Abstract
Subcortical hyperintensities (SH) on neuroimaging are a prominent feature of vascular dementia (VaD) and SH severity correlates with cognitive impairment in this population. Previous studies demonstrated that SH burden accounts for a degree of the cognitive burden among VaD patients, although it remains unclear if individual factors such as cognitive reserve influence cognitive status in VaD. To address this issue, we examined 36 individuals diagnosed with probable VaD (age = 77.56; education = 12). All individuals underwent MMSE evaluations and MRI brain scans. We predicted that individuals with higher educational attainment would exhibit less cognitive difficulty despite similar levels of SH volume, compared to individuals with less educational attainment. A regression analysis revealed that greater SH volume was associated with lower scores on the MMSE. Additionally, education moderated the relationship between SH volume and MMSE score, demonstrating that individuals with higher education had higher scores on the MMSE despite similar degrees of SH burden. These results suggest that educational attainment buffers the deleterious effects of SH burden on cognitive status among VaD patients.
Keywords: Dementia, Neuroimaging, Epidemiology, Aging, Ischemia, Cerebrovascular disorders
INTRODUCTION
Vascular dementia (VaD) refers to cognitive impairment secondary to cerebral vascular disease that is of sufficient severity to impact activities of daily living. VaD is believed by some to represent the second most prevalent type of dementia in the United States after Alzheimer’s disease (AD; Roman, Erkinjuntti, Wallin, Pantoni, & Chui, 2002). The prevalence of VaD has been the subject of debate, particularly in terms of the independence of VaD from AD (Di Iorio, Zito, Lupinetti, & Abate, 1999; Jellinger, 2003; Jellinger & Attems, 2003; Wallin, 1998). However, many studies have reported that these diseases are characterized by unique neuropsychological profiles. VaD patients normally have a more intact recognition memory system, and greatly impaired executive functions, compared to AD patients (Graham, Emery, & Hodges, 2004; Kertesz & Clydesdale, 1994; Tierney et al., 2001; Traykov et al., 2005).
The expression of VaD is highly variable and dependent upon lesion size and location. The most commonly affected areas are the subcortical regions of the gray and white matter, likely due to the vulnerability of the small anastamosing vessels that perfuse these regions and are compromised by aging. As noted above, common cognitive deficits associated with subcortical VaD are slowed processing speed, memory impairment, executive dysfunction, and impaired language fluency (Jokinen et al., 2006; Paul, Garrett, & Cohen, 2003), although heterogeneity is the rule as the cognitive profile will depend on the affected underlying neural circuits.
Subcortical hyperintensities (SH), a hallmark feature of subcortical VaD, are believed to represent necrotic or inflamed tissue due to small subcortical strokes visualized as areas of bright white regions on T2 or fluid-attenuated inversion recovery (FLAIR) MRI (Fischer et al., 2007). The development of SH appears to be related to both vascular factors, such as hypertension, as well as degenerative factors, such as brain shrinkage, due to aging and disease (Fischer et al., 2007). Other possible causes have been suggested including blood–brain barrier failure and endothelial leakage (Warlaw, Sandercock, Dennis, & Starr, 2003). Several studies have demonstrated that SH in VaD populations are associated with impairment in processing speed and executive function (Bombois et al., 2007; Jokinen et al., 2005). Additionally, areas of SH in healthy, nondemented older individuals have been shown to be related to declines in processing speed (Nebes et al., 2006; Paul et al., 2005; Schmidt et al., 1993).
In other dementia populations such as AD, the level of cognitive impairment associated with neuropathological burden appears to be mitigated by higher levels of educational attainment. This level of discrepancy between neuropathological features of disease and conserved behavioral competence may be attributed to modifying factors that support brain function despite advanced disease. The concept of cognitive reserve (CR) has been proposed to explain the discrepancy between individuals with similar degrees of pathology, but with varying functional capacities (Stern, 2002). Several studies indicate that this discrepancy may be accounted for by individual life experiences, such as educational attainment (McDowell, Xi, Lindsay, & Teirney, 2007; Mortimer, Snowdon, & Markesbery, 2003; Wilson et al., 2009), IQ (Sole-Padulles et al., 2009), linguistic ability (Riley, Snowdon, Desrosiers, & Markesbery, 2005; Snowdon et al., 1996), active social lifestyles (Fratiglioni, Paillard-Borg, & Winblad, 2004), and leisure activities (Helzner, Scarmeas, Cosentino, Portet, & Stern, 2007; Scarmeas & Stern, 2003).
