Abstract
Background
A hordeolum is a common, painful inflammation of the eyelid margin that is usually caused by a bacterial infection. The infection affects oil glands of the eyelid and can be either internal or external. In many cases, the lesion drains spontaneously and resolves without treatment; however, the inflammation can spread to other ocular glands or tissues, and recurrences are common. If unresolved, an acute internal hordeolum can become chronic, or can develop into a chalazion. External hordeola, also known as styes, were not included in the scope of this review.
Objectives
The objective of this review was to investigate the effectiveness, and when possible, the safety, of non‐surgical treatments for acute internal hordeola compared with observation or placebo.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register (2016; Issue 12)), MEDLINE Ovid, MEDLINE Ovid Epub Ahead of Print, MEDLINE Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Ovid Daily (January 1946 to December 2016), Embase (January 1947 to December 2016), PubMed (1948 to December 2016), Latin American and Caribbean Literature on Health Sciences (LILACS (January 1982 to December 2016)), the metaRegister of Controlled Trials (mRCT; www.controlled‐trials.com (last searched 26 July 2012)), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 December 2016.
Selection criteria
The selection criteria for this review included randomized or quasi‐randomized clinical trials of participants diagnosed with an acute internal hordeolum. Studies of participants with external hordeola (styes), chronic hordeola, or chalazia were excluded. Non‐surgical interventions of interest included the use of hot or warm compresses, lid scrubs, antibiotics, or steroids compared with observation, placebo, or other active interventions.
Data collection and analysis
Two review authors independently assessed the references identified by electronic searches for inclusion in this review. No relevant studies were found. The reasons for exclusion were documented.
Main results
No trials were identified for this review. Most of the references identified through our search reported on external hordeola or chronic internal hordeola. The few references specific to acute internal hordeola reported recommendations for treatment, were reports of interventional case series, case studies, or other types of observational study designs, and were published more than 20 years ago.
Authors' conclusions
We did not find any evidence for or against the effectiveness of non‐surgical interventions for the treatment of an internal hordeolum. Controlled clinical trials would be useful to determine which interventions are effective for the treatment of acute internal hordeola.
Plain language summary
Interventions for an acute internal hordeolum
What is the aim of this review? The aim of this Cochrane review was to investigate whether treatments such as warm compresses, over‐the‐counter topical medications and lid scrubs, antibiotics, steroids, and lid massages were useful treatments for an internal hordeolum (a swelling that develops on the inside of the eyelid). Cochrane researchers searched for all relevant studies to answer this question and found no studies.
Key messages Many common treatments are available to treat an internal hordeolum. At present, there is no evidence to show whether any of these treatments work.
What was studied in this review? A hordeolum is a common, painful lump in the eyelid that is usually caused by a bacterial infection. The infection affects the oil glands in the eyelid and results in a lump. Often, the infected lump drains and heals by itself, with no treatment. However, the infection can sometimes spread to other glands in the eyes, and can become long lasting. It can also turn into a cyst (known as a chalazion). Hordeola can be internal (on the inside of the eyelid), or external (on the outside of the eyelid near the eyelashes). A hordeolum on the outside of the eyelid is known as a stye. Hordeola can also be acute (appearing suddenly and healing in a short time), or chronic (long lasting). Common treatments for hordeola include warm compresses applied at home, topical medications and lid scrubs available over‐the‐counter, prescribed antibiotics or steroids, and lid massages.
What are the main results of the review? Cochrane researchers looked for studies of people with an acute internal hordeolum. They did not look for studies of people with styes or long‐lasting hordeola. They found no relevant studies that had compared treatments. Thus, no evidence was found for or against using any of the common treatments for hordeola.
How up‐to‐date is this review? Cochrane researchers searched for studies that had been published up to 2 December 2016.
Background
Description of the condition
A hordeolum is a common inflammation of the eyelid margin. It presents as a red, painful, swollen furuncle with an acute onset, and is usually caused by a staphylococcal infection (Mueller 2008; Peralejo 2008; Skorin 2002). The inflammation can be internal, affecting the meibomian glands, or external, affecting the glands of Zeis or Moll (Wald 2004). External hordeola are more commonly known as styes. In many cases, the lesion drains spontaneously and resolves untreated; however, the inflammation can spread to other ocular glands or tissues (i.e., cellulitis), and recurrences are common. If unresolved, an acute internal hordeolum can become chronic or can develop into a chalazion (De Jesus 2004; Hudson 1981; Mueller 2008; Rubin 1995).
A hordeolum is one of the most common diseases of the eye; therefore, many people can be affected, and many causative factors are known to be related to the disease. Blepharitis (Fuchs 1911; Skorin 2002), acne rosacea (De Jesus 2004), trichiasis, and cicatricial ectropion (Moriarty 1982), are conditions frequently associated with internal hordeola. Incidence rates for hordeola are not available because most cases are not reported. Hordeola tend to occur in younger people, but are not limited to any age, gender, or racial group (Fuchs 1911; Lederman 1999; Roodyn 1954). Internal hordeola tend to be more painful and longer lasting than external hordeola (Barza 1983; Fuchs 1911; Olson 1991; Wilkie 1956). Onset is spontaneous (idiopathic), but may be related to lid hygiene, an underlying condition, or a systemic infection (Mathew 1966; Wald 2004). When due to infection, the size of the swelling is a direct indicator of the severity of the infection (Lebensohn 1950). Cases of recurrent hordeola are usually the result of failure to eliminate bacteria completely, rather than resulting from new infections (Roodyn 1954).
Most cases of internal hordeola resolve on their own; therefore, people with hordeola often do not seek professional medical treatment (Olson 1991). Home therapies, including heated compresses, lid scrubs, and over‐the‐counter medications, are often used without consulting a medical professional. For times when medical care is sought, a general practitioner or a family physician may be consulted before an ophthalmologist or an optometrist is seen (Fraunfelder 1971; Lebensohn 1950).
