Scholar]
- 137.Matsuda M, et al. Cellular migration and morphology in corneal endothelial wound repair. Invest Ophthalmol Vis Sci. 1985;26:443–449. [PubMed] [Google Scholar]
- 138.Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M. Corneal endothelial cell density in glaucoma. Cornea. 1997;16:314–318. [PubMed] [Google Scholar]
- 139.Olsen T. Changes in the corneal endothelium after acute anterior uveitis as seen with the specular microscope. Acta Ophthalmol (Copenh) 1980;58:250–256. doi: 10.1111/j.1755-3768.1980.tb05718.x. [DOI] [PubMed] [Google Scholar]
- 140.Liesegang TJ. The response of the corneal endothelium to intraocular surgery. Refract Corneal Surg. 1991;7:81–86. [PubMed] [Google Scholar]
- 141.Irvine AR., Jr The role of the endothelium in bullous keratopathy. AMA Arch Ophthalmol. 1956;56:338–351. doi: 10.1001/archopht.1956.00930040346003. [DOI] [PubMed] [Google Scholar]
- 142.Schultz RO, Glasser DB, Matsuda M, Yee RW, Edelhauser HF. Response of the corneal endothelium to cataract surgery. Arch Ophthalmol. 1986;104:1164–1169. doi: 10.1001/archopht.1986.01050200070053. [DOI] [PubMed] [Google Scholar]
- 143.Ventura AC, Walti R, Bohnke M. Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol. 2001;85:18–20. doi: 10.1136/bjo.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soumittra N, et al. Biosynthetic and functional defects in newly identified SLC4A11 mutants and absence of COL8A2 mutations in Fuchs endothelial corneal dystrophy. J Hum Genet. 2014;59:444–453. doi: 10.1038/jhg.2014.55. [DOI] [PubMed] [Google Scholar]
- 145.Loganathan SK, Casey JR. Corneal dystrophy-causing SLC4A11 mutants: suitability for folding-correction therapy. Hum Mutat. 2014;35:1082–1091. doi: 10.1002/humu.22601. [DOI] [PubMed] [Google Scholar]
- 146.Hamill CE, Schmedt T, Jurkunas U. Fuchs endothelial cornea dystrophy: a review of the genetics behind disease development. Semin Ophthalmol. 2013;28:281–286. doi: 10.3109/08820538.2013.825283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bourne WM, Johnson DH, Campbell RJ. The ultrastructure of Descemet’s membrane. III. Fuchs’ dystrophy. Arch Ophthalmol. 1982;100:1952–1955. doi: 10.1001/archopht.1982.01030040932013. [DOI] [PubMed] [Google Scholar]
- 148.Gottsch JD, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46:1934–1939. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 149.Lee WR, Marshall GE, Kirkness CM. Corneal endothelial cell abnormalities in an early stage of the iridocorneal endothelial syndrome. Br J Ophthalmol. 1994;78:624–631. doi: 10.1136/bjo.78.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weller JM, et al. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Invest Ophthalmol Vis Sci. 2014;55:3700–3708. doi: 10.1167/iovs.14-14154. [DOI] [PubMed] [Google Scholar]
- 151.Biswas S, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 152.Zhang C, et al. Immunohistochemistry and electron microscopy of early-onset fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation. Transactions of the American Ophthalmological Society. 2006;104:85–97. [PMC free article] [PubMed] [Google Scholar]
- 153.Jun AS, et al. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2012;21:384–393. doi: 10.1093/hmg/ddr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meng H, et al. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Invest Ophthalmol Vis Sci. 2013;54:1887–1897. doi: 10.1167/iovs.12-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet. 2009;10:173–183. doi: 10.1038/nrg2520. [DOI] [PubMed] [Google Scholar]
- 156.Klintworth GK. The molecular genetics of the corneal dystrophies--current status. Front Biosci. 2003;8:d687–713. doi: 10.2741/1018. [DOI] [PubMed] [Google Scholar]