Individual differences in head circumference, brain volume, and synaptic density may account for the impact of CR on behavioral outcomes (Kesler, Adams, Blasey, & Bigler, 2003; Mortimer et al., 2003). Other models suggest that these effects are due to redundancy in the normal brain networks, providing more cognitive strength and flexibility after damage (Helzner et al., 2007; Riley et al., 2005; Scarmeas, Albert, Manly, & Stern, 2006; Stern et al., 2005).
The construct of CR has been most extensively studied in AD (McGurn, Deary, & Starr, 2008; Roselli et al., 2009; Stern, 2006; Wolf, Julin, Gertz, Winblad, & Wahlund, 2004). Other studies have identified evidence for the role of CR in traumatic brain injury (Kesler et al., 2003), Lewy body dementia (Del Ser, Hachinski, Merskey, & Munoz, 1999), and hepatitis C (Bieliauskas et al., 2007). Several studies have examined CR in the context of vascular disease among nondemented individuals. Results from these studies revealed that greater CR variables were associated with better cognitive outcome. A study by Dufouil, Alperovitch, and Tzourio (2003) revealed that individuals with greater education withstood greater vascular insult to the brain. Another study by Nebes et al. (2006) revealed that education provided protection against poor processing speed due to SH in normal aging.
To date, studies have not examined the impact of CR in the context of VaD, yet the issue is important in terms of identifying factors associated with disease status. The current study investigated the relationship between educational attainment, SH volume and global cognitive status in a sample of patients with VaD. The Mini-Mental State Exam (MMSE) is a brief measure of global cognitive function, and is used regularly as a cognitive screening tool by physicians. We hypothesized that individuals with greater educational attainment would perform better on the MMSE than individuals with less education, even if the higher educated individuals have greater amounts of SH damage.
METHODS
Participants
The study consisted of 36 participants (mean age = 77.56; SD = 5.71; mean education = 12.03; SD = 3.38, education range = 6–20 years; see Table 1) diagnosed with probable VaD. All participants were extracted from a larger cohort of individuals that were originally enrolled in a longitudinal late phase drug trial to treat VaD. Results of the trial revealed no significant drug-effects (Cohen, Browndyke, et al., 2003), and therefore, the treatment and placebo groups were collapsed for the present study. The current sample was extracted from the larger cohort based on the presence of complete baseline data.
Table 1.
Demographics
| Summary details of VaD sample (N = 36)
| |||
|---|---|---|---|
| Variable | Mean (SD) | Minimum | Maximum |
| Education (years) | 12.03 (3.38) | 6.00 | 20.00 |
| MMSE | 20.00 (4.23) | 9.00 | 24.00 |
| SH volumea | 0.04 (0.02) | 0.01 | 0.12 |
Note. VaD = vascular dementia; MMSE = Mini-Mental State Examination; SH = subcortical hyperintensities.
SH volume is calculated as a ratio using total subcortical and periventricular hyperintensity volumes (mm3) over whole brain volume (mm3).
All participants were diagnosed with probable VaD by consensus and met criteria for both the NINDS-AIREN and DSM-IV criteria. To establish these diagnoses, a thorough neuropsychological and neurological examination was performed that included a complete medical history from the patient and an additional informant, and structural neuroimaging scans. Due to this study being a pharmacological study in origin, rigorous efforts were made to identify individuals with probable pure VaD, rather than probable mixed dementia. To increase the reliability of the diagnoses, all VaD diagnoses were made by group consensus between experts in the field. All participants were between the ages of 67 and 88 years old and had a score below 24 on the MMSE. Exclusionary criteria included terminal systemic illnesses, other neurological disorders, major psychiatric disorders, and contraindications for MRI. Written informed consent was obtained from each of the participants. All aspects of the study protocol were approved by the local IRB, and all research was conducted in compliance with the Helsinki Declaration.
Procedures
All subjects underwent an MRI, and they were administered the MMSE as part of a larger neuropsychological battery. Education was used as a proxy measure of cognitive reserve. Education was quantified as a continuous variable using highest level of years of education completed. Previous studies have used education as a proxy measure of CR with robust results (Dufouil et al., 2003; McDowell et al., 2007; Nebes et al., 2006; Roselli et al., 2009).