Practice standards for the initial treatment of hordeola are conservative, typically limited to the application of warm compresses several times a day, if any treatment is recommended at all (Barza 1983; Fuchs 1911; Olson 1991; Panicharoen 2011; Sethuraman 2009; Wilkie 1956). A topical antibiotic may be prescribed in conjunction with warm compresses (Diegel 1986; Lebensohn 1950; Lederman 1999; Panicharoen 2011; Wald 2004). If the condition is severe and is resistant to topical antibiotics, systemic antibiotics, or surgical incision and drainage may be implemented (Moriarty 1982; Mueller 2008; Panicharoen 2011; Rubin 1995; Skorin 2002).
Description of the intervention
Nonsurgical treatments for hordeola include the application of warm or hot compresses, the use of lid scrubs and digital massage, the administration of antibiotics or steroids, or alternative medicine such as acupuncture and autohemotherapy. Typically, the intent of these interventions is to reduce healing time while relieving the symptoms associated with the lesion. Interventions of interest would be provided during the first week after onset. Beyond one week, it is believed that internal hordeola may resolve on their own, or may require surgical incision and curettage. In addition to resolving the presenting hordeolum, other aims of the interventions are to minimize the risk that the inflammation may worsen, may spread to other areas, or may become recurrent.
How the intervention might work
The natural history of an acute internal hordeolum generally spans one to two weeks, beginning with the appearance of an abscess and concluding with draining of the abscess. Initial treatments for hordeola have been aimed at promoting the drainage of pus from the abscess and removing the meibomian gland obstruction. The application of a warm or hot compress may facilitate drainage by softening the granuloma (Diegel 1986; Fuchs 1911; Moriarty 1982; Skorin 2002). Heated compresses are typically employed for five to 10 minutes, several times a day, until the hordeolum is resolved.
Lid scrubs consist of mild shampoos or saline solutions, and are applied while the affected area is gently massaged. The theory underlying the use of lid scrubs is that they promote lid hygiene and prepare the physical environment for drainage by clearing debris from the lid margin (Driver 1996; Skorin 2002). Creating a clear channel is believed to initiate drainage, similar to the epilation of an eyelash in cases of an external hordeolum (Hudson 1981). Also, ingredients used in shampoos break down bacterial membranes, which further decreases the presence of bacteria at the infection site (McCulley 1984). Lid scrubs are commonly recommended in the treatment of other ocular bacterial infections, such as blepharitis, and may prevent the spread of infection (Avisar 1991). In conjunction with lid scrubs, lid massage has been proposed to physically express secretions from the infected glands (Driver 1996; Scobee 1942).
Antibiotics can be administered locally, at the site of infection, or may be given systemically. Most cases of hordeola are caused by one of the staphylococcal species; therefore, antibiotics should be effective against the bacteria. Application of topical antibiotics may reduce healing time by fighting against the causative bacterial infection and reducing inflammation. Many topical medications include ingredients that relieve the symptomatic pain of an internal hordeolum. Antibiotics can also be applied locally by injection. Systemic antibiotics are sometimes used when local antibiotics are not effective, or when the infection is not localized.
Steroids can be applied topically as ointments or eyedrops. Internal hordeola have a short course; therefore, as little as one steroid treatment could be effective in reducing healing time and relieving symptoms associated with the inflammation (King 1986; Palva 1983).
Alternative medicine treatments, such as acupuncture, may be used alone or complementary to traditional interventions to treat hordeola. Acupuncture for the treatment of hordeola is evaluated in a separate Cochrane review (Cheng 2014).
Why it is important to do this review
An acute internal hordeolum is a common disease experienced by a wide population. Although the course of the disease is relatively short, instances of internal hordeola are painful and bothersome. Furthermore, improper management of the underlying cause of the infection may lead to recurrent infections, or to the development of other diseases. Despite the common recommendation to use heated compresses, their efficacy in treating hordeola has not been systematically reviewed. If heated compresses are indeed sufficient to treat hordeola, then more rigorous interventions, such as antibiotics or steroids, may not be warranted for initial treatment. Conversely, comparing the effectiveness and safety of all available interventions, to determine which may be most beneficial to the individual, is also important. A summary of the evidence should assist patients and professionals to determine preferred methods of treatment.
Objectives
The objective of this review was to investigate the effectiveness, and when possible, the safety, of non‐surgical treatments for acute internal hordeola compared with observation or placebo.
Methods
Criteria for considering studies for this review
Types of studies
This review was limited to randomized and quasi‐randomized clinical trials. Examples of quasi‐randomized allocation include using participants' birth dates, medical record numbers, or order of enrolment to determine treatment groups.
Types of participants
We were interested in studies of participants with a diagnosis of an acute internal hordeolum. Studies of participants with only an external hordeolum (stye), chronic hordeola, or chalazia were excluded.
Types of interventions
Non‐surgical interventions were the primary focus of this review. We included trials that compared the use of hot or warm compresses, lid scrubs, antibiotics, or steroids with observation, placebo, or another active intervention for the treatment of acute internal hordeola. Complementary and alternative therapies, such as acupuncture and bloodletting, were outside the scope of this review (see Cheng 2014).
Types of outcome measures
Primary outcomes
The primary outcome for this review was the proportion of participants with complete resolution of a hordeolum seven days after diagnosis. The seven‐day period for resolution was selected because most cases of hordeola resolve on their own at between one and two weeks. We had also planned to analyze the proportion of participants with complete resolution of hordeola after 14 days as a secondary outcome, when these data were available.
Secondary outcomes
The proportion of participants requiring surgical incision and drainage after the treatment period, or seven days after diagnosis.
The incidence of chalazia after the treatment period or seven days after diagnosis.
The incidence of recurrence of hordeola after six months, and after one year. A recurrent case was considered to be any hordeolum that occurred after one month from the resolution of the initial hordeolum, at any location on the same eyelid, or as defined by the included study.
The incidence of a secondary hordeolum during or after the treatment period, or seven days after diagnosis. A secondary hordeolum was defined as a hordeolum that occurred within one month of the initial hordeolum, at a different location than the initial hordeolum, or as defined by the included study.
Adverse outcomes
We had planned to report all adverse effects related to the treatment of hordeola that were reported in the primary studies. Specific adverse outcomes of interest included conjunctivitis; eye irritation; discoloration of the eyelid, conjunctiva, and lens; and corneal damage.
Economic data
We had planned to report economic data.