- 157.Chung DW, Frausto RF, Ann LB, Jang MS, Aldave AJ. Functional impact of ZEB1 mutations associated with posterior polymorphous and Fuchs’ endothelial corneal dystrophies. Invest Ophthalmol Vis Sci. 2014;55:6159–6166. doi: 10.1167/iovs.14-15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baratz KH, et al. E2-2 protein and Fuchs’s corneal dystrophy. N Engl J Med. 2010;363:1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- 159.Chang PC, Sulik GI, Soong HK, Parkinson WC. Galvanotropic and galvanotaxic responses of corneal endothelial cells. J Formos Med Assoc. 1996;95:623–627. [PubMed] [Google Scholar]
- 160.Vilas GL, et al. Oligomerization of SLC4A11 protein and the severity of FECD and CHED2 corneal dystrophies caused by SLC4A11 mutations. Hum Mutat. 2012;33:419–428. doi: 10.1002/humu.21655. [DOI] [PubMed] [Google Scholar]
- 161.Riazuddin SA, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012;90:533–539. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Son HS, Villarreal G, Jr, Meng H, Eberhart CG, Jun AS. On the origin of ‘guttae’. Br J Ophthalmol. 2014;98:1308–1310. doi: 10.1136/bjophthalmol-2014-305069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xia D, et al. The Ultrastructures and Mechanical Properties of the Descement’s Membrane in Fuchs Endothelial Corneal Dystrophy. Sci Rep. 2016;6:23096. doi: 10.1038/srep23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thomasy S, VKR, Ali M, Murphy C. The Association for Research in Vision and Ophthalmology. Seattle, WA: 2016. [Google Scholar]
- 165.Black LD, Allen PG, Morris SM, Stone PJ, Suki B. Mechanical and failure properties of extracellular matrix sheets as a function of structural protein composition. Biophys J. 2008;94:1916–1929. doi: 10.1529/biophysj.107.107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hwang ES, Brodsky B. Folding delay and structural perturbations caused by type IV collagen natural interruptions and nearby Gly missense mutations. J Biol Chem. 2012;287:4368–4375. doi: 10.1074/jbc.M111.269084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hwang ES, Thiagarajan G, Parmar AS, Brodsky B. Interruptions in the collagen repeating tripeptide pattern can promote supramolecular association. Protein Sci. 2010;19:1053–1064. doi: 10.1002/pro.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muiznieks LD, Keeley FW. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim Biophys Acta. 2013;1832:866–875. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 169.Mehta JS, et al. ARVO 2016 Annual Meeting. [Google Scholar]
- 170.Tocce EJ, et al. The ability of corneal epithelial cells to recognize high aspect ratio nanostructures. Biomaterials. 2010;31:4064–4072. doi: 10.1016/j.biomaterials.2010.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bergmanson JP, Sheldon TM, Goosey JD. Fuchs’ endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol Opt. 1999;19:210–222. doi: 10.1046/j.1475-1313.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 172.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286:17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Thomasy SM, et al. In Vivo Imaging of Corneal Endothelial Dystrophy in Boston Terriers: A Spontaneous, Canine Model for Fuchs’ Endothelial Corneal DystrophyA Canine Model of Fuchs’ Endothelial Corneal Dystrophy. Invest Ophth Vis Sci. 2016;57:OCT495–OCT503. doi: 10.1167/iovs.15-18885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bozkurt B, Irkec M, Mocan MC. In vivo confocal microscopic findings in posterior polymorphous corneal dystrophy. Cornea. 2013;32:1237–1242. doi: 10.1097/ICO.0b013e31828e324d. [DOI] [PubMed] [Google Scholar]
- 175.Henriquez AS, et al. Morphologic characteristics of posterior polymorphous dystrophy. A study of nine corneas and review of the literature. Surv Ophthalmol. 1984;29:139–147. doi: 10.1016/0039-6257(84)90171-1. [DOI] [PubMed] [Google Scholar]
