Abstract
Conceptualizations of links between stress and cellular aging in childhood suggest that accumulating stress predicts shorter leukocyte telomere length (LTL). At the same time, several models suggest that emotional reactivity to stressors may play a key role in predicting cellular aging. Using intensive repeated measures, we tested whether exposure or emotional “reactivity” to conflict and warmth in the family were related to LTL. Children (N=39; 30 target children and 9 siblings) between 8 and 13 years of age completed daily diary questionnaires for 56 consecutive days assessing daily warmth and conflict in the marital and the parent-child dyad, and daily positive and negative mood. To assess exposure to conflict and warmth, diary scale scores were averaged over the 56 days. Mood “reactivity” was operationalized by using multilevel modeling to generate estimates of the slope of warmth or conflict scores (marital and parent-child, separately) predicting same-day mood for each individual child. After diary collection, a blood sample was collected to determine LTL. Among children aged 8-13 years, a stronger association between negative mood and marital conflict, suggesting greater negative mood reactivity to marital conflict, was related to shorter LTL (B = -1.51, p < .01). A stronger association between positive mood and marital affection, suggesting positive mood reactivity, was related to longer LTL (B = 1.15, p < .05). These effects were independent of exposure to family and marital conflict and warmth, and positive and negative mood over a two-month period. To our knowledge, these findings, although cross-sectional, represent the first evidence showing that links between children's affective responses and daily family interactions may have implications for telomere length.
Keywords: family, marital, conflict, warmth, telomere length, biological aging
1. Introduction
Harmony and disharmony within the home have consequences for children's health and well-being (see Repetti et al., 2002; Troxel and Matthews, 2004 for a review). Family conflict promotes children's emotional dysregulation (Davies and Cummings, 1994), risky health behaviors, and dysregulation of stress-responsive biological systems. On the other hand, family warmth and affection may have protective effects, including effective emotion regulation, adopting health-promoting behaviors (Repetti et al., 2002), and developing adaptive biological responses to stress (Bai and Repetti, 2015). In this paper, we examine associations between daily family environments and a marker of cellular aging, leukocyte telomere length (LTL), which may also explain links between family functioning and health.
Within cells, chromosomes (organized structures formed by our DNA) are capped at the end by telomeres, which shorten with each cell division; this process can be accelerated by factors that include oxidative damage to DNA (Blackburn, 2000; von Zglinicki, 2002). Once a critical telomere length is reached, the cell ceases dividing and enters replicative senescence, in which the cell has altered function including dysfunctional mitochondrial activity and increased production of proinflammatory signals (Campisi and d'Adda di Fagagna, 2007; Chou and Effros, 2013; Sahin et al., 2011). In humans, the telomere within the leukocyte pool and in other bodily tissues progressively shortens over the lifespan (Daniali et al., 2013), and epidemiological evidence shows that with age, the number of cells with shorter LTL increases (Calado and Young, 2009; Frenck et al., 1998). Shorter LTL is a hallmark indicator of cellular aging (López-Otín et al., 2013), and predicts risk for poor health outcomes in adults, including increased risk for cardiovascular disease and type 2 diabetes (D'Mello et al., 2015).
Several studies have reported that greater exposure to childhood adversity is related to shorter LTL in adulthood (reviewed in Shalev, 2012), and recent studies of telomere length in childhood have appeared in the literature. Thus far, in cross-sectional studies, shortened buccal cell telomere length (BTL; from cheek cells) in middle childhood and early adolescence appears related to exposure to major stressful life events including time spent in institutional care (Drury et al., 2012), family violence, suicide, or incarceration (Drury et al., 2014), and maternal depression (Gotlib et al., 2015). Greater cumulative exposure to home and school violence over five years predicted BTL shortening from age 5 to 10 (Shalev et al., 2013).
Thus far, much of the work on family environments and TL has focused on maltreatment and abuse. At the same time, children are also exposed to less “toxic” stressors (Shonkoff et al., 2012). Our interest was associations between moderate intensity stressors, such as everyday arguing and punishment, and cellular aging. Work in African-American adolescents suggests that possibility, as parent reports of high parent-child conflict and low parent-child warmth were related to shorter LTL at 5 year follow-up (Beach et al., 2014). In the same sample, children whose families participated in a family-based parenting skills intervention showed longer LTL compared to a control group (Brody et al., 2015). Thus, exposure to either less conflict or greater parent support may protect against cellular aging.
Another relevant approach to studying the impact of daily, moderate intensity stressful events within the family involves children's psychological responses to those events. Emotional security theory specifically proposes that greater exposure to marital conflict influences children's emotional reactivity to future conflict (Davies and Cummings, 1994; Davies and Martin, 2013). Notably, that model is consistent with frameworks that emphasize children's heightened emotional responses to stressors as a mechanism explaining how family environments influence health (Miller et al., 2011; Repetti et al., 2011; Troxel and Matthews, 2004). Similarly, the multisystem resiliency model of cellular aging suggests that emotional reactivity to stress has implications for LTL (Puterman and Epel, 2012), and is supported by one work showing associations between short LTL and greater threat appraisals during a laboratory stressor (O'Donovan et al., 2012). However, no other studies to our knowledge have examined associations between naturalistic assessments of children's emotional reactivity to stressors and LTL. Moreover, studies of reactivity to daily stressful events have primarily focused on adults (e.g., Cichy et al., 2012) and on negative emotional responses to daily stressors or family conflict.