Quality of life data
We had planned to report quality of life data.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register (2016; Issue 12 in The Cochrane Library)), MEDLINE Ovid, MEDLINE Ovid Epub Ahead of Print, MEDLINE Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Ovid Daily (January 1946 to December 2012), Embase (January 1947 to December 2016), PubMed (1948 to December 2016), Latin American and Caribbean Literature on Health Sciences (LILACS (January 1982 to December 2016)), the metaRegister of Controlled Trials (mRCT; www.controlled‐trials.com (last searched 26 July 2012)), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 December 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and the ICTRP (Appendix 8).
Searching other resources
We reviewed the reference lists from potentially eligible studies to identify further studies. In addition, we used the Science Citation Index to search for references that cited potentially eligible studies (last searched on 9 December 2016; no relevant studies were identified).
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts from the electronic literature searches and the manual search to identify possible trials of interest, according to the 'Criteria for considering studies for this review'. We designated each reference identified from the searches as (a) relevant, (b) possibly relevant, or (c) not relevant for this review. We retrieved full‐text copies of the articles if an abstract was classified as (a) or (b). Each article was then independently assessed by two people and was classified as (1) include in review, (2) awaiting classification, or (3) exclude from review. We resolved discrepancies between authors by consensus.
Data extraction and management
As no studies were identified for inclusion in this review, no data extraction or assessment of risk of bias was performed. If, in the future, relevant studies become available, we will undertake the following methods for updating this review.
Two review authors will independently extract data using the data extraction forms created by Cochrane Eyes and Vision. For each included study, we will extract data on study characteristics, interventions, outcomes, cost, quality of life, and other relevant information. One review author will enter the data into Review Manager (RevMan 2014) and a second review author will verify the data entry. Discrepancies between review authors will be resolved by the third review author.
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of bias of included studies, based on the methods provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Sources of potential bias affecting the methodological quality of a study will be divided into six domains that include:
Selection bias: sequence generation and allocation concealment.
Performance bias: masking (blinding) of participants and study personnel.
Detection bias: masking (blinding) of outcome assessors.
Attrition bias: incomplete outcome data and rates of missing data among treatment groups.
Reporting bias: selective outcome reporting.
Other sources of bias: funding source and other potential sources of bias.
For every study included in the review, we will assess each domain to have (a) low risk of bias, (b) unclear or not reported risk of bias, or (c) high risk of bias. Discrepancies between review authors will be resolved by a third review author. For studies classified as unclear or not reported, we will contact the authors of the study for further information, in an attempt to reclassify the quality of the study. If no informative response is received within six weeks, we will assess the study on the basis of available information.
Measures of treatment effect
The measures of treatment effect will depend on the types of data presented in the included studies and will be identified by the definitions given in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Dichotomous data
The primary outcome of interest, the proportion of participants with complete resolution of hordeola at seven days after diagnosis, will be analyzed as a dichotomous variable: resolved versus not resolved. Data on the proportion of participants requiring surgical incision and drainage after treatment, the proportion of participants developing a chalazion after treatment, the proportion of participants with recurrent hordeola, and the number of secondary hordeola will also be analyzed as dichotomous data. We will report dichotomous data as a summarized risk ratio with 95% confidence interval.
Continuous data
We will report continuous data as a mean difference with its standard deviation. We anticipate that available economic and quality of life data will be analyzed as continuous data.
Ordinal data
We will summarize ordinal data qualitatively.
Counts and rate data
We will summarize counts and rate data in rate ratios when the event is rare, and as continuous outcome data when the event is more common. We will analyze adverse events data as counts and rates.
Unit of analysis issues
The unit of analysis for this review will be an eyelid of an individual participant.
Dealing with missing data
We will contact authors of included studies in an attempt to obtain missing data. We will set the response time at six weeks and will document any communications with study authors. If data cannot be retrieved, we will not impute data and use only the reported data in the study. We will report loss to follow‐up when available.
Assessment of heterogeneity
We will test for statistical heterogeneity using the I² statistic and will examine the overlap of confidence intervals of individual trial effects on forest plots.
Assessment of reporting biases
We will use funnel plots to assess the possibility of reporting biases, if more than ten studies are available.
Data synthesis
If limited heterogeneity is suggested (defined here as I² < 50%), we will perform meta‐analyses using the random‐effects model, unless there are three or fewer trials, in which case, we will use the fixed‐effect model. If heterogeneity is detected, and sufficient data are available, we will combine trial results by relevant, less heterogeneous subgroups. Otherwise, we will describe the results individually.
Subgroup analysis and investigation of heterogeneity
We will investigate heterogeneity by conducting subgroup analyses, provided sufficient information is available. Subgroups of interest include sex, age, use of contact lenses, including soft lenses versus hard lenses, and the frequency of hordeola occurrences, co‐infections, and other comorbidities at baseline.
Sensitivity analysis
We will investigate the impact of studies with a high likelihood of bias, or with missing data, as well as the impact of unpublished studies, using sensitivity analyses.
Summary of findings
We will present a "Summary of findings" table to summarize the comparative effects between treatments for outcomes evaluated in the review. For each outcome, we will use the GRADE approach to assess the certainty of evidence (gradeworkinggroup.org/). The GRADE approach examines five criteria that may affect the certainty of effect estimates: risk of bias in individual trials, indirectness, heterogeneity, imprecision (wide confidence intervals), and publication bias. Two review authors will independently grade the body of evidence supporting each outcome of interest as very low, low, moderate, or high, and document reasons for downgrading as indicated. We will resolve any discrepancy by discussion.
Results
Description of studies
Results of the search
The electronic searches identified a total of 517 references, as of 21 June 2010, for the original publication of the review (Lindsley 2010). After the review authors screened the titles and abstracts, they classified 19 references as being potentially relevant. After reviewing the full text, they excluded all 19 references, which reported on 18 unique studies.
For the first update of this review, as of 26 July 2012, we identified 427 additional references through electronic searches (Lindsley 2013). After we screened the titles and abstracts, we classified six references as being potentially relevant. After reviewing the full text, we excluded all six references, which reported on five unique studies.
For this update (2017), we identified 901 additional references through electronic searches (Figure 1). We excluded 899 records after screening titles and abstracts, and two records after reviewing the full‐text report (NCT01763437; NCT02338648). We excluded an additional two studies identified by searching the Science Citation Index for references that cited potentially eligible studies (Gordon 1970; Laibson 1981). No trial was included in this review.