MRI Acquisition
SH volume was determined by MRI using a 1.5 Tesla Siemens Magnatom Vision scanner within 1 week of neuropsychological assessment. FLAIR pulse sequences [repetition time (TR) = 6000 ms; echo time (TE) = 105 ms] were used to acquire 5-mm-thick axial slices with a 2-mm gap between slices. Field of view was 24 × 24 cm, with a matrix of 192 × 256 voxels. MedVision System software was used to download and reconstruct the neuroimaging data. SH quantification is described in more detail in previous studies (Cohen et al., 2002; Garrett et al., 2004). Briefly, SH volumes were quantified with a semi-automated thresholding routine performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Brain volume and SH volume were calculated using a semi-automatic thresholding technique that categorized bandwidths of light as white, black, or gray based on an intensity histogram that was generated for each individual. A threshold for each light peak was set manually to represent brain tissue, SH, and cerebrospinal fluid, and the threshold was then applied across all scan slices to render volume summations. Brain volume was calculated by summing all pixels classified as brain tissue (excluding space) and a ratio of SH was defined as the total SH value in subcortical and periventricular areas over whole brain volume. Calculated SH volumes were expressed as a ratio of total subcortical and periventricular hyperintensity over whole brain volume, excluding ventricular space. Intrarater reliability of the SH quantification exceeded .96.
Analyses
Moderated multiple regression was used to determine if the relationship between MMSE score and SH volume was dependent on level of education, an indication of CR. Two regressions were estimated, one we will call the “main effects” model and the other the “interaction” model. SH volume and level of education were mean centered before creation of an interaction term to facilitate interpretation and to reduce multicollinearity that would occur for the first order variables with the interaction term (Aiken & West, 1991). For the first model, MMSE was regressed onto the mean centered SH volume and the mean centered education level. The interaction term was then added to the regression as a moderator in a second model to determine if the relationship between SH volume and MMSE scores were dependent on level of education. Results of both models are presented below.
RESULTS
Education Moderates SH Volume and MMSE Score
The regression of MMSE onto mean centered SH volume and mean centered education was significant (F(2,33) = 5.77; p <.05) accounting for 26% of the variance in MMSE (see Table 2). Without accounting for the interaction, SH volume had an overall negative relationship with MMSE and education had an overall positive. However, these effects should only be interpreted with the following model which includes the interaction. The interaction accounted for an additional 13% (R2 = .39) of the variance (F(3,32) = 6.76; p <.05). The effect size is modest at f2 = .21. The most important point to note for this interaction model is the form of the interaction. Figure 1 shows the simple slopes for the relationship between MMSE and SH volume when education is high (defined as the mean + 1 standard deviation ~15.4 years) and when education is low (defined as the mean − 1 standard deviation ~7.6 years). These values are arbitrary and sample dependent, but this method of illustrating the effects is well-documented and accepted (Aiken & West, 1991; Cohen, Cohen, Aiken, & West, 2003). Figure 1 shows that the overall relationship of SH volume with MMSE scores when education is low is negative. That is, when individuals with lower education experience high SH volume, they are more inclined to experience lower MMSE. On the contrary, when individuals have higher education the relationship between SH volume and MMSE scores is attenuated. The overall pattern expressed in Figure 1 is consistent with a buffering hypothesis (Cohen, Cohen et al., 2003) whereby education buffers the relationship between SH volume and MMSE.
Table 2.
Moderation
| Summary of regression analysis for the moderating effects of education on SH and MMSE scores
| ||||
|---|---|---|---|---|
| Variable | B | SE | B | SE |
| Intercept | 20.03* | 0.63 | 20.33* | 0.59 |
| SH vol. | −61.23* | 26.25 | 0.075 | 33.85 |
| Education | 0.40* | 0.19 | 0.313 | 0.178 |
| SH* Education | 26.81* | 10.34 | ||
| R2 | 0.259 | 0.388 | ||
Note. N = 36. SH vol. is mean centered. Education is mean centered. SH*Education is the interaction of mean centered SH and mean centered Education. MMSE = Mini-Mental State Examination; SH = subcortical hyperintensities.
p < .05.
Fig. 1.