Given that greater daily family support is related to greater positive mood (Weinstein et al., 2006), and more positive interactions with parents and peers are associated with greater positive mood (Flook, 2011), positive emotional responses to parental expressions of warmth or involvement may also have implications for cellular aging. Higher positive mood reactivity to family warmth and involvement may indicate the building of inter- and intrapersonal resources (Bai and Repetti, 2015), while low positive mood reactivity may indicate social withdrawal (Rubin et al., 2009). Unfortunately, positive mood reactivity has received little attention in terms of biobehavioral pathways related to health.
Purpose of the study
Most approaches to studying family functioning and children's health have employed “snapshot” single-occasion, retrospective assessments of inter-parental and parent-child relationships. Naturalistic methods, such as daily diary designs, allow for examining family relationships as they unfold over time and minimize retrospection bias (Repetti et al., 2015; Robles et al., 2013). Moreover, such designs allow for examining psychological mechanisms. With multiple days of parent-child interactions and mood reports, within-person associations between parent-child conflict and negative mood can be computed, which in previous work has been used as a proxy for emotional reactivity (e.g., Cichy et al., 2012)1. For example, children who show a stronger within-person association (steeper slope) between parent-child conflict and negative mood may have stronger emotional reactivity to conflict compared to children who show a weaker within-person association (flatter slope). This study examined associations between daily family environments, focused on child-reported parent-child and marital conflict and warmth, and LTL in children. We tested hypotheses stemming from two models of how mild to moderate stressors (family and marital conflict) may be related to children's LTL: an exposure model, where family conflict over a specific period of time may be related to shorter LTL; and a “reactivity” model2, where stronger associations between children's negative mood and family conflict may be related to shorter LTL. In addition, we examined potential associations between positive family functioning, including parental warmth and involvement as well as marital affection, and LTL. Experiencing greater family warmth may be related to longer LTL, or in a “reactivity” model, greater children's positive emotional responses to family warmth may be related to longer LTL.
2. Material and methods
2.1. Participants
Families with children between 8 and 13 years of age were recruited through schools, community centers, medical clinics, and direct mailings using a marketing list. At least one parent and one child in the target age range from each family were required for the family to participate, although both parents were encouraged to do so. All participants were screened for medical conditions known to confound endocrine and immune measures, including chronic lung conditions (e.g., asthma); endocrine disorders (e.g., Cushing's disease); metabolic disease; immunodeficiency; and heart disease or chronic heart conditions.
A total of 47 target children (19 boys, 28 girls, mean age = 11.3, SD = 1.5), and 12 siblings (5 boys, 7 girls, mean age = 10.0, SD = 1.5) from 47 families participated. Target children participated in all study procedures, including interviews, questionnaires, diaries, saliva sampling, and blood draws. Siblings were included if they were in the same age range as the target child, but were younger than the target child, and completed diaries, questionnaires, saliva sampling, and blood draws. Ethnicity was as follows: 21 white, 16 mixed ethnicity (e.g., non-Hispanic white/Native American, black/Puerto Rican, and non-Hispanic white/Filipino), 9 black, 7 Latino, and 6 Asian. Parents' median education was 13 years, and parents' median self-reported individual income fell within a $32,000-$82,000 bracket and ranged from below $8,725 to above $171,850.
Because the study procedures constituted significant participant burden, the blood draw was not required. Consequently, this paper focuses on the 39 children (30 target, 9 siblings) that provided blood draws and complete diary data (one child did not report marital conflict and affection on the diaries). Children who provided blood samples were marginally more ethnically diverse, χ2(4) = 8.14, p = .09, compared to children who did not provide samples (15 of mixed ethnicity vs. 1, 6 vs. 3 black, and 5 vs. 2 Latino). Children who provided blood samples did not significantly differ from children who did not provide blood samples in age, gender distribution, parental education, parent income, BMI, daily diary measures, or within-person family interaction-mood associations described below.
2.2. Procedures
After two home visits that involved interviews and training on daily diaries and saliva collection (full study details can be found at Robles et al., 2013), families began completing the 8-week daily diary online (SurveyMonkey.com) each evening prior to bedtime. Paper diaries were available in case of technical difficulties. After the 8-week daily diary, children provided a blood sample at UCLA (one provided a sample 2 days before the end of the 8 weeks, the remaining samples were collected between 1 to 85 days after the end of the diary period, Mdn = 15 days after). LTL was not associated with the number of days between the end of the diary and the blood draw. Each child could earn up to $300 for completing all study procedures, including a $5 bonus gift card per week of 100% diary compliance (i.e., all diaries were completed on the evening due or before 9 AM the next morning). Data collection took place from October to May in 2009 through 2012. The protocol was approved by UCLA's Institutional Review Board.
2.3. Measures
2.3.1. Child diary measures
The average child completed 51.8 diaries (range 24 – 58) with an average completion time of 4.41 ±3.14 min (Reynolds et al., under review). Reliability was estimated using a Generalizability Theory framework (Shrout and Lane, 2012). Reliability of between-person differences was .99 and above for all measures. Reliability of meaningful within-person day-to-day change is reported below as RC.
