Abstract
Background
Despite effective combination antiretroviral therapy (cART) HIV infected individuals develop co-morbidities including cardiovascular disease (CVD), where activated macrophages play a key role. To date, few therapies target activated monocytes and macrophages.
Methods
We evaluated a novel oral form of the polyamine biosynthesis inhibitor methylglyoxal-bis-guanylhydrazone (MGBG) on cardiovascular inflammation, carotid artery intima-media thickness (cIMT), and fibrosis in a SIV infection model of AIDS. Eleven SIV-infected animals received MGBG (30 mg/kg) once daily and 8 received a placebo control both beginning at 21 dpi. Animals were time sacrificed (49 dpi), sacrificed when matched placebos controls developed AIDS (63, 70, 77, 80), or at the study endpoint (84 dpi). Aorta, carotid artery and cardiac tissues were analyzed. Quantitative analysis of macrophage populations and T-lymphocytes were done and correlated with cIMT and fibrosis.
Results
MGBG treatment resulted in a 2.19 (CD163+), 1.86 (CD68+), 2.31 (CD206+), and 2.12-fold (MAC387+) decrease in macrophages in carotid arteries and significant 2.07 (CD163+), 1.61 (CD68+), 1.95 (MAC387+) and 1.62-fold (CD206+) decrease in macrophages in cardiac tissues. CIMT (1.49-fold) and fibrosis (2.05-fold) also were significantly decreased with MGBG treatment. Numbers of macrophage and the degree of fibrosis in treated animals were similar to uninfected animals. A positive correlation between decreased macrophage in the carotid artery and CIMT, and cardiac macrophages and fibrosis was found.
Conclusions
These data demonstrate directly targeting macrophages with MGBG can reduce cardiovascular inflammation, CIMT, and fibrosis. They suggest therapies targeting macrophages with HIV could be used in conjunction with cART.
INTRODUCTION
Effective combination anti-retroviral therapy (cART) has increased the lifespan of HIV+ individuals1–3 and can successfully decrease viral load to low or undetectable levels.4,5 While effective in reducing plasma viral load, chronic immune activation and inflammation can persist leading to non-AIDS morbidities including cardiovascular disease (CVD).6 HIV+ individuals on cART have elevated levels of soluble CD14 (sCD14), C-reactive protein (CRP), tumor necrosis factor (TNF)-α, and soluble CD163 (sCD163) that are myeloid derived and markers of activated monocytes and macrophages.7 Elite controllers who have never been on cART also have elevated plasma sCD163, activated monocyte/macrophages and CVD, despite low level viremia.8–10 Thus with chronic HIV infection activated monocytes and macrophages demonstrated by elevated sCD14 and sCD163 correlate with CVD, increased aortic macrophage inflammation underscoring their role in chronic immune activation with low level plasma virus.
HIV+ individuals have increased risk of cardiovascular disease (CVD) compared to the general population.11,12 Growing evidence suggests that chronic immune activation and inflammation are a cause for increased CVD risk.13,14 HIV+ individuals have increased percentages of activated nonclassical (CD14+CD16++) and intermediate (CD14++CD16+) monocytes as do non HIV infected individuals with CVD.15 Using FDG-PET imaging, we have shown that increased arterial inflammation in the ascending aorta correlates with increased plasma sCD16316 Similarly, monocyte activation markers sCD163, sCD14 and CCL2 are elevated in HIV+ individuals and associated with atherosclerosis.17
Monocytes and macrophages have been demonstrated to play roles in the development of atherosclerosis, vascular calcification, and myocarditis. Macrophages secrete tumor necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β), which can enhance calcification in vitro18. Macrophages are associated with calcium deposits in carotid plaques19 and longitudinal studies provide evidence that inflammation precedes such calcification.20,21 In the heart, macrophages secrete pro-inflammatory and pro-fibrotic factors, which correlate with the extent of fibrosis.22 Unpublished data from our lab showed increase macrophage accumulation in the aorta with HIV infection and plasma sCD163 correlate with increased aortic intima-media thickness (aIMT). Using a SIV-infected, CD8+ T-lymphocyte depletion model of rapid and consistent AIDS, we found macrophage infiltration of cardiac parenchymal tissue correlates with increased cardiac fibrosis.23 Blocking monocyte/macrophage traffic to the heart, using an anti-α4 antibody, decreased macrophage accumulation in cardiac tissues and correlated with decreased cardiac fibrosis.24 These results suggest that targeting macrophage accumulation can decrease HIV-associated cardiovascular inflammation and decrease HIV-associated CVD.