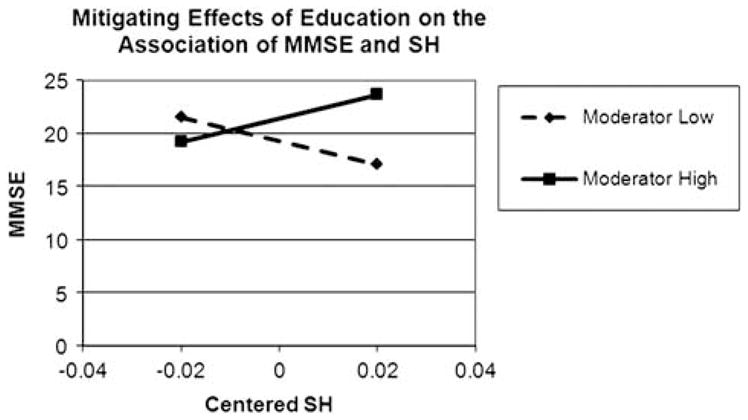
Moderator high represents individuals with 1 standard deviation above the mean in educational attainment. Moderator low represents individuals with 1 standard deviation below the mean in educational attainment. Individuals with a higher level of education perform better on the MMSE, despite greater amounts of SH. By contrast, individuals with lower levels of education perform worse on the MMSE with greater SH volumes. MMSE = Mini-Mental State Examination; SH = subcortical hyperintensities.
DISCUSSION
Our cross-sectional results suggest that education, a proxy measure of CR, provides protection against decline in cognitive function due to SH damage in the brain. To our knowledge, these are the first data that describe a relationship between CR and SH burden in a VaD cohort. Additionally, our results are consistent with previous studies indicating that increased SH volume has a negative outcome on global cognitive function in patients diagnosed with VaD.
The main hypothesis investigating whether the protection of CR extended to a VaD group was supported in this study. On a test of overall cognitive function, individuals with higher educational attainment, a proxy measure of CR, performed better than individuals with similar degrees of SH pathology. To the extent that educational attainment serves as a valid proxy for mental activity, our findings indicate that greater amounts of mental activity in young adulthood are beneficial later in life. These effects may provide a buffer against functional decline in the face of damage to the brain. This is important because it provides a means by which an individual can modify their risk factors for dementia. Furthermore, the results may provide information regarding their prognosis following clinical diagnosis.
A few studies have investigated the role of CR in populations with varying degrees of vascular burden. A study by McGurn et al. (2008) reported that individuals who scored higher on an early life test of premorbid ability were less likely to develop VaD later in life. Nebes et al. (2006) reported that white matter lesions in a healthy older population were related to deficits in processing speed. This relationship was further influenced by education level, but only in individuals with less than a college level education. Of interest is that the Nebes et al. (2006) study included individuals with greater than 12 years of education. Dufouil et al. (2003) also reported that education moderated the relationship between neuropsychological performance and SH burden among healthy older adults with SH. Our data suggest that the protective effect of education is evident among individuals with a range of educational attainment, and a clinical diagnosis of dementia.
While the exact mechanisms of CR are largely unknown several studies have revealed that greater brain volume and head circumference may provide a buffer, likely by supporting synaptic complexity (Kesler et al., 2003; Mortimer et al., 2003). However, this passive model, or brain reserve model, does not take into account individual variability in response to damage or compensation, which has led to the introduction of “active” models of reserve (Stern, 2002). These active models postulate that the systems involved in processing a task are more efficient among individuals with greater reserve, perhaps through redundancy, allowing an individual to better cope with damage (Stern, 2006, 2009). Our study provides further support for the active model of reserve. We observed that it may not be simple neuronal or synaptic density that accounts for the differences in cognitive ability, since some individuals with greater education may still have less brain volume. Instead, there appears to be supportive value to the greater amount of education that these individuals have received. While the above discussion is of interest, it is important to note that our study was not designed to determine the mechanisms associated with CR and therefore future work in this area is needed.
It should be noted that in the many VaD cases reviewed at autopsy, the pathology is of mixed origin and often shared with AD pathology. Significant efforts were undertaken in the parent pharmaceutical study to ensure that the sample included individuals with VaD rather than a mixed sample. Nevertheless, a measure of caution is warranted since it is not possible to determine the fidelity of the diagnosis in the absence of autopsy data. Additionally, since we believe this to be a pure VaD group, the current findings would only be applicable to individuals with pure VaD, and it is unknown if these findings can be generalized to a mixed pathology group.