2.3.1.1. Parent-child interactions
Items were based on the Youth Everyday Social Interaction and Mood measure (Repetti, 1996). Child-reported parent-child conflict (three items: “My [Mom/Dad] got mad at me today,” “I was angry at my [Mom/Dad] today,” and “My [Mom/Dad] punished me today”) and warmth (three items: “I had fun with my [Mom/Dad] today,” “My [Mom/Dad] and I got along well today,” and “My [Mom/Dad] gave me love and attention today”) were rated on the following scale: 1 = not at all, 2 = some, 3 = a lot. Child reports of mother and father behavior were averaged each day, yielding a daily parent-child warmth (RC = .72) and conflict score (RC = .74).
2.3.1.2. Marital interactions
Items were based on the Child Home Data Questionnaire (Margolin, 1990). Child-reported marital conflict (two items: “My mom and dad seemed angry with each other today”, “My mom and dad argued today”) and affection (“My mom and dad kissed or hugged today”) were rated on the same three-point response scale described above. Items were averaged for each day, yielding a daily marital affection (RC not computed because of the single-item measure) and conflict score (RC = .90).
2.3.1.3. Daily mood
Child-reported negative (sad, mean, unhappy, tense, angry, worried) and positive mood (lively, happy, relaxed, full of energy, cheerful, calm, proud, loved) were adapted from previous research (Cohen et al., 2003). Items were rated on the following scale: 1 = not at all, 2 = some of the day, 3 = most of the day, 4 = all day, and averaged to create daily positive (RC = .81) and negative mood scores (RC = .72).
2.3.2. Leukocyte telomere length
At the beginning of the final visit (occurring between noon and 7 pm), blood was collected from participating children at the UCLA Clinical Laboratory through antecubital venipuncture in PAXgene Blood DNA tubes (8.5 ml; Qiagen), which were chilled and transported to the UCLA Health Psychology Laboratory for storage at -80° C. Assessment of LTL in whole blood was performed at the UCLA Cousins Center for Psychoneuroimmunology Inflammatory Biology Core laboratory using established real-time quantitative polymerase chain reaction (PCR) methodology as described in previously published protocols (Carroll et al., under review; Cawthon, 2002). Briefly, genomic DNA from leukocytes was extracted using PAXgene Blood DNA kits (Qiagen), and diluted to 5ng/10uL. Samples were run in triplicate on two 96-well plates, estimating the ratio of relative telomere (T) gene expression to single copy (S) gene beta hemoglobin (HGB) expression (T/S ratio). Each sample well included 5ng of sample, iQ SYBR Green PCR Master Mix (BioRad), and primers (Invitrogen) for Tel1b and Tel2b for the telomere plate (which amplify the repeating DNA sequences of the telomere), or HGB1 and HGB2 for the single copy HGB plate. A no template control was included in all reactions and used for background subtraction. Real time PCR was performed using the Biorad iCycler thermal cycler, equipped to read fluorescence at each cycle. The cycling thresholds (Ct) derived for each sample reflect the number of PCR cycles required for the sample DNA to express enough product to meet the threshold of magnitude of the selected fluorescent signal. Standard curves were generated on each plate using serial dilution of TaqMan CTL Genomic DNA (15 ng-0.94 ng; Applied Biosystems), and were used to calculate relative telomere length by plotting the Ct of the other samples against the control standard curve, to confirm PCR efficiency for all plates, and to control for plate-to-plate variation. Triplicate values were assessed for reliability and averaged for analysis. Intra-assay and inter-assay variability were ≤5%.
2.3.3. Covariates
Potential covariates were based on previous research (Drury et al., 2014; Mitchell et al., 2014; Needham et al., 2012; Shalev et al., 2013). Demographics included child age, gender, and parent educational status. Body mass index (BMI) was computed by dividing weight (kg, balance beam scale) by height (m2, stadiometer). BMI percentile (within age group) was used as a potential covariate, as it is the normative approach to quantifying BMI in children, and is appropriate for cross-sectional analyses. Variations in leukocyte cell subset composition may influence estimated LTL (Aubert & Lansdorp, 2008; Weng, 2001), and recent work in adults suggests that leukocyte composition may account for some of the age differences in LTL (Lin et al., 2015). Thus, we evaluated neutrophil, lymphocyte, monocyte, eosinophil, and basophil percentage (of total white blood cells), obtained by complete blood count with differential obtained by the UCLA Clinical Laboratory and Pathology Services using standard clinical laboratory methods, as possible covariates (one child did not have CBC data).
2.4. Data analysis
Our sample included 9 families with nested data, where including the target child and sibling in ordinary least squares regressions would violate statistical assumptions of independence. Indeed, the intraclass correlation coefficient for indistinguishable dyads (Kenny et al., 2006) was .45, p = .09. Given our low power to conduct the test of nonindependence (9 families), Kenny et al. (2006) recommend assuming nonindependence. Thus, we used the more appropriate route of multilevel modeling accounting for nesting within families (Krull, 2007), using Satterthwaite degrees of freedom in SAS PROC MIXED as recommended (Kenny et al., 2006). Child LTL was the dependent variable of interest, and one outlier greater than 3 SD above the mean was winsorized to the 95%ile. Due to our small sample size, we first ran unadjusted models with the primary independent variables of interest, followed by models with covariates that were associated with LTL in univariate analyses at p < .10.