- 176.Mahmood MA, Teichmann KD. Corneal amyloidosis associated with congenital hereditary endothelial dystrophy. Cornea. 2000;19:570–573. doi: 10.1097/00003226-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 177.Vemuganti GK, Sridhar MS, Edward DP, Singh S. Subepithelial amyloid deposits in congenital hereditary endothelial dystrophy: a histopathologic study of five cases. Cornea. 2002;21:524–529. doi: 10.1097/00003226-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 178.Pandrowala H, Bansal A, Vemuganti GK, Rao GN. Frequency, distribution, and outcome of keratoplasty for corneal dystrophies at a tertiary eye care center in South India. Cornea. 2004;23:541–546. doi: 10.1097/01.ico.0000126324.58884.b9. [DOI] [PubMed] [Google Scholar]
- 179.Al-Shehah A, Al-Rajhi A, Alkatan H. Amyloid corneal deposition in corneal buttons of congenital hereditary endothelial dystrophy (CHED) - A clinical and histopathological case series. Saudi J Ophthalmol. 2010;24:111–118. doi: 10.1016/j.sjopt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schmid E, et al. A new, X-linked endothelial corneal dystrophy. American journal of ophthalmology. 2006;141:478–487. doi: 10.1016/j.ajo.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 181.Sacchetti M, et al. Diagnosis and Management of Iridocorneal Endothelial Syndrome. Biomed Res Int. 2015;2015:763093. doi: 10.1155/2015/763093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Levy SG, et al. Descemet’s membrane in the iridocorneal-endothelial syndrome: morphology and composition. Exp Eye Res. 1995;61:323–333. doi: 10.1016/s0014-4835(05)80127-7. [DOI] [PubMed] [Google Scholar]
- 183.Zhang J, Patel DV. The pathophysiology of Fuchs’ endothelial dystrophy--a review of molecular and cellular insights. Exp Eye Res. 2015;130:97–105. doi: 10.1016/j.exer.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 184.Gjorevski N, Boghaert E, Nelson CM. Regulation of Epithelial-Mesenchymal Transition by Transmission of Mechanical Stress through Epithelial Tissues. Cancer Microenviron. 2012;5:29–38. doi: 10.1007/s12307-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang YH, Zhao CQ, Jiang LS, Dai LY. Substrate stiffness regulates apoptosis and the mRNA expression of extracellular matrix regulatory genes in the rat annular cells. Matrix Biol. 2011;30:135–144. doi: 10.1016/j.matbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 186.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kothapalli D, et al. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep. 2012;2:1259–1271. doi: 10.1016/j.celrep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tseng SC, Savion N, Gospodarowicz D, Stern R. Characterization of collagens synthesized by cultured bovine corneal endothelial cells. J Biol Chem. 1981;256:3361–3365. [PubMed] [Google Scholar]
- 189.Perlman M, Baum JL. Synthesis of a collagenous basal membrane by rabbit corneal endothelial cells in vitro. Arch Ophthalmol. 1974;92:238–239. doi: 10.1001/archopht.1974.01010010246015. [DOI] [PubMed] [Google Scholar]
- 190.Kay EP, Gu X, Ninomiya Y, Smith RE. Corneal endothelial modulation: a factor released by leukocytes induces basic fibroblast growth factor that modulates cell shape and collagen. Invest Ophthalmol Vis Sci. 1993;34:663–672. [PubMed] [Google Scholar]
- 191.Lee JG, Ko MK, Kay EP. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp Eye Res. 2012;95:35–39. doi: 10.1016/j.exer.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 192.Raphael B, Lange T, Wood TO, McLaughlin BJ. Growth of human corneal endothelium on altered Descemet’s membrane. Cornea. 1992;11:242–249. [PubMed] [Google Scholar]
- 193.Choi JS, et al. In vitro evaluation of the interactions between human corneal endothelial cells and extracellular matrix proteins. Biomed Mater. 2013;8:014108. doi: 10.1088/1748-6041/8/1/014108. [DOI] [PubMed] [Google Scholar]
- 194.Palchesko RN, Funderburgh JL, Feinberg AW. Biomedical Engineering Society. [Google Scholar]