A limitation of the current study is the small sample size used for the regression analyses. It is worth noting that in general, it is difficult to detect interactions in small sample sizes. Thus, given both the form and magnitude of this interaction, the sample size becomes less a concern. It is very unlikely that the effects determined here are spurious (see Evans, 1985). To address concerns about the stability of the results, we used a bootstrapping technique to provide further evidence of the interaction reported in the results (Davison & Hinkley, 1999; Efron & Tibshirani, 1993). By replicating the procedure using random sampling with replacement 999 times, we determined that the effect size of the interaction shows little bias (median f2 = .22; median R2 = .42). Another weakness in the current study is the use of the MMSE as an overall measure of cognitive function. We chose to use the MMSE because it is a highly used test in clinical practice, however it may not be ideal for a VaD sample due to the limited assessment of executive function using this scale. This is important as the executive domain is relatively more impaired than other domains in some forms of VaD (Boyle, Paul, Moser, & Cohen, 2004; Jokinen et al., 2006; Paul et al., 2003). Additionally, our study provides evidence that even with the limitations of the MMSE in a VaD population, the impact of CR on this test is evident and therefore impacts clinical performance. Relatedly, previous studies of healthy adults indicate that assessment of individual cognitive domains rather than global function may provide additional insight into the nature of the relationships between CR and neuropsychological performance (Kaplan et al., 2009).
Finally, previous studies have indicated that the effects of education on the vascular system and cognitive performance are not related to higher socioeconomic status or health advantages in more educated individuals (McDowell et al., 2007). Our study did not investigate whether the influence of education would still be significant when these other factors are taken into account. Future studies should focus on controlling the effects of these factors to investigate if the CR hypothesis remains valid in a VaD population.
The findings of this study suggest that individuals with a greater amount of educational attainment are able to cognitively withstand greater amounts of damage related to VaD than individuals with comparable damage and less education. There are several implications that can be derived from these findings. First, individuals should be encouraged to engage in mental activity to mitigate the neuropsychological compromise associated with vascular pathology. While education was the focus of the current study, education is believed to represent a proxy for focused mental activity. Second, our data may aid clinicians in determining with greater certainty the prognosis of patients diagnosed with VaD based on educational attainment.
Acknowledgments
This work was supported by a grant to RA Cohen from Indevus Pharmaceuticals.
Footnotes
There are no conflicts of interest for any of the authors of this study.
References
- Aiken LS, West SG. Multiple regression: Testing and interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Wright EC, Kronfol Z, Lok ASF The Halt-C Trial Group. Cognitive reserve and neuropsychological functioning in patients infected with Hepatitis C. Journal of the International Neuropsychological Society. 2007;13(4):687–692. doi: 10.1017/S1355617707070877. [DOI] [PubMed] [Google Scholar]
- Bombois S, Debette S, Delbeuck X, Bruandet A, Lepoittevin S, Delmaire C, Pasquier F. Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke. 2007;38:2595–2597. doi: 10.1161/STROKEAHA.107.486407. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Paul RH, Moser DJ, Cohen RA. Executive impairments predict functional declines in vascular dementia. The Clinical Neuropsychologist. 2004;18(1):75–82. doi: 10.1080/13854040490507172. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Hillsdale: Erlbaum; 2003. [Google Scholar]
- Cohen RA, Browndyke JN, Moser DJ, Paul RH, Gordon N, Sweet L. Long-term Citicoline (Cytidine Diphosphate Choline) use in patients with vascular dementia: Neuroimaging and neuropsychological outcomes. Cerebrovascular Diseases. 2003;16:199–204. doi: 10.1159/000071116. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul RH, Ott BR, Moser DJ, Zawacki TM, Stone W, Gordon N. The relationship of subcortical MRI hyperintensities and brain volume to cognitive function in vascular dementia. Journal of the International Neuropsychological Society. 2002;8:743–752. doi: 10.1017/s1355617702860027. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. London: Cambridge University Press; 1999. [Google Scholar]
- Del Ser T, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain. 1999;122:2309–2319. doi: 10.1093/brain/122.12.2309. [DOI] [PubMed] [Google Scholar]
- Di Iorio A, Zito M, Lupinetti M, Abate G. Are vascular factors involved in Alzheimer’s disease? Facts and theories. Aging Clinical and Experimental Research. 1999;11:345–352. doi: 10.1007/BF03339811. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Evans MG. A Monte-Carlo study of the effects of correlated method variance in moderated multiple regression analysis. Organizational Behavior and Human Decision Processes. 1985;36:305–323. [Google Scholar]