In the exposure model, we tested whether average scores over the 56 days in the two domains of interest (marital, parent-child), types of behaviors of interest (conflict, warmth), and mood (positive, negative) predicted child LTL. Average diary scale scores were computed rather than sums over 56 days because diary completion rates varied across children (range 28 – 58 diaries). In the “reactivity” model, we estimated within-person associations between mood and family interactions using multilevel models (PROC MIXED in SAS) to compute slopes of marital or parent-child warmth/conflict, specified as a random variable, predicting similarly-valenced mood (i.e., conflict predicting negative mood, warmth predicting positive mood). We then generated empirical Bayes estimates of slopes for each child, which were then used as independent variables predicting LTL; see Cohen et al. (2008) and Mohr et al. (2013) for a similar “slopes as predictors” approach. In the “reactivity” model, we tested whether within-person associations (slopes) between mood and family interactions were associated with LTL. The within-person slopes included conflict – negative mood, and warmth/affection – positive mood slopes for the two domains of interest (marital, parent-child). We then tested exposure predictors and “reactivity” model predictors in the same model. Average diary scale scores and within-person slopes that were greater or less than 3 SD from the mean were winsorized (set to 95%ile).
3. Results
The left side of Table 1 shows descriptive statistics for average child-reported conflict and warmth in parent-child and marital relationships, as well as average positive and negative mood across the diary period. As expected, child-reported parent-child and marital conflict was infrequent relative to warmth, and negative mood was low relative to positive mood. The right side of Table 1 shows average within-person associations. On average, greater parent-child and marital conflict were related to higher negative mood, and greater parent-child and marital affection were related to higher positive mood.
Table 1. Descriptive Statistics for Daily Diary Measures and Within-person Slopes.
| Measure | Averaged over diary period, within-child | Within-person slopes | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| M | SD | Range | M | 95% CI | SD | Range | |
| Conflict | Negative mood | ||||||
| Parent-child | 1.15 | 0.17 | 1.00 – 1.86 | 0.44 | 0.36, 0.53 | 0.25 | 0.02 – 1.13 |
| Marital | 1.12 | 0.17 | 1.00 – 1.94 | 0.14 | 0.07, 0.20 | 0.12 | -0.06 – 0.42 |
| Warmth | Positive mood | ||||||
| Parent-child | 2.44 | 0.39 | 1.54 – 3.00 | 0.48 | 0.40, 0.57 | 0.26 | 0.06 – 1.10 |
| Marital | 1.84 | 0.52 | 1.00 – 3.00 | 0.07 | 0.006, 0.13 | 0.15 | -0.20 – 0.61 |
| Negative mood | 1.26 | 0.27 | 1.01 – 2.28 | ||||
| Positive mood | 2.94 | 0.66 | 1.64 – 3.99 | ||||
Note. Slope mean estimates were the fixed effects in multilevel models predicting mood as a function of the relationship predictor of interest (e.g., parent-child warmth). The mean slope estimates were statistically greater than zero as indicated by the 95% CI.
Although the magnitude and direction of the association between age and LTL was similar to previous studies, it was not statistically significant, r = -.13, 95% CI [-.42, .19], p = .37, due to a combination of our restricted 5 year age range and sample size. LTL did not differ between male (M = 1.26, n = 16) and female (M = 1.30, n = 24) children, 95% CI for mean difference [-0.39, 0.23]. LTL was not significantly related to parent education, rmother = -.10, 95% CI [-.40, .22], rfather = -.17, 95% CI [-.46, .15], or BMI percentile, r = .17, 95% CI [-.15, .46]. LTL was not correlated with any leukocyte subset percentages (95% CI lower bounds from -.41 to -.23, upper bounds from .20 to .39) except for basophil percentages, with greater basophil percentages related to longer LTL, r = .44, 95% CI [.14, .66], p = .005. Basophil percentages were also related to longer LTL with a similar magnitude when including all five subsets (lymphocytes, monocytes, neutrophils, eosinophils, basophils) as predictors of LTL. Thus, subsequent analyses with covariates included basophil percentages only.
3.1. Exposure and “reactivity” models
3.1.1. Exposure models
As shown in the top of Table 2, a model with average conflict and warmth in the parent-child and marital relationship, and average positive and negative mood over two months did not predict LTL, both before and after including basophil percentage as a covariate.
Table 2. Exposure and “Reactivity” Multilevel Models Predicting LTL.