Few therapies exist that directly targeting macrophages and residual immune activation in conjunction with cART in HIV infection.6 Gluoccocorticoid treatment of SIV-infected animals depleted pro-inflammatory and pro-thrombic CD14+CD16++ monocytes.25 Minocycline, a tetracycline antibiotic that prevents the development of SIV encephalitis (SIVE),26,27 decreased activated monocytes and monocyte/macrophage inflammation in lymph nodes28 Minocycline treatment in humans did not decrease HIV-RNA in CSF or decrease cognitive impairment but has not been tested in HIV associated CVD.29–31 SIV-infected monkeys treated with a CCR5 inhibitor maraviroc had decreased CD163 expression on macrophages in the myocardium32 and in humans, maraviroc in conjunction with effective cART decreased vascular cell adhesion molecule-1 (VCAM-1) suggesting a possible mechanism to inhibit monocyte/macrophage traffic to the heart.33 More recently, a clinical trial using low dose methotrexate treatment to reduce macrophage-mediated inflammation has been initiated (NCT01949116).
Methylglyoxal-bis-guanylhydrazone (MGBG) is a polyamine biosynthesis inhibitor that inhibits S-adenosine methionine decarboxylase (SAMDC) resulting in decreased intracellular concentrations of spermine and spermidine in monocytes/macrophages.34,35 Polyamines are necessary for macrophage activation, proliferation, and differentiation, suggesting MGBG might be useful in targeting macrophages to alleviate macrophage-mediated diseases associated with HIV infection.36,37 Previously, MGBG administered intravenously (i.v.) was used to treat HIV-associated lymphomas38 but was discontinued due in part to GI associated toxicity.39 More recently we have studied the MGBG formulated for oral administration. Initial studies showed MGBG inhibited HIV-p24 expression and DNA integration in human monocytes/macrophages in vitro without cell associated toxicities.40 Further, we found that MGBG is taken up and concentrated in macrophages, but not by T-lymphocytes. This is potentially important because most ART agents target T-lymphocytes and have limited uptake and effect on macrophages that are more resistant to antiretroviral agents41
We have used a CD8+ T-lymphocyte depletion model of rapids AIDS with consistent CNS and cardiac pathology, and found that increased macrophage accumulation in cardiac tissues and vessels leads to cardiac fibrosis and cardiomyocyte injury.23 In the current study used 19 SIV-infected, CD8+ lymphocyte depleted rhesus macaques and 6 uninfected controls, to examine if oral administration of MGBG treatment decreased macrophage inflammation in the aorta, carotid artery, and left ventricle (cardiac tissue). Additionally, we determined if decreased cardiac inflammation decreased cIMT and cardiac fibrosis. We found that MGBG treatment significantly decreased macrophage associated inflammation and fibrosis in cardiac tissues to levels found in uninfected animals. Compared to SIV-infected placebo controls, MGBG treatment resulted in a trend towards decreased inflammation in the carotid artery and a significantly decreased cIMT. These results suggest that MGBG directly targeting monocyte/macrophages with ART could reduce HIV-associated CVD.