- Fischer P, Krampla W, Mostafaie N, Zehetmayer S, Rainer M, Jungworth S, Tragl KH. VITA study: White matter hyperintensities of vascular and degenerative origin in the elderly. Journal of Neural Transmission, Supplement. 2007;72:181–188. doi: 10.1007/978-3-211-73574-9_23. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Garrett KD, Cohen RA, Paul RH, Moser DJ, Malloy PF, Shah P, Haque O. Computer-mediated measurement and subjective ratings of white matter hyperintensities in vascular dementia: Relationships to neuropsychological performance. The Clinical Neuropsychologist. 2004;18(1):50–62. doi: 10.1080/13854040490507154. [DOI] [PubMed] [Google Scholar]
- Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Archives of Neurology. 2007;64(12):1749–1754. doi: 10.1001/archneur.64.12.1749. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Is Alzheimer’s disease a vascular disorder? Journal of Alzheimer’s Disease. 2003;5:247–250. doi: 10.3233/jad-2003-5308. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Incidence of cerebrovascular lesions in Alzheimer’s disease: A postmortem study. Acta Neuropathologica. 2003;105:14–17. doi: 10.1007/s00401-002-0634-5. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Mantyla R, Pohjasvaara T, Ylikoski R, Hietanen M, Erkinjuntti T. Cognitive profile of subcortical ischaemic vascular disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:28–33. doi: 10.1136/jnnp.2005.069120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Mantyla R, Ylikoski R, Heitanen M, Pohjasvaara T, Erkinjuntti T. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1229–1233. doi: 10.1136/jnnp.2004.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RF, Cohen RA, Muscufo N, Guttmann C, Chasman J, Buttaro M, Wolfson L. Demographic and biological influences on cognitive reserve. Journal of Clinical and Experimental Neuropsychology. 2009;31(7):868–876. doi: 10.1080/13803390802635174. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Clydesdale S. Neuropsychological deficits in vascular dementia vs Alzheimer’s disease: Frontal lobe deficits in vascular dementia. Archives of Neurology. 1994;51:1226–1231. doi: 10.1001/archneur.1994.00540240070018. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: An investigation of the cognitive reserve hypothesis. Applied Neuropsychology. 2003;10(3):153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- McDowell I, Xi G, Lindsay J, Teirney M. Mapping the connections between education and dementia. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: Findings from the Nun Study. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA, DeKosky ST. The relation of white matter hyperintensities to cognitive performance in the normal old: Education matters. Aging, Neuropsychology, and Cognition. 2006;13:326–340. doi: 10.1080/138255890969294. [DOI] [PubMed] [Google Scholar]
- Paul RH, Garrett K, Cohen R. Vascular dementia: A diagnostic conundrum for the clinical neuropsychologist. Applied Neuropsychology. 2003;10(3):129–136. doi: 10.1207/S15324826AN1003_01. [DOI] [PubMed] [Google Scholar]
- Paul RH, Haque O, Gunstad J, Tate DF, Grieve SM, Hoth K, Gordon E. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Archives of Clinical Neuropsychology. 2005;20:697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the Nun Study. Neurobiology of Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaeic vascular dementia. Lancet Neurology. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Roselli F, Tartaglione B, Federico F, Lepore V, Defazio G, Livrea P. Rate of MMSE score change in Alzheimer’s disease: Influence of education and vascular risk factors. Clinical Neurology and Neurosurgery. 2009;111:327–330. doi: 10.1016/j.clineuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Offenbacher H, Dusek T, Zach E, Grieshofer RP, Lechner H. Neuropsychological correlates of MRI white matter hyperintensities: A study of 150 normal volunteers. Neurology. 1993;43:2490–2494. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: Findings from the Nun Study. Journal of the American Medical Association. 1996;275(7):528–532. [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Molinuevo JL. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging. 2009;30:1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MC, Black SE, Szalat JP, Snow G, Fisher RH, Nadon G, Chui H. Recognition memory and verbal fluency differentiate probable Alzheimer’s disease from subcortical ischemic vascular dementia. Archives of Neurology. 2001;58:1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- Traykov L, Baudic S, Raoux N, Latour F, Rieu D, Smagghe A, Rigaud AS. Patterns of memory impairment and perseverative behavior discriminate early Alzheimer’s disease from subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229–230:75–79. doi: 10.1016/j.jns.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wallin A. The overlap between Alzheimer’s disease and vascular dementia: The role of white matter changes. Dementia and Geriatric Cognitive Disorders. 1998;9(Suppl 1):30–35. doi: 10.1159/000051187. [DOI] [PubMed] [Google Scholar]
- Warlaw JM, Sandercock PAG, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukaraiosis, and dementia? Stroke. 2003;34(3):806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Julin P, Gertz HJ, Winblad B, Wahlund LO. Intracranial volume in mild cognitive impairment, Alzheimer’s disease and vascular dementia: Evidence for brain reserve? International Journal of Geriatric Psychiatry. 2004;19:995–1007. doi: 10.1002/gps.1205. [DOI] [PubMed] [Google Scholar]