| Predictor | Unadjusted model | With basophil % covariate | Exposure and “reactivity” models combined, with basophil % as a covariate | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| B | 95% CI | SE | t | B | t | B | t | |
| Exposure modela | ||||||||
| Parent-child | ||||||||
| Conflict | 0.14 | -1.39, 1.67 | 0.75 | 0.19 | 0.29 | 0.41 | 0.18 | 0.31 |
| Warmth | -0.41 | -1.08, 0.26 | 0.33 | -1.26 | -0.32 | -1.03 | 0.16 | 0.51 |
| Marital | ||||||||
| Conflict | -0.44 | -1.56, 0.67 | 0.55 | -0.81 | -0.19 | -0.37 | 0.17 | 0.36 |
| Affection | 0.10 | -0.16, 0.36 | 0.13 | 0.78 | 0.05 | 0.43 | -0.00 | -0.00 |
| Mood | ||||||||
| Negative | 0.15 | -0.16, 0.47 | 0.15 | 1.03 | -0.09 | -0.24 | -0.11 | -0.36 |
| Positive | -0.02 | -0.81, 0.77 | 0.39 | -0.05 | 0.15 | 1.02 | -0.09 | -0.27 |
| “Reactivity” modelb | ||||||||
| Parent-child | ||||||||
| Conflict – Negative mood | -0.33 | -0.86, 0.19 | 0.26 | -1.28 | -0.08 | -0.29 | -0.08 | -0.27 |
| Warmth – Positive mood | 0.50 | 0.05, 0.94 | 0.22 | 2.27* | 0.41 | 1.91 | 0.50 | 1.84 |
| Marital | ||||||||
| Conflict – Negative mood | -1.51 | -2.49, -0.53 | 0.48 | -3.16** | -1.76 | -3.83*** | -1.80 | -3.46** |
| Affection – Positive mood | 1.15 | 0.32, 1.97 | 0.38 | 2.99* | 0.86 | 2.21* | 0.88 | 1.68 |
Note. All coefficients are unstandardized. N = 39 for unadjusted models. For model with basophil % as a covariate,
basophil %, B = 0.44, t = 2.78, p = .01, N = 38,
basophil % B = 0.34, p = .02, N = 38. For the model combining exposure and “reactivity” predictors, basophil % B = 0.34, t = 2.21, p = .04, N = 38.
p < .05,
p < .01,
p < .001
3.1.2. “Reactivity” models
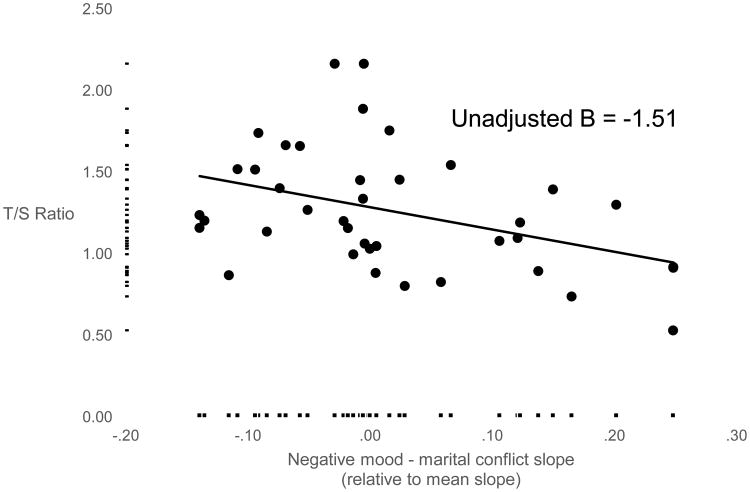
As shown in the bottom of Table 2, in a model with the four within-person slopes, stronger associations between positive mood and parent-child warmth (B = 0.50, p = .03), negative mood and marital conflict (B = -1.51, p = .004), and positive mood and marital affection (B = 1.15, p = .01) were related to LTL. Children who showed steeper positive mood - daily parent-child warmth slopes and positive mood – daily marital affection slopes had longer LTL, and children who showed steeper negative mood - daily marital conflict slopes had shorter LTL (Figure 1). The observed associations for negative mood – daily marital conflict slopes and positive mood – marital affection slopes in the unadjusted model remained statistically significant even after including basophil percentage as a covariate, while the positive mood – family warmth slopes were not related LTL after controlling for basophil percentage. Finally, as shown in the last two columns of Table 2, even after controlling for basophil % and including predictors from the exposure model (average conflict, warmth, and negative and positive mood), the marital conflict-negative mood “reactivity” model continued to predict LTL.
Figure 1.
Scatterplots depicting the association between negative mood - marital conflict slope and LTL. Dots correspond to the scatterplot, and markers along the X- and Y-axes indicate the distribution of the X- and Y- variables. LTL was indexed by the ratio of relative telomere (T) gene expression to single copy (S) gene beta hemoglobin expression (T/S ratio). One LTL outlier greater than M + 3 SD was winsorized to the 95%ile. Outliers for all independent variables were winsorized to the 95%ile. The unstandardized, unadjusted B is also shown in Table 2.
3.1.3. Sensitivity analyses
Several additional analyses evaluated the robustness of our results. First, we repeated the unadjusted multilevel models with data from 31 target children only (i.e., excluding data from nine siblings). Stronger negative mood - marital conflict slopes remained significantly associated with shorter LTL, B = -1.35, 95% CI [2.61, -0.08], SE = 0.61, t = -2.19, p = .04. Greater positive mood – family warmth slopes showed a trend-level association with longer LTL, B = 0.2, 95% CI [-0.04, 1.08], SE = 0.27, t = 1.91, p = .07. Positive mood – marital affection slopes were not significantly associated with LTL, t = 0.75, p = .46.