METHODS
Ethics Statement
All animals used in this study were handled in strict accordance with the American Association for Accreditation of Laboratory Animal Care with the approval of the Institutional Animal Care and Use Committee of Harvard University and housed at the New England Primate Research Center (NERPC) (Southborough, MA). The NEPRC Protocol Number for this study was 04420 and the Animal Welfare Assurance Number was A3431-01. When control animals developed clinical symptoms consistent with the development of AIDS, they and their corresponding MGBG treated cohorts were anesthetized with ketamine-HCl and euthanized by an intravenous pentobarbital overdose and exsanguinated. Animals were sacrificed on the basis of the following guidelines for euthanasia of SIV-infected rhesus macaques: i) weight loss >15% in 2 weeks, 30% body weight in 2 months, or 25% overall, ii) documented opportunistic infection, iii) persistent anorexia >3 to 5 days without explicable cause, iv) severe intractable diarrhea (ie, nonresponsive to standard treatment and results in dehydration and debilitation of the animal), v) progressive neurological signs (ie, instability on the perch bar, depression, head tilt, nystagmus, ataxia, stupor, or depression), vi) significant cardiac and/or pulmonary signs (ie, dyspnea, open-mouthed breathing, or severe, previously unrecognized, cardiac murmur, especially if resulting in pulmonary edema), vii) persistent leukopenia, viii) progressive or persistent anemia, ix) signs of progressive immunosuppressive disease, x) body condition score >1.5/5 with weight loss, or xi) any other serious illness. The development of simian AIDS was determined post-mortem by the presence of opportunistic infections and tumors and included the development of SIV giant cell pneumonia, CMV pneumonia, SIV encephalitis with giant cells (SIVE), pneumocystis carnii, and lymphoma (Table 1).
Table 1.
Animals Used in the Study.
| Tissue Sections
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Animal ID | Survival (dpi) | SIV/AIDS | AIDS Defining Criteria | Carotid Artery | Left Ventricle | Treatment | CD8 Depletion | |
| Group 1 | 373–03 | NA | SIV− | – | N | Y | – | NA |
| 257–00 | NA | SIV− | – | N | Y | – | NA | |
| 417–08 | NA | SIV− | – | N | Y | – | NA | |
| 454–08 | NA | SIV− | – | N | Y | – | NA | |
| 96–10 | NA | SIV− | – | N | Y | – | NA | |
| 428–08 | NA | SIV− | – | N | Y | – | NA | |
|
| ||||||||
| Group 2 | 5035 | 49 | SIV+ | SIVE, CMV pneumonia | N | Y | Placebo | P |
| 5038 | 49 | SIV+ | SIVE, CMV pneumonia | N | Y | Placebo | P | |
| 5041 | 49 | SIV+, AIDS | CMV pneumonia | N | Y | MGBG | P | |
| 5042 | 49 | SIV+, No AIDS | – | N | Y | MGBG | P | |
|
| ||||||||
| Group 3 | 299–10 | 63 | SIV+, AIDS | SIV pneumonia, CMV pneumonia | Y | Y | Placebo | P |
| 264–10 | 63 | SIV+, AIDS | CMV brain, CMV lung | Y | Y | MGBG | P | |
| 186–10 | 63 | SIV+, No AIDS | – | Y | Y | MGBG | P | |
|
| ||||||||
| Group 4 | 296–10 | 70 | SIV+ | SIV giant cell lung, P.carinii | Y | Y | Placebo | P |
| 291–10 | 70 | SIV+, No AIDS | – | Y | Y | MGBG | R | |
| 273–10 | 70 | SIV+, No AIDS | – | Y | Y | MGBG | P | |
|
| ||||||||
| Group 5 | 291–09 | 77 | SIV+ | SIVE | Y | Y | Placebo | R |
| 275–10 | 77 | SIV+, AIDS | CMV pneumonia, adenovirus in jejunum | Y | Y | MGBG | P | |
| 328–10 | 77 | SIV+ No AIDS | – | Y | Y | MGBG | P | |
|
| ||||||||
| Group 6 | 326–10 | 83 | SIV+, AIDS | Lymphoma | Y | Y | Placebo | P |
| 201–10 | 83 | SIV+, No AIDS | – | Y | Y | MGBG | P | |
| 272–10 | 83 | SIV+, AIDS | Y | Y | MGBG | P | ||
|
| ||||||||
| Group 7 | 434–10 | 84 | SIV+, AIDS | P. carinii in lung | Y | Y | Placebo | R |
| 262–09 | 84 | SIV+, No AIDS | – | Y | Y | Placebo | R | |
| 272–09 | 84 | SIV+, No AIDS | – | Y | Y | MGBG | R | |
Twenty-five animals were used in this study to examine the effects of MGBG treatment on cardiovascular inflammation. Six animals were uninfected (group 1). The remaining 19 animals (groups 2–7) were either treated daily with MGBG or given a placebo control. Animals were grouped and sacrificed at the indicated days post infection (dpi) when placebo controls developed AIDS or at the end of the study (84 dpi). AIDS was assessed based on the presence of SIVE, (SIV-encephalitis) or the following opportunistic infections (OIs) Cytomegalovirus (CMV), pneumocystis carinii (P. carinii), and lymphoma. For sections of carotid artery and left ventricle, N indicates no section was taken from the indicated animal, Y indicates that a tissue section was taken. CD8+ T-lymphocyte were either persistently (P) depleted from SIV-infected animals or they returned ®. Persistently depleted animals are defined as animals where numbers of CD8+ T-lymphocytes in circulation at necropsy are less than 75% of the number of CD8+ T-lymphocytes at 0dpi. Nine of 11 (81.8%) MGBG treated and 5 of 8 placebo control (62.5%) animals in this study were persistently depleted.