1.
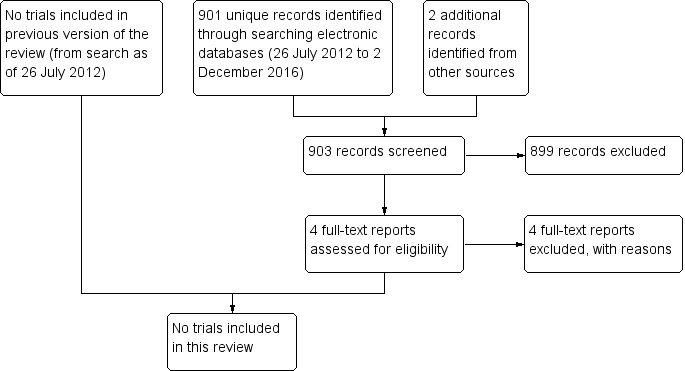
Study flow diagram.
Excluded studies
Overall, we excluded 27 studies from this review. The reasons for exclusion are described in the 'Characteristics of excluded studies' table.
Of the 27 excluded studies, six were randomized controlled trials (RCTs) that included patients with acute internal hordeola. The first included pediatric participants with lid inflammation, and was conducted to evaluate the safety of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension (Zylet®) in the pediatric population (Comstock 2012). As safety was the primary focus of the trial, the study population comprised participants with varying ocular inflammatory conditions, and data were not collected by study investigators for specific conditions. The second study compared the effectiveness of a combined antibiotic ophthalmic solution with placebo after surgical incision and curettage, in participants with internal and external hordeola (Hirunwiwatkul 2005). All participants had been newly diagnosed and untreated before undergoing incision and curettage. A total of 14 participants were randomly assigned to each group, and results for participants with internal and external hordeola were not reported separately. The study authors concluded that there was no evidence that suggested differences in pain score, mass size, or duration of cure between groups. The remaining four RCTs evaluated complementary and alternative therapies, such as acupuncture and bloodletting (Chen 2000; Gao 2001; Takama 2006; Xu 2004), which were not within the scope of this review, but are assessed in a separate Cochrane review (Cheng 2014).
Risk of bias in included studies
No studies were included in this review, thus no 'Risk of bias' assessment was done.
Effects of interventions
No studies were included in this review, thus no effects of interventions were reported.
Discussion
Summary of main results
No trials were identified for this review.
Overall completeness and applicability of evidence
Most of the references identified from the literature search for this review were related to external hordeola (styes) or chalazia. By and large, the few references specific to acute internal hordeola either reported recommendations for treatment without cited evidence, or were reports of interventional case series, case studies, or other types of observational study designs. The only clinical trials we found that included participants with acute internal hordeola were not eligible for the review because they included multiple conditions and did not stratify by specific diagnoses, they included participants who underwent surgical treatment as a criterion for study enrolment, or they evaluated treatments that were not within the scope of this review. Furthermore, the bulk of the literature was published more than 20 years ago.
Potential biases in the review process
We had anticipated that the primary source of bias for this review would be selection bias, specifically, the identification and inclusion of relevant studies. Before beginning the review process, we expected that few trials had been published on hordeola, that various authors used different terminologies when referring to different classifications of hordeola (i.e. hordeolum, stye, chalazion, etc.), and that relevant studies may be found in older publications. Therefore, we designed a broad search strategy for the electronic databases, to facilitate the identification of potentially relevant studies. We also manually searched the reference lists of potentially relevant studies, to identify older studies that may not be included in electronic databases.
To minimize bias during the process of selecting studies for this review, two review authors screened the references from the electronic search and independently classified them for inclusion or exclusion. We included potentially relevant references that mentioned any type of hordeolum or external eye inflammation for assessment at the full‐text level. Inclusion and exclusion were determined by using the definition of the disease given in the full‐text article. Furthermore, one review author screening the studies had a clinical background (JN), and one had a methodological background (KL).
Agreements and disagreements with other studies or reviews
Although it is the most commonly recommended therapy for hordeola, the application of warm compresses has not been shown to be effective in accelerating healing time or reducing symptoms associated with a hordeolum in a controlled trial. Moreover, there is no evidence that indicates that warm compresses alone would eliminate the infection. It is also unclear whether medical treatment or lid hygiene is effective in treating acute internal hordeola.
Authors' conclusions
Implications for practice.
Common interventions for the treatment of an acute internal hordeolum include warm compresses applied at home, over‐the‐counter topical medications and lid scrubs, prescribed antibiotics or steroids, and lid massages. At this time, there was insufficient evidence to support or refute the effectiveness of these non‐surgical interventions for treating an acute internal hordeolum. Clinical practice decisions should be based on physician judgment, and available treatment options should be discussed with patients.
Implications for research.
Generally, RCTs are considered the gold standard for comparing the efficacy of interventions. However, because of the relative mildness and short duration of the disease, study participants may be limited to more severe cases that are not representative of the general population; recruitment of participants at onset may be challenging. Even with these considerations, controlled clinical trials would be useful to determine which interventions are effective for the treatment of acute internal hordeola.