Finally, we tested the possibility that the associations between slopes reflecting reactivity to daily family interactions and LTL might be driven by individual differences in variability in either family conflict/warmth or mood. For each child we computed within-person SDs in parent-child and marital conflict and warmth, as well as positive and negative mood, as indicators of within-person variability. Only positive mood variability was systematically related to LTL, B = 0.98, 95% CI[0.35, 1.60], SE = 0.31, t = 3.18, p = .003; children with greater positive mood variability over the two-months had longer LTL. Even after including positive mood variability in the “reactivity” model, the daily negative mood - marital conflict slope, B = -1.46, 95% CI[-2.49, -0.43], SE = 0.50, t = -2.90, p = .007, and the daily positive mood – marital affection slope, B = 1.14, 95% CI[0.25, 2.03], SE = 0.40, t = 2.83, p = .02, were related to LTL. In that model positive mood variability was not related to LTL, B = 0.20, 95% CI [-0.51, 1.00], SE = 0.39, t = 0.50.
4. Discussion
We tested two potential models of how family environments may be associated with cellular aging in middle childhood, and found preliminary support for a reactivity model. LTL was shorter in children for whom daily negative mood was more strongly related to perceptions of daily conflict between their parents. While our data suggested that children for whom daily positive mood was more strongly related to perceptions of daily parent-child warmth and marital affection also had longer LTL, those findings were not robust after including additional covariates. Links between within-person negative mood - marital conflict slopes and LTL were independent of exposure to family and marital conflict, parent-child warmth, and marital affection, and positive and negative mood over the same two-month period. To our knowledge, these cross-sectional findings represent the first evidence showing that children's affective responses to daily family interactions have implications for telomere length.
Our findings for negative mood reactivity to daily inter-parental conflict are consistent with theories that emphasize the marital relationship as a major contributor to children's well-being (Davies and Cummings, 1994; Davies and Martin, 2013; Troxel and Matthews, 2004). Several models suggest that children who show greater negative emotional responses to conflict should also show heightened immune responses to infectious challenges (Miller et al., 2011; Repetti et al., 2011). Accordingly, children who are especially reactive to marital conflict may have shorter LTL because their immune system is primed to respond quickly to infections. Rapid immune responses increase rates of immune cell division and may promote faster aging of immune cells by increasing the number of successive cell divisions (Chou and Effros, 2013). Another possible mechanism is health-compromising behaviors like substance use (Beach et al., 2014; Shalev, 2012), though our young, healthy sample precluded testing that specific behavioral mechanism.
Previous work examining associations between supportive parenting and TL focused on children experiencing early or ongoing adversity, including children referred to child welfare agencies as infants (Asok et al., 2013), and African-American children living in a low-income household (Beach et al., 2014). In the latter study, participation in a prevention program designed in part to increase parental warmth reduced the association between nonsupportive parenting and LTL that was observed in the control group (Brody et al., 2015). In the unadjusted models, average daily positive parent-child interactions over a two-month period were not related to LTL. Instead, children who reported greater positive mood on days they reported more positive parent-child interactions or marital affection had longer LTL.
Despite evidence that positive parenting has benefits for childhood health the short-term daily processes by which positive parenting lead to health gains have been rarely explored (Bai and Repetti, 2015). This study sheds light on a plausible mechanism: individual differences in the way that children emotionally react to positive parent-child interactions. Notably, that finding and our results for marital conflict are consistent with the differential susceptibility framework, which posits that children vary in their susceptibility to both beneficial and adverse aspects of the rearing environment, due to individual differences in temperament and genetics (Belsky and Pluess, 2009). Negative emotion reactivity suggests vulnerability to stress, and positive emotion reactivity may reflect an ability to maximize social resources in the environment.
4.1. Limitations, Strengths, and Future Directions
We have interpreted the covariation between daily mood and the degree of association between day-to-day fluctuations in child-reported family interactions as indicating greater reactivity. However, there are other plausible interpretations for our findings. For example, according to a mood-congruent recall interpretation (Kihlstrom et al., 2000), individual differences in recall biases (i.e., recalling inter-parental conflict when in a negative mood state, and recalling positive parent-child interactions in a positive mood state) might be the key variables that relate to LTL. A transactional processes interpretation (Kuczynski and Parkin, 2007) would focus on how children's negative moods could help evoke greater parent-child or even marital conflict (Schermerhorn et al., 2010). Perhaps some children's moods have a stronger impact on the family social climate: those whose negative moods can push family interactions in a more conflictual direction may have shorter LTL. On the other hand, children whose daily positive moods can shape warm parent-child interactions, may have longer LTL. Although neither of these alternative explanations can be ruled out given our study design, both interpretations support the relevance of the degree of covariation between family interactions and mood as predictors in future research. Experience sampling designs that assess family interactions and mood at multiple points each day, rather than once a day, could help tease apart reactivity, mood-congruent recall, and transactional process interpretations (Repetti et al., 2015).