Animals, SIV infection, CD8+ T-lymphocyte depletion, MGBG treatment
Twenty-five animals were used in this study. Six animals were uninfected controls and 19 animals SIV-infected and CD8 lymphocyte depleted. Animals were infected with the SIVmac251 viral swarm provided by Dr. Ron Desroisiers. Of these, 11 received MGBG (30 mg/kg) (provided by Pathologica, LLC; formulated as syrup by Wedgewood Pharmacy, Swedesboro, NJ) orally, once daily beginning at 21 days post infection (dpi), when significant CNS and cardiovascular inflammation is known to occur in this model.24,42 Eight received on oral placebo-syrup control at the same time point. We had determined in prior pilot studied that an effective dose of 0.7μM MGBG in plasma and tissues is reached in animals receiving a daily oral dose of 30mg/kg.40 Infected animals receiving MGBG or placebo where assigned to one of six groups with at least one placebo control per group. Six uninfected animals were group 1. Animals in each group were sacrificed at 49 dpi (Group 2), when placebo controls developed AIDS (Groups 3–6), or at the end of the study (84 days) (Group 7) (Table 1).
Assessment of cardiovascular pathology
Following exsanguination a full SIV necropsy was performed and major organs were collected in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm. Sections of the carotid artery, aorta, and left ventricle (cardiac tissue) were stained with hematoxylin and eosin and graded blindly by a veterinary pathologist. The carotid artery and aorta was assessed based on two criteria; inflammation and carotid artery or aortic degeneration. Degeneration of the carotid artery or aorta was based on the alignment of smooth muscle fibers in the tunica intima. Cardiac tissues were assessed based on the degree of inflammation, fibrosis, and cardiomyocyte degeneration. Sections were graded for each category based on the degree of change in cardiac tissues and scored as either having no significant findings (NSF) or being mild, moderate, or severe. Data are presented as percent of animals per treatment group with the corresponding level of inflammation, fibrosis and cardiomyocyte degeneration.
Immunohistochemistry
Numbers of macrophages in the carotid artery (n=11 MGBG treated, n=8 placebo) and left ventricle (cardiac tissue) (n=11 MGBG treated, n=8 placebo, n=6 SIV negative) were assessed using immunohistochemistry and cell counting as previously described.24 Formalin-fixed, paraffin-embedded sections of carotid artery and cardiac tissue were deparaffinized in xylenes and rehydrated in graded ethanols, followed by incubation with peroxidase block (Dako) for 5 minutes. Sections were incubated with serum free protein block (Dako) for 30 minutes then incubated with antibodies against CD163+, CD68+, CD206+, MAC387+ macrophage markers, and CD3+ T-lymphocytes for 1 hour at RT or overnight at 4°C. Tissue sections were rinsed and incubated with an anti-mouse secondary antibody conjugated to horseradish peroxidase (Dako). The reaction product was visualized using 3, 3′-diaminobenzidine tetrahydrochloride (DAB, Dako). The average number of immune positive macrophages/mm2 plus or minus the standard error of the mean (SEM) was determined by counting the number of positive macrophages from twenty non-overlapping 200× fields of view (field area=0.148mm2) per section, using a Zeiss Axio Imager M1 microscope with Plan-Apochromat x20/0.8 Korr objectives (Carl Zeiss Microimaging Inc., Thornwood, NY).