What's new
| Date | Event | Description |
|---|---|---|
| 2 December 2016 | New search has been performed | Issue 1 2017: The electronic searches were updated on 2 December 2016 |
| 2 December 2016 | New citation required but conclusions have not changed | Issue 1 2017: No new trials that met the inclusion criteria were identified |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 10 January 2013 | New citation required but conclusions have not changed | Updated searches yielded no new trials. |
| 10 January 2013 | New search has been performed | The electronic searches were amended to include broader terms for hordeolum. |
Acknowledgements
We thank Iris Gordon and Lori Rosman for devising and implementing the electronic search strategy for the review. We thank the Cochrane Eyes and Vision editorial team for assisting with preparation of the protocol, review, and updates to the review. We also thank Barbara Hawkins, Karen Blackhall, Daniel Ezra, and John Bladen for their comments; Takeshi Iwase and Sueko Matsumura for their assistance with evaluating Japanese‐language articles; and Tsung Yu and Xue Wang for their assistance with evaluating Chinese‐language articles. We especially thank Nancy Fitton for her work on writing the Plain Language Summary, and Sarah Money for editing the Plain Language Summary.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Hordeolum explode all trees #2 (Hordeol* or stye or styes) #3 MeSH descriptor Eye explode all trees #4 (sty) #5 (#3 AND #4) #6 MeSH descriptor Meibomian Glands explode all trees #7 (Meibomian* adj3 (gland* or cyst* or infection* or inflammat*)) #8 (tarsal adj3 (gland* or cyst* or infection* or inflammat*)) #9 (palpebral adj3 (gland* or cyst* or infection* or inflammat*)) #10 (conjunctiv* adj3 (gland* or cyst*)) #11 (gland* adj5 (zeis* or Moll*)) #12 (lid* or eyelid* or "eye margin") adj3 inflammat* #13 (lid* or eyelid* or "eye margin") adj3 infection* #14 (#1 OR #2 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp hordeolum/ 13. (Hordeol* or stye or styes).tw. 14. sty.tw. 15. exp eyes/ 16. 14 and 15 17. exp meibomian glands/ 18. (Meibomian* adj3 (gland* or cyst* or inflammat* or infection*)).tw. 19. (tarsal adj3 (gland* or cyst* or inflammat* or infection*)).tw. 20. (palpebral adj3 (gland* or cyst* or inflammat* or infection*)).tw. 21. (conjunctiv* adj3 (gland* or cyst*)).tw. 22. (gland* adj5 (zeis* or Moll*)).tw. 23. ((lid* or eyelid* or eye margin) adj3 inflammat*).tw. 24. ((lid* or eyelid* or eye margin) adj3 infection*).tw. 25. 12 or 13 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26. 11 and 25
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'hordeolum'/exp #34 hordeol*:ab,ti OR stye:ab,ti OR styes:ab,ti #35 sty:ab,ti #36 'eye'/exp #37 #35 AND #36 #38 'meibomian gland'/exp #39 (meibomian* NEAR/3 (gland* OR cyst* OR inflammat* OR infection*)):ab,ti #40 (tarsal NEAR/3 (gland* OR cyst* OR inflammat* OR infection*)):ab,ti #41 (palpebral NEAR/3 (gland* OR cyst* OR inflammat* OR infection*)):ab,ti #42 (conjunctiv* NEAR/3 (gland* OR cyst*)):ab,ti #43 (gland* NEAR/5 (zeis* OR moll*)):ab,ti #44 ((lid* OR eyelid* OR 'eye margin') NEAR/3 inflammat*):ab,ti #45 ((lid* OR eyelid* OR 'eye margin') NEAR/3 infection*):ab,ti #46 #33 OR #34 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 #47 #32 AND #46
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (Hordeol*[tw] OR stye[tw] OR styes[tw]) NOT Medline[sb] 3. (Meibomian*[tw] AND (gland*[tw] OR cyst*[tw] OR infection*[tw] OR inflammat*[tw])) NOT Medline[sb] 4. (tarsal[tw] AND (gland*[tw] OR cyst*[tw] OR infection*[tw] OR inflammat*[tw])) NOT Medline[sb] 5. (palpebral[tw] AND (gland*[tw] OR cyst*[tw] OR infection*[tw] OR inflammat*[tw])) NOT Medline[sb] 6. (conjunctiv*[tw] AND (gland*[tw] OR cyst*[tw])) NOT medline[sb] 7. (gland*[tw] AND (zeis*[tw] OR Moll*[tw])) NOT Medline[sb] 8. ((lid*[tw] OR eyelid*[tw] OR "eye margin"[tw]) AND inflammat*[tw]) NOT Medline[sb] 9. ((lid*[tw] OR eyelid*[tw] OR "eye margin"[tw]) AND infection*[tw]) NOT Medline[sb] 10. (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) 11. #1 AND #10
Appendix 5. LILACS search strategy
MH:C01.252.354.400$ OR MH:C01.539.375.354.400$ OR MH:C11.294.354.400$ OR MH:C11.338.648$ OR Hordeol$ OR stye OR styes OR sty OR MH:A09.371.337.614$ OR MH:A10.336.827.600$ OR Meibomian$ OR (Tarsal gland$) OR (tarsal cyst$) OR (tarsal inflammat$) OR (tarsal infection$) OR (palpebral gland$) OR (palpebral cyst$) OR (palpebral inflammat$) OR (palpebral infection$) OR (conjunctiv$ AND gland$) OR (conjunctiv$ AND cyst$) OR (zeis$ AND gland$) OR (moll$ AND gland$) OR (Eyelid$ AND inflammat$) OR (Eyelid$ AND infection$)
Appendix 6. metaRegister of Controlled Trials search strategy
Hordeolum OR hordeola OR stye OR styes OR sty OR Meibomian OR Eyelid inflammation
Appendix 7. ClinicalTrials.gov search strategy
Hordeolum OR Meibomian OR Eyelid inflammation OR Tarsal gland OR tarsal cyst OR tarsal inflammation or tarsal infection OR palpebral gland OR palpebral cyst OR palpebral inflammation OR palpebral infection OR conjunctiva gland OR conjunctiva cyst
Appendix 8. ICTRP search strategy
Hordeolum OR hordeola OR stye OR styes OR Meibomian OR Eyelid inflammation OR Tarsal gland$ OR tarsal cyst$ OR tarsal inflammation or tarsal infection$ OR palpebral gland$ OR palpebral cyst$ OR palpebral inflammation OR palpebral infection$ OR conjunctiva gland$ OR conjunctiva cyst$
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bahgat 1986 | Population not eligible: CCT of participants with chalazia, defined as chronic lipogranulomas with duration of one month to three years before therapy. Participants were stratified by type of chalazia and were assigned to injections of corticosteroids with or without antibiotics, or to control. |
| Chen 2000 | Intervention not eligible: RCT of participants with internal and external hordeolum randomly assigned to bloodletting of the ear or no treatment. |
| Comstock 2012 | Population not eligible: RCT of pediatric participants with lid inflammation to evaluate the safety and efficacy of Zylet® compared with vehicle. As the focus of the trial was primarily safety of Zylet® in pediatric patients, eligibility criteria were not strict and subgroup data for specific diagnoses were not collected. |
| Copeman 1958 | Population not eligible: CCT of participants with recurrent external hordeola, defined as at least one previous stye of the eyelash follicle within the last month. Patients alternately assigned to antibiotic ointment or control applied to the anterior nares. |
| Gao 2001 | Intervention not eligible: RCT of participants with hordeolum randomly assigned to wrist‐ankle acupuncture or oral ofloxacin plus topical chloramphenicol. |