- 195.Castaldo C, et al. Cardiac fibroblast-derived extracellular matrix (biomatrix) as a model for the studies of cardiac primitive cell biological properties in normal and pathological adult human heart. Biomed Res Int. 2013;2013:352370. doi: 10.1155/2013/352370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zavala J, Lopez Jaime GR, Rodriguez Barrientos CA, Valdez-Garcia J. Corneal endothelium: developmental strategies for regeneration. Eye (Lond) 2013;27:579–588. doi: 10.1038/eye.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Progress in retinal and eye research. 2013;35:1–17. doi: 10.1016/j.preteyeres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 198.Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:2930–2935. [PubMed] [Google Scholar]
- 199.Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001;20:731–737. doi: 10.1097/00003226-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 200.Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–824. [PubMed] [Google Scholar]
- 201.Mimura T, Yokoo S, Araie M, Amano S, Yamagami S. Treatment of rabbit bullous keratopathy with precursors derived from cultured human corneal endothelium. Invest Ophthalmol Vis Sci. 2005;46:3637–3644. doi: 10.1167/iovs.05-0462. [DOI] [PubMed] [Google Scholar]
- 202.Bayyoud T, et al. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr Eye Res. 2012;37:179–186. doi: 10.3109/02713683.2011.644382. [DOI] [PubMed] [Google Scholar]
- 203.Ishino Y, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45:800–806. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]
- 204.Yoeruek E, et al. Human anterior lens capsule as carrier matrix for cultivated human corneal endothelial cells. Cornea. 2009;28:416–420. doi: 10.1097/ICO.0b013e31818c2c36. [DOI] [PubMed] [Google Scholar]
- 205.Gruschwitz R, et al. Alignment and cell-matrix interactions of human corneal endothelial cells on nanostructured collagen type I matrices. Invest Ophthalmol Vis Sci. 2010;51:6303–6310. doi: 10.1167/iovs.10-5368. [DOI] [PubMed] [Google Scholar]
- 206.Mimura T, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 207.Madden PW, et al. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials. 2011;32:4076–4084. doi: 10.1016/j.biomaterials.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 208.Gao X, Liu W, Han B, Wei X, Yang C. Preparation and properties of a chitosan-based carrier of corneal endothelial cells. J Mater Sci Mater Med. 2008;19:3611–3619. doi: 10.1007/s10856-008-3508-0. [DOI] [PubMed] [Google Scholar]
- 209.Desgranges P, Tardieu M, Loisance D, Barritault D. Extracellular matrix covered biomaterials for human endothelial cell growth. Int J Artif Organs. 1992;15:722–726. [PubMed] [Google Scholar]
- 210.Underwood PA, Bennett FA. The effect of extracellular matrix molecules on the in vitro behavior of bovine endothelial cells. Exp Cell Res. 1993;205:311–319. doi: 10.1006/excr.1993.1091. [DOI] [PubMed] [Google Scholar]
- 211.Blake DA, Yu H, Young DL, Caldwell DR. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest Ophthalmol Vis Sci. 1997;38:1119–1129. [PubMed] [Google Scholar]
- 212.Wang TJ, Wang IJ, Chen S, Chen YH, Young TH. The phenotypic response of bovine corneal endothelial cells on chitosan/polycaprolactone blends. Colloids Surf B Biointerfaces. 2012;90:236–243. doi: 10.1016/j.colsurfb.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 213.Chen J, et al. Electrospun nanofibrous SF/P(LLA-CL) membrane: a potential substratum for endothelial keratoplasty. Int J Nanomedicine. 2015;10:3337–3350. doi: 10.2147/IJN.S77706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hsiue GH, Lai JY, Chen KH, Hsu WM. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation. 2006;81:473–476. doi: 10.1097/01.tp.0000194864.13539.2c. [DOI] [PubMed] [Google Scholar]
- 215.Gotze T, et al. Cultivation of an immortalized human corneal endothelial cell population and two distinct clonal subpopulations on thermo-responsive carriers. Graefes Arch Clin Exp Ophthalmol. 2008;246:1575–1583. doi: 10.1007/s00417-008-0904-6. [DOI] [PubMed] [Google Scholar]
- 216.Hadlock T, Singh S, Vacanti JP, McLaughlin BJ. Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng. 1999;5:187–196. doi: 10.1089/ten.1999.5.187. [DOI] [PubMed] [Google Scholar]