Measurement of carotid artery intima-media thickness (cIMT)
Sections of carotid artery were deparraffinzed and rehydrated in xylenes and graded ethanol, followed by staining with a Verhoeff Van Gieson elastic stain (Sigma) to differentiate the intima, media, and adventitia of the carotid artery wall.43 The cIMT was measured optically using ImageJ Analysis Software for 10 non-overlapping fields of view per section. Data was expressed as the average cIMT plus or minus the SEM.
Measurement of cardiac fibrosis
Cardiac tissue were stained using a modified Massons Trichrome (Newcomer Supply, Middleton, WI) and the percent of collagen per tissue area.44,45 The percent collagen (blue dye) per tissue area was determined using ImageJ Analysis software from 20 non-overlapping 200× fields of view (field area=0.148mm2). Data was expressed as the average percent collagen per tissue area plus or minus the SEM.
In Situ hybridization for SIV-RNA
In situ hybridization for SIV-RNA was performed using digoxigenin-labeled antisense riboprobes from Lofstrand Labs (Gaithersburg, MD), as previously described.46 Formalin-fixed, paraffin-embedded sections of carotid artery and cardiac tissues were deparffinized and rehydrated in xylenes and graded ethanols. Sections were treated with antigen unmasking solution (Vector Labs, Burlingame, CA) for 20 minutes at high heat, washed, and prehybridized at 45°C for 1 hour with prehybridization buffer containing denatured herring sperm DNA and yeast tRNA at concentrations of 10mg/mL. Digoxigenin-labeled probes (10ng/mL) were diluted in hybridization buffer and hybridized overnight at 45°C. Following stringency washes with decreasing concentrations of SSC buffer, sections were blocked with a 1% blocking solution (Roche Diagnostics) for 30 minutes. Digoxigenin-labeled probes were detected with NBT/BCIP (Roche Diagnostics) with levamisole (Dako) to inhibit alkaline phosphatases. The NBT/BCIP substrate produces a visible blue reaction product.
Plasma SIV-RNA was quantified using real-time PCR for all animals used in this study, as previously described.47 500μL of EDTA plasma was collected and SIV virions were pelleted by centrifugation at 20,000 g for 1 hour. The threshold sensitivity was 100 copy Eq/mL, with an average interassay coefficient variation of less than 25%.
Statistical Analysis
Statistical analyses were done using Prism version 5.0 (Graphpad Software Inc., San Diego, CA). For the carotid artery, comparisons of the mean number of macrophages/mm2 and carotid artery intima-media thickness (cIMT) were made between SIV-infected placebo controls, and SIV-infected MGBG treated animals using a non-parametric Mann-Whitney t-test with significance accepted at p<0.05. For cardiac tissues, comparisons of the mean number of macrophages/mm2 and the percent collagen per tissue area were made between uninfected animals, SIV-infected placebo controls, and SIV-infected MGBG treated animals using analysis of variance (ANOVA). If the ANOVA was significant (p<0.05) a posthoc non-parametric Mann-Whitney t-test was performed. A Spearman rank correlation analysis was used to determine if changes in cIMT and the percent collagen per total tissue area correlated with decreased macrophage numbers in the carotid artery and cardiac tissues of MGBG treated animals. A Fisher’s exact test was used to categorically and numerically compare the degree of cardiac and cardiac vessel inflammation, fibrosis and degeneration as assessed by a veterinary pathologist on H&E tissue sections.