| Garrett 1988 | Population not eligible: RCT of participants with chalazia, defined as chronic inflammation of the meibomian glands. Participants randomly assigned to warm compresses and lid scrubs, intralesional steroid injections, or both treatments. |
| Gordon 1970 | Population not eligible: RCT of participants with acute, subacute, or chronic external eye infections. Participants randomly assigned to treatment with topical gentamicin or topical combination of neomycin, bacitracin, and polymyxin. Of the 104 participants studied, none were diagnosed with hordeolum. |
| Hatano 1969 | Population not eligible: interventional case series of participants with ophthalmic infection. Of the 28 participants studied, 10 were diagnosed with hordeolum, but internal and external cases were not specified. All participants received topical minocycline. |
| Hatano 1974 | Population not eligible: interventional case series of participants with ophthalmic infection. Of the 40 participants studied, 10 were diagnosed with hordeolum, but internal and external cases were not specified. Four of these participants underwent surgery at the time of first consult, and six received the antibiotic amikacin. |
| Hirunwiwatkul 2005 | Intervention not eligible: RCT of participants with internal and external hordeolum after undergoing surgical incision and curettage. Participants randomly assigned to treatment with antibiotic ophthalmic solution or placebo after surgery. |
| Jacobs 1984 | Population not eligible: RCT of participants with noninfectious chalazia present for two or more weeks. Participants randomly assigned to injection with triamcinolone or incision and curettage. |
| Kastl 1987 | Population not eligible: RCT of participants with active eyelid infection (styes and blepharitis) determined by cultures taken from the base of the eyelashes. Participants randomly assigned to treatment with yellow mercuric oxide or placebo. |
| Laibson 1981 | Population not eligible: RCT of participants with acute ocular infections. Participants randomly assigned to tobramycin ophthalmic solution or gentamicin ophthalmic solution. Of the 68 participants studied, none were diagnosed with hordeolum. |
| Magnuson 1967 | Population not eligible: interventional case series of participants with various eye infections, with most having conjunctivitis, meibomianitis, or blepharitis. Of the 131 participants studied, as many as three may have had a stye. All participants received gentamicin sulfate and hot and cold compresses. |
| Manabe 1981 | Population not eligible: CCT of participants with infection of the anterior portion of the eye. Participants were assigned by order of enrolment to treatment with tobramycin ophthalmic solution or gentamicin ophthalmic solution. Of the 504 participants studied, 43 were diagnosed with hordeolum, but internal and external cases were not specified. Data were not reported separately for hordeolum cases. |
| Mathew 1966 | Population not eligible: interventional case series of participants with external hordeola, defined as acute inflammation of the glands situated at the root of eye lashes. All participants received penicillin and streptomycin plus a polyvalent antigen (Munomycin). |
| NCT01763437 | Population not eligible: RCT of participants with chalazia of longer than one week duration. Participants assigned to injection with tetracycline or observation. |
| NCT02338648 | Population not eligible: RCT of participants with chalazia, defined as visible single granulomas in the upper or lower eyelid. Participants assigned to an investigation transdermal patch or placebo. |
| Oishi 1973 | Not a controlled trial: interventional case series of 26 children with ophthalmic infection, three of whom had internal hordeolum. All participants received topical clindamycin‐2‐palmitate. |
| Panda 1987 | Population not eligible: CCT of participants with chalazia, defined as chronic inflammatory granulomas. Participants assigned to injection with one of three corticosteroids: dexamethasone, hydrocortisone, or triamcinolone. |
| Sawae 1971 | Population not eligible: interventional case series of participants with ophthalmic infection. Of the 22 participants studied, one was diagnosed with hordeolum, but it was not specified as being internal or external. All patients received clindamycin. |
| Takama 2006 | Intervention not eligible: RCT of participants with hordeolum randomly assigned to receive Chinese herbal medicine or no Chinese herbal medicine supplementary to topical ofloxacin and fluorometholone. |
| Vácha 1987 | Population not eligible: observational study of participants with chalazia, defined as granulomas. Treatment with kenalog injections was compared with incision and curettage. |
| Wang 1983 | Population not eligible: CCT of participants with chalazia, defined as tarsal cyst. Participants assigned to local injection of one of two corticosteroids. |
| Watson 1984 | Population not eligible: CCT of participants with chalazia, defined as chronic granulomas. Participants alternately assigned to injection with triamcinolone acetonide or incision and curettage. |
| Willcox 2008 | Population not eligible: case report of a participant treated with malian ointment for a stye. |
| Xu 2004 | Intervention not eligible: RCT of participants with hordeolum randomly assigned to acupuncture plus bloodletting of the ear or sham therapy. |
CCT: controlled clinical trial RCT: randomized clinical trial
Differences between protocol and review
We added methods related to the production of a 'Summary of findings' table, and assessment of the certainty of the body of evidence, using the GRADE approach. These methods were not included in the protocol or previous versions of this review.
Contributions of authors
Conceiving the review: KD, KL Designing the review: KL, JJN Co‐ordinating the review: KL
Data collection for the review:
Designing and undertaking electronic search strategies: Iris Gordon and Lori Rosman
Screening search results: KL, JJN
Organizing retrieval of papers: KL
Screening retrieved papers against inclusion criteria: KL, JJN
Appraising risk of bias of papers: KL, JJN
Extracting data from papers: KL, JJN
Writing to authors of papers for additional information: KL
Providing additional data about papers: KL, JJN
Obtaining and screening data on unpublished studies: KL, JJN
Data management for the review:
Entering data into RevMan: KL, JJN
Analysis of data: KL, JJN, KD
Interpretation of data:
Providing a methodological perspective: KL, KD
Providing a clinical perspective: JJN
Providing a policy perspective: JJN
Writing the review: KL, JJN, KD Providing general advice on the review: KL, JJN, KD Securing funding for the review: KD
Updating the review:
KL reviewed search results, extracted data, and wrote the review
JJN and KD revised and edited the review
We acknowledge Sueko Matsumura and Chris Khanoyan for assistance in screening search results for the update.