Additional study limitations suggest directions for future research. Future work would benefit from larger number of children, as our sample size precludes drawing firm conclusions about the magnitude of effects, especially in the final model including exposure and “reactivity” predictors. That being said, our predictors of interest were obtained from two months of daily diary data, providing greater precision in our measures. We cannot draw conclusions about telomere shortening or lengthening over time due to our cross-sectional assessment. Prospective longitudinal designs are therefore critical to address change in telomere length over time, particularly in youth (Shalev, 2012). Our small sample size and restricted range of family adversity relative to other studies limited our statistical power to detect between-child differences. That said, our findings are consistent with the negative consequences of witnessing family conflict, particularly marital conflict, for cellular aging (Drury et al., 2014). Finally, multiple biological mechanisms may explain how childhood experiences may be related to cellular aging (Robles and Carroll, 2011; Shalev, 2012), including telomere shortening (DNA damage, inflammation, oxidative stress) and lengthening (telomerase activity, antioxidants, diet-related factors); we were unable to assess these mechanisms in this study. Despite these limitations, having 56 days of diary reports allowed for prospective assessments of daily family interactions and mood that went well beyond single-occasion “snapshots” of functioning. Intensive repeated measures also allowed for computing individual slopes reflecting between-child differences in how tightly mood was associated with family interactions. Finally, this study is one of only a few that could rule out the possibility that leukocyte cell subsets contributed to observed differences in LTL.
4.1.2. Conclusions
Several independent groups have now documented that early adversity during childhood is related to LTL in adulthood (Shalev, 2012). Studies in childhood have focused on childhood abuse and maltreatment (Shalev, 2012), and circumstances like low socioeconomic status (Beach et al., 2014; Needham et al., 2012), although not all studies replicate (Glass et al., 2010). While approaches to studying stressful life events and cellular aging, based in part on the allostatic load model (Carroll et al., 2013; Shalev, 2012), suggest that exposure to major life stressors over long periods of time (years) leads to greater wear-and-tear over time, this study begins to fill a large gap in the existing literature by turning attention to everyday family interactions, children's affective experience, and cellular aging. Greater within-person associations between negative mood and marital conflict were related to shorter LTL in children, suggesting that daily emotional reactivity to family interactions in childhood may be related to cellular aging, independent of average levels of family and marital conflict, parent-child warmth, marital affection, and positive and negative mood. Ideally, future work should examine whether measures of emotional reactivity to marital conflict or family warmth during middle childhood, as well as shortened telomere length during the same period, predict mental and physical health outcomes in adolescence and adulthood. In other words, an important next step is longitudinal data that informs whether emotional reactivity and cellular aging may be potential precursor outcomes that presage the “long shadow” of deleterious health outcomes cast by childhood adversity (Kiecolt-Glaser et al., 2011).
Highlights.
The magnitude of within-person associations between daily mood and family interactions was related to leukocyte telomere length
Exposure to family interactions, and self-reported positive and negative mood were not significantly related to telomere length in children.
Children who showed a stronger association between daily negative mood and daily child-reported marital conflict had shorter leukocyte telomere length.
Acknowledgments
This research was supported by a Research Grant (9333) from the William T. Grant Foundation, a UCLA Academic Senate Faculty Research Grant, and the Cousins Center for Psychoneuroimmunology; preparation of this manuscript was supported in part by T32MH015750. We would like to thank Paul Chung, Richard Slatcher, and Gayla Margolin for their invaluable contributions to the project. Most of all, we want to thank the parents and children in the UCLA Families and Health study for their participation, and the graduate students, laboratory staff, and undergraduate research assistants for their efforts.
Role of the funding source: This research was supported by a Research Grant (9333) from the William T. Grant Foundation, a UCLA Academic Senate Faculty Research Grant, and the Cousins Center for Psychoneuroimmunology; preparation of this manuscript was supported in part by National Institutes of Health training grant T32MH015750. The financial sponsors had no role in the study design, collection, analysis, and interpretation of the data, writing of the report, or decision to submit the article for publication.
Footnotes
The major limitation to this approach is that in a daily diary design (as opposed to an ecological momentary assessment design involving multiple reports per day) one cannot infer that self-reported negative mood was preceded or “caused” by parent-child conflict. As we discuss in the Limitations section of the Discussion, other equally plausible inferences besides reactivity could be made from within-person associations, such as mood-congruent recall.
Throughout the remainder of the manuscript, we use “reactivity” in quotation marks for simplicity. Our use of the term reactivity originates from prior studies that operationalized emotional reactivity based on daily diary data, with the same limitations as those described in the previous footnote. The quotation marks underscore that a within-person association is an imperfect operationalization of the emotional reactivity concept.
Conflicts of interest: None
Contributors: The substantive contributions of each author to each phase of paper preparation is described below.