RESULTS
MGBG treatment resulted in a trend toward decreased s cardiovascular pathology in SIV-infected rhesus macaques
Aorta and carotid artery
Cardiovascular pathology was assessed blindly by a veterinary pathology using H&E stained tissue sections. The aorta and carotid artery were graded and the degree of inflammation, carotid artery degeneration, and cIMT scored. Neither the MGBG treated nor placebo control animals had significant findings in the aorta. MGBG treated animals had decreased inflammation in the carotid artery where 5 of 9 (55%) MGBG treated animals had no inflammation compared to 2 of 6 (33%) placebo controls. Four of the 9 (44%) MGBG and 4 of the 6 (66%) placebo controls had mild carotid artery inflammation. Six of the 9 (66%) MGBG treated animals had no significant carotid artery degeneration compared to 3 of 6 placebo controls (50%). cIMT was noted in only 1 of 9 (11%) MGBG treated animals compared to 3 of 6 (50%) placebo controls.
Cardiac Tissue
Cardiac tissues (left ventricle) were scored blindly by a veterinary pathology based on inflammation, fibrosis, and cardiomyocyte degeneration using the same criteria described above. MGBG treatment resulted in a frequency and severity of inflammation in cardiac tissues. Five of 11 (45%) MGBG treated animals had no inflammation while placebo controls had either mild (7 of 8, 87%) or moderate (1 of 8, 13%) inflammation. There was no cardiac fibrosis in 7of 11 (63%) MGBG treated and 2 of 8 (25%) of placebo controls had cardiac fibrosis. Four of 11 (37%) MGBG treated animals had mild fibrosis and 6 of 8 (75%) remaining placebo controls had mild to moderate fibrosis. Five of 11 (45%) MGBG treated animals had no cardiomyocyte degeneration compared to only 1 of 8 (13%) of the placebo control animals, suggesting that MGBG decreased the frequency of cardiomyocyte degeneration. A Fischer’s exact statistical analysis was used to categorically and numerically compare MGBG treated versus controls with no significant findings (NSF) versus mild and moderate aortic, carotid artery, and cardiac tissue and the degree of inflammation, fibrosis, or cardiomyocyte damage. We did not find significant categorical differences between groups. This can be due to the small sample size and variation as well as the results being a relatively non-sensitive comparison between H&H tissue sections. Further comparisons using cIMT measurements, and immunohistochemical analysis and the measurement of the number of macrophages and amount of collagen deposition (fibrosis) are presented below.
MGBG decreases macrophage inflammation in the carotid artery and cardiac tissue in SIV-infected rhesus macaques
MGBG treatment resulted in a trend of decreased macrophage numbers in the carotid artery compared to placebo controls. There was a 2.19-fold (CD163+), 1.86-fold (CD68+), 2.12-fold (MAC387+), and 2.31-fold (CD206) decrease in the numbers of macrophages in the carotid artery of MGBG treated animals compared to placebo controls (Fig. 1A). There were no differences between the numbers of CD3+ T-lymphocytes in the carotid artery in placebo controls compared to MGBG treated animals (data now shown). MGBG treatment significantly decreased cardiac tissue macrophages compared to placebo controls. There was a significant 2.07-fold (CD163+), 1.61-fold (CD68+), 1.95-fold (MAC387+) and 1.62-fold (CD206+) decrease in numbers of macrophages in cardiac tissues of MGBG treated animals (Fig. 1B). Similar to the carotid artery, there was no difference in numbers of CD3+ T-lymphocytes in cardiac tissues of placebo controls and treated animals. There were significantly increased numbers of macrophages in placebo controls compared to uninfected animals. The numbers of CD163+, MAC387+ and CD206+ macrophages in cardiac tissues of MGBG treated animals were similar to the numbers the same macrophages in uninfected animals (Fig. 1B).
Figure 1. Daily treatment with MGBG results in a trend of decreased inflammation in the carotid artery and a significant decrease in inflammation in cardiac tissues from SIV-infected CD8+ T-lymphocyte depleted rhesus macaques.