Sources of support
Internal sources
No sources of support supplied
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV editorial base in London.
The views expressed in this publication are those of the review authors and not necessarily those of the NIHR, the NHS or the Department of Health.
Declarations of interest
KL: none known. JJN: none known. KD: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Bahgat 1986 {published data only}
- Bahgat MM. Comparative study of local injection therapy of chalazia. Orbit 1986;5(3):219‐22. [Google Scholar]
Chen 2000 {published data only}
- Chen H. Effective observation on 111 cases of hordeolum treated by bloodleting in the point of ear. Journal of Guiyang College of Traditional Chinese Medicine 2000;22(1):29. [Google Scholar]
Comstock 2012 {published and unpublished data}
- Comstock TL, Paterno MR, Bateman KM, Decory HH, Gearinger M. Safety and tolerability of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension in pediatric subjects. Paediatric Drugs 2012;14(2):119‐30. [DOI] [PubMed] [Google Scholar]
Copeman 1958 {published data only}
- Copeman PW. Treatment of recurrent styes. Lancet 1958;2(7049):728‐9. [DOI] [PubMed] [Google Scholar]
Gao 2001 {published data only}
- Gao Q. Treatment of hordeolum by wrist‐ankle acupuncture. Shanghai Journal of Acupuncture and Moxibustion 2001;20(5):19. [Google Scholar]
Garrett 1988 {published data only}
- Garrett GW, Gillespie ME, Mannix BC. Adrenocorticosteroid injection vs. conservative therapy in the treatment of chalazia. Annals of Ophthalmology 1988;20(5):196‐8. [PubMed] [Google Scholar]
Gordon 1970 {published data only}
- Gordon DM. Gentamicin sulfate in external eye infections. American Journal of Ophthalmology 1970;69(2):300‐6. [DOI] [PubMed] [Google Scholar]
Hatano 1969 {published data only}
- Hatano H, Saito T, Takahashi N. Ophthalmological application of minocycline. Japanese Journal of Antibiotics 1969;22(6):522‐5. [PubMed] [Google Scholar]
Hatano 1974 {published data only}
- Hatano H, Tokuda H, Kayaba C. Evaluation of amikacin (BB‐K8) in ophthalmological field. Japanese Journal of Antibiotics 1974;27(4):451‐5. [PubMed] [Google Scholar]
Hirunwiwatkul 2005 {published and unpublished data}
- Hirunwiwatkul P, Wachirasereechai K. Effectiveness of combined antibiotic ophthalmic solution in the treatment of hordeolum after incision and curettage: a randomized, placebo‐controlled trial: a pilot study. Journal of the Medical Association of Thailand 2005;88(5):647‐50. [PubMed] [Google Scholar]
Jacobs 1984 {published data only}
- Jacobs PM, Thaller VT, Wong D. Intralesional corticosteroid therapy of chalazia: a comparison with incision and curettage. British Journal of Ophthalmology 1984;68(11):836‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kastl 1987 {published data only}
- Kastl PR, Ali Z, Mather F. Placebo‐controlled, double‐blind evaluation of the efficacy and safety of yellow mercuric oxide in suppression of eyelid infections. Annals of Ophthalmology 1987;19(10):376‐9. [PubMed] [Google Scholar]
Laibson 1981 {published data only}
- Laibson P, Michaud R, Smolin G, Okumoto M, Rosenthal A, Cagle G. A clinical comparison of tobramycin and gentamicin sulfate in the treatment of ocular infections. American Journal of Ophthalmology 1981;92(6):836‐41. [DOI] [PubMed] [Google Scholar]
Magnuson 1967 {published data only}
- Magnuson RH, Suie T. Gentamicin sulfate in external eye infections. JAMA 1967;199(6):427‐8. [PubMed] [Google Scholar]
Manabe 1981 {published data only}
- Manabe R, Moriyama H, Suda T. A double‐blind study of tobramycin eye drops on infectious diseases of anterior portion of the eye. Comparison with gentamicin eye drops. Folia Ophthalmologica Japonica 1981;32(4):1041‐65. [Google Scholar]
Mathew 1966 {published data only}
- Mathew M. Munomycin in hordeolum externum. Indian Practitioner 1966;19(10):689‐90. [PubMed] [Google Scholar]
NCT01763437 {published data only}
- NCT01763437. Intralesional tetracycline injection in the treatment of chalazia (TET). clinicaltrials.gov/ct2/show/NCT01763437 (accessed 9 December 2016).
NCT02338648 {published data only}
- NCT02338648. Safety and efficacy study of SUN 131 TDS as compared to placebo TDS in adult patients with a chalazion. clinicaltrials.gov/ct2/show/NCT02338648 (accessed 9 December 2016).