Conception and design of the study: Theodore F. Robles, Rena L. Repetti, Bridget M. Reynolds
Acquisition of data: Theodore F. Robles, Rena L. Repetti, Bridget M. Reynolds, Sunhye Bai
Acquisition of telomere data: Judith E. Carroll, Stephanie Esquivel
Analysis of data: Theodore F. Robles, Judith E. Carroll, Bridget M. Reynolds, Sunhye Bai, Stephanie Esquivel, Rena L. Repetti
Drafting the article or revising it critically for intellectual content: Theodore F. Robles, Judith E. Carroll, Bridget M. Reynolds, Sunhye Bai, Rena L. Repetti
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Bai S, Repetti RL. Short-term resilience processes in the family. Fam Relat. 2015;64:108–119. doi: 10.1111/fare.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Brody GH, Yu TY, Philibert RA. Nonsupportive parenting affects telomere length in young adulthood among African Americans: Mediation through substance use. J Fam Psychol. 2014;28:967–972. doi: 10.1037/fam0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu TY, Beach SRH, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prev Sci. 2015;16:171–180. doi: 10.1007/s11121-014-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;362:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A. 2013;110:17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative pcr. Nucleic Acids Res. 2002;30 doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichy KE, Stawski RS, Almeida DM. Racial differences in exposure and reactivity to daily family stressors. J Marriage Fam. 2012;74:572–586. doi: 10.1111/j.1741-3737.2012.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, Parrish BP, Wenze SJ, Beck JS. Negative affective spillover from daily events predicts early response to cognitive therapy for depression. J of Consul and Clin Psychol. 2008;76:955–965. doi: 10.1037/a0014131. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosom Med. 2003;65:652–657. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- D'Mello MJJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ Cardiovasc Gen. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai KK, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Cummings EM. Marital conflict and child adjustment - an emotional security hypothesis. Psychol Bull. 1994;116:387–411. doi: 10.1037/0033-2909.116.3.387. [DOI] [PubMed] [Google Scholar]
- Davies PT, Martin MJ. The reformulation of emotional security theory: The role of children's social defense in developmental psychopathology. Dev Psychopathol. 2013;25:1435–1454. doi: 10.1017/S0954579413000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP. The association of telomere length with family violence and disruption. Pediatrics. 2014;134:E128–E137. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JYY, Fox NA, Zeanah CH, Nelson CA. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook L. Gender differences in adolescents' daily interpersonal events and well-being. Child Dev. 2011;82:454–461. doi: 10.1111/j.1467-8624.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68:e21–22. doi: 10.1016/j.biopsych.2010.02.026. author reply e23-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, Lin J, Wolkowitz OM. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2015;20:615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cooke WL. Dyadic data analysis. Guildford Press; New York: 2006. [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlstrom JF, Eich E, Sandbrand D, Tobias BA. Emotion and memory: Implications for self-report. Lawrence Erlbaum Assoc Publ; Mahwah: 2000. [Google Scholar]
- Krull JL. Using multilevel analyses with sibling data to increase analytic power: An illustration and simulation study. Dev Psychol. 2007;43:602–619. doi: 10.1037/0012-1649.43.3.602. [DOI] [PubMed] [Google Scholar]
- Kuczynski L, Parkin CM. Agency and bidirectionality in socialization: Interactions, transactions, and relational dialects. In: Grusec JE, Hastings PD, editors. Handbook of socialization: Theory and research. Guilford Press; New York: 2007. pp. 259–283. [Google Scholar]
- Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, Morris C, Longo DL, Zhan M, Ferrucci L, Hodes RJ, Weng NP. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci. 2015;128:367–377. doi: 10.1042/CS20140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin G. Child home data questionnaire. University of Southern California; Los Angeles, CA: 1990. [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children's telomere length. Proc Natl Acad Sci U S A. 2014;111:5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr CD, Brannan D, Wendt S, Jacobs L, Wright R, Wang M. Daily mood-drinking slopes as predictors: A new take on drinking motives and related outcomes. Psychol Addict Behav. 2013;27:944–955. doi: 10.1037/a0032633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH. Socioeconomic status and cell aging in children. Soc Sci Med. 2012;74:1948–1951. doi: 10.1016/j.socscimed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Epel E. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6:807–825. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL. The effects of perceived daily social and academic failure experiences on school-age children's subsequent interactions with parents. Child Dev. 1996;67:1467–1482. [PubMed] [Google Scholar]
- Repetti RL, Reynolds BM, Sears MS. Families under the microscope: Repeated sampling of perceptions, experiences, biology, and behavior. J Marriage Fam. 2015;77:126–146. [Google Scholar]
- Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;23:921–938. doi: 10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Robles TF, Carroll JE. Restorative processes and health. Soc Personal Psychol Compass. 2011;5:518–537. doi: 10.1111/j.1751-9004.2011.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Reynolds BM, Repetti RL, Chung PJ. Using daily diaries to study family settings, emotions, and health in everyday life. J Soc Pers Relat. 2013;30:179–188. [Google Scholar]
- Rubin KH, Coplan RJ, Bowker JC. Social withdrawal in childhood. Annu Rev Psychol. 2009:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermerhorn AC, Chow SM, Cummings EM. Developmental family processes and interparental conflict: Patterns of micro level influences. Dev Psychol. 2010;46:869–885. doi: 10.1037/a0019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I. Early life stress and telomere length: Investigating the connection and possible mechanisms. Bioessays. 2012;34:943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Mol Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Garner AS, McGuinn L, Pascoe J, Wood DL. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:E232–E246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Lane SP. Psychometrics. In: Mehl MR, Conner TS, editors. Handbook of research methods for studying daily life. Guilford Press; New York, NY: 2012. pp. 302–320. [Google Scholar]
- Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children's physical health? Clin Child Fam Psychol Rev. 2004;7:29–57. doi: 10.1023/b:ccfp.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ, Hedeker D, Hankin BL, Flay BR. The time-varying influences of peer and family support on adolescent daily positive and negative affect. J Clin Child Adolesc Psychol. 2006;35:420–430. doi: 10.1207/s15374424jccp3503_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N. Interplay between telomere length and telomerase in human leukocyte differentiation and aging. J Leukoc Biol. 2001;70:861–7. [PubMed] [Google Scholar]