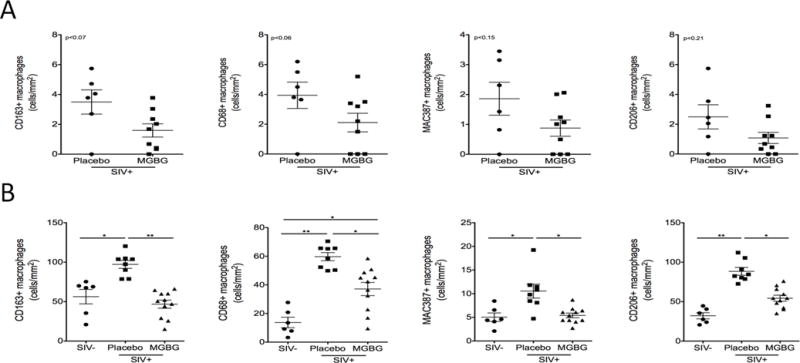
(A) Sections of carotid artery from MGBG treated (n=9) and placebo control (n=6) SIV-infected CD8+ T-lymphocyte rhesus macaques were immunohistochemically stained with antibodies recognizing CD163+, CD68+, CD206+, and MAC387+ macrophages. MGBG treatment resulted in a trend of decreased numbers of CD163+ (2.19-fold), CD68+ (1.86-fold), MAC387+ (2.12-fold), and CD206+ (2.31-fold) macrophages present in the carotid artery when compared to placebo controls. Statistical analysis was performed using a non-parametric Mann-Whitney t-test with significance accepted at p<0.05. (B) Sections of cardiac tissue from SIV- (n=6) MGBG treated (n=11) and placebo controls (n=8) rhesus macaques were immunohistochemically stained with the same antibodies. MGBG treated animals had significantly decreased numbers of CD163+ (2.07-fold), CD68+ (1.61-fold), and MAC387+ (1.95-fold) and CD206+ (1.62-fold) macrophages when compared to placebo controls. There was no difference in numbers of CD163+, MAC387+, and CD206+ macrophages when comparing uninfected and MGBG treated animals. For cardiac tissues statistical analysis was performed between uninfected animals, SIV-infected placebo controls, and SIV-infected MGBG treated animals using analysis of variance (ANOVA). If the ANOVA was significant (p<0.05) between two groups, a posthoc non-parametric Mann-Whitney t-test was performed. * p<0.05, ** p<0.01.
MGBG treatment results in a reduction of carotid artery intima-media (cIMT)
MGBG treatment resulted in a significant 1.49-fold decrease of carotid artery intima-media thickness (cIMT) compared to placebo controls. The average cIMT for placebo controls was 0.41±0.05mm (n=6) compared to 0.28±0.02mm MGBG treated animals (n=9) (Fig 2A, 2B). Spearman rank correlation analysis of tissues from placebo controls and MGBG treated animals demonstrated a positive correlation between increased numbers of CD68+ (r=.52, p<0.05), CD206+, and MAC387+ (r=.42, p<0.05) macrophages present in the carotid artery and increased cIMT. There was no correlation between the number of CD163+ macrophages and increased cIMT (Fig 2C).
Figure 2. MGBG treatment prevents an increase in carotid artery intima-media thickness which correlates with a decreased trend in macrophage inflammation.
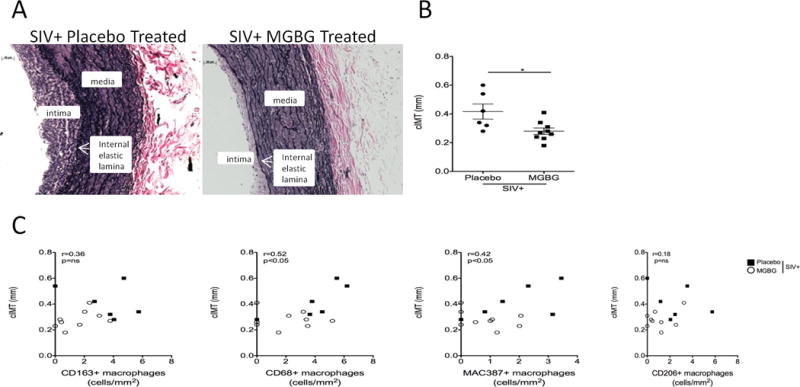
(A) Sections of carotid artery were stained with a Verhoeff Van Gieson elastic stain in order to measure the carotid artery intima-media thickness (cIMT). The cIMT was measured using calipers and ImageJ Analysis Software from 10 random non-overlapping fields of view with average cIMT calculated and expressed as plus or minus the standard error of the mean (SEM). (B) There was a significant 1.49-fold decrease in cIMT when comparing placebo control animals to MGBG treated animals. Statistical analysis was performed using a non-parametric Mann-Whitney t-test comparing placebo controls to MGBG treated animals with significance accepted at p<0.05. (C) Using a Spearman rank correlation analysis, in the carotid artery, there was a positive correlation between increased numbers of CD68+ (r=0.52) and MAC387+ (r=0.42) macrophages and increasing cIMT. There was no correlation between numbers of CD163+ and CD206+ macrophages and cIMT. * p<0.05. Scale bar equals 50 μm.