Oishi 1973 {published data only}
- Oishi M, Imai M, Takahashi T, Motoyama M, Tanaka M. Clinical evaluation of clindamycin‐2‐palmitate for ophthalmic infections of children. Japanese Journal of Antibiotics 1973;26(6):535‐9. [PubMed] [Google Scholar]
Panda 1987 {published data only}
- Panda A, Angra SK. Intralesional corticosteroid therapy of chalazia. Indian Journal of Ophthalmology 1987;35(4):183‐5. [PubMed] [Google Scholar]
Sawae 1971 {published data only}
- Sawae Y, Inoue H, Shiraishi M, Fujimura T, Takemori K. Laboratory and clinical evaluation of clindamycin. Japanese Journal of Antibiotics 1971;24(2):51‐8. [PubMed] [Google Scholar]
Takama 2006 {published data only}
- Takama N, Fujiwara T. The efficacy of hainou‐san‐kyu‐to for internal hordeolum. Ganka Rinsho Iho (Japanese Review of Clinical Ophthalmology) 2006;100:9‐11. [Google Scholar]
Vácha 1987 {published data only}
- Vácha J, Bodnár M. Kenalog injection-one possibility in the treatment of chronic chalazion. Ceskoslovenska Oftalmologie 1987;43(5):374‐6. [PubMed] [Google Scholar]
Wang 1983 {published data only}
- Wang TM. Treatment of tarsal cyst by local injection of cortisone. Zhonghua Yan Ke Za Zhi (Chinese Journal of Ophthalmology) 1983;19(3):168‐9. [PubMed] [Google Scholar]
Watson 1984 {published data only}
- Watson AP, Austin DJ. Treatment of chalazions with injection of a steroid suspension. British Journal of Ophthalmology 1984;68(11):833‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willcox 2008 {published data only}
- Willcox M, Bengaly T, Lopez V, Falquet J, Lambert B, Diallo D. Traditional malian ointment for styes. Journal of Alternative and Complementary Medicine 2008;14(5):461‐4. [DOI] [PubMed] [Google Scholar]
Xu 2004 {published data only}
- Xu W. Clinical observation on 67 cases of hordeolum treated by acupuncture and blood letting on ear. Hunan Guiding Journal of Traditional Chinese Medicine 2004;10(5):47. [Google Scholar]
Additional references
Avisar 1991
- Avisar R, Savir H, Deutsch D, Teller J. Effect of I‐Scrub on signs and symptoms of chronic blepharitis. DICP: the Annals of Pharmacotherapy 1991;25(4):359‐60. [DOI] [PubMed] [Google Scholar]
Barza 1983
- Barza M, Baum J. Ocular infections. Medical Clinics of North America 1983;67(1):131‐52. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng K, Wang X, Guo M, Wieland LS, Shen X, Lao L. Acupuncture for acute hordeolum. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD011075] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jesus 2004
- Jesus JM. Treating hordeola with systemic medications: first‐line therapy with oral antibiotics for the treatment of hordeola may provide a more permanent resolution. Pacific Optometry. www.opt.pacificu.edu/test/journal/Articles/deJesus%20hordeola/hordeola.html (accessed 4 June 2008).
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Diegel 1986
- Diegel JT. Eyelid problems. Blepharitis, hordeola, and chalazia. Postgraduate Medicine 1986;80(2):271‐2. [DOI] [PubMed] [Google Scholar]
Driver 1996
- Driver PJ, Lemp MA. Meibomian gland dysfunction. Survey of Ophthalmology 1996;40(5):343‐67. [DOI] [PubMed] [Google Scholar]
Fraunfelder 1971
- Fraunfelder FT, Roy FH. How to treat common external eye problems. American Family Physician 1971;3(4):104‐9. [PubMed] [Google Scholar]
Fuchs 1911
- Fuchs E. Textbook of Ophthalmology. JB Lippincott Company, 1911. [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hudson 1981
- Hudson RL. Treatment of styes and meibomian cysts. Practical procedures. Australian Family Physician 1981;10(9):714‐5. [PubMed] [Google Scholar]
King 1986
- King RA, Ellis PP. Treatment of chalazia with corticosteroid injections. Ophthalmic Surgery 1986;17(6):351‐3. [PubMed] [Google Scholar]
Lebensohn 1950
- Lebensohn JE. Treatment of hordeola. Postgraduate Medicine 1950;7(2):133. [PubMed] [Google Scholar]
Lederman 1999
- Lederman C, Miller M. Hordeola and chalazia. Pediatrics in Review 1999;20(8):283‐4. [DOI] [PubMed] [Google Scholar]
McCulley 1984
- McCulley JP. Blepharoconjunctivitis. International Ophthalmology Clinics 1984;24(2):65‐77. [DOI] [PubMed] [Google Scholar]
Moriarty 1982
- Moriarty PA, Collin JR. Eyelid problems. Practitioner 1982;226(1367):901‐23. [PubMed] [Google Scholar]
Mueller 2008
- Mueller JB, McStay CM. Ocular infection and inflammation. Emergency Medicine Clinics of North America 2008;26(1):57‐72. [DOI] [PubMed] [Google Scholar]
Olson 1991
- Olson MD. The common stye. Journal of School Health 1991;61(2):95‐7. [DOI] [PubMed] [Google Scholar]
Palva 1983
- Palva J, Pohjanpelto PEJ. Intralesional corticosteroid injection for the treatment of chalazia. Acta Ophthalmologica 1983;61(5):933‐7. [DOI] [PubMed] [Google Scholar]
Panicharoen 2011
- Panicharoen C, Hirunwiwatkul P. Current pattern treatment of hordeolum by ophthalmologists in Thailand. Journal of the Medical Association of Thailand 2011;94(6):721‐4. [PubMed] [Google Scholar]
Peralejo 2008
- Peralejo B, Beltrani V, Bielory L. Dermatologic and allergic conditions of the eyelid. Immunology and Allergy Clinics of North America 2008;28(1):137‐68. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roodyn 1954
- Roodyn L. Staphylococcal infections in general practice. British Medical Journal 1954;2(4900):1322‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 1995
- Rubin S, Hallagan L. Lids, lacrimals, and lashes. Emergency Medicine Clinics of North America 1995;13(3):631‐48. [PubMed] [Google Scholar]
Scobee 1942
- Scobee RG. The role of the meibomian glands in recurrent conjunctivitis. American Journal of Ophthalmology 1942;25:184‐92. [Google Scholar]
Sethuraman 2009
- Sethuraman U, Kamat D. The red eye: evaluation and management. Clinical Pediatrics 2009;48(6):588‐600. [DOI] [PubMed] [Google Scholar]
Skorin 2002
- Skorin L. Hordeolum and chalazion treatment: the full gamut. Optometry Today 2002, issue June 28:25‐7.
Wald 2004
- Wald ER. Periorbital and orbital infections. Infectious Disease Clinics of North America 2004;25(9):312‐20. [DOI] [PubMed] [Google Scholar]
Wilkie 1956
- Wilkie JL. Styes. Practitioner 1956;176(1053):318‐21. [PubMed] [Google Scholar]
References to other published versions of this review
Lindsley 2010
- Lindsley K, Nichols JJ, Dickersin K. Interventions for acute internal hordeolum. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsley 2013
- Lindsley K, Nichols JJ, Dickersin K. Interventions for acute internal hordeolum. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007742.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