MGBG treatment results in decreased cardiac fibrosis in SIV-infected rhesus macaques
There was a significant 2.05-fold decrease in the percent collagen per tissue area in MGBG treated animals compared to placebo controls. The average percent collagen per tissue area for MGBG treated animals was 5.79±0.67% (n= 11) compared to 11.85±0.98% for placebo controls (n=8) (Fig. 3). SIV negative animals had a mean percent collagen per tissue area of 6.74±1.91% (n=6). Placebo control animals had a significant 1.76-fold increase in the percent collagen per tissue area compared to uninfected animals. MGBG treated animals showed no significant differences in the percent collagen per tissue area compared to uninfected animals. Spearman rank analysis of tissues from uninfected, placebo controls, and MGBG treated animals demonstrated positive correlations between increased numbers of CD163+ (r=0.69, p<0.01), CD68+ (r=0.63, p<0.05), CD206+ (r=0.54, p<0.01), and MAC387+ (r=0.53, p<0.05) macrophages in cardiac tissues and increased fibrosis in cardiac tissues (Fig 3C).
Figure 3. MGBG treatment prevents an increase in cardiac fibrosis in cardiac tissues similar to levels seen in uninfected controls.
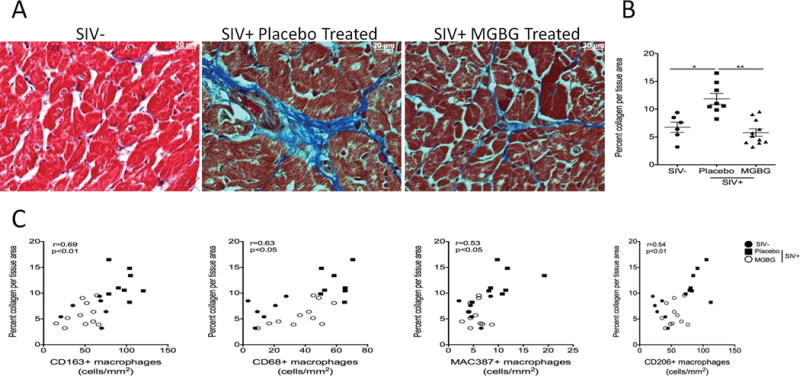
(A) Collagen, a marker of fibrosis, in cardiac tissues was measured by staining cardiac tissue with a trichrome stain. ImageJ Analysis software was used to determine the percent collagen (blue dye) per total tissue area from 20 200× non-overlapping fields of view with the average percent collagen per total tissue area calculated for each animal. (B) There was a significant 2.05-fold decrease in the percent collage per total tissue area when comparing placebo controls to MGBG treated animals. There was no significant difference between the percent collagen per total tissue area between uninfected and MGBG treated animals. Statistical analysis was performed using analysis of variance (ANOVA). If the ANOVA was significant (p<0.05) between two groups, a posthoc non-parametric Mann-Whitney t-test was performed. (C) Using Spearman rank correlation analysis, we found positive correlations between increasing numbers of CD163+ (r=0.69), CD68+ (r=0.63), MAC387+ (r=0.53) and CD206+ (r=0.54) macrophages and increasing percent collagen per total tissue area. * p<0.05, ** p<0.01. Scale bar equals 20 μm.
In situ hybridization for SIV-RNA
We did not find SIV-RNA or SIV-p28+ cells in the carotid artery or cardiac tissues of SIV-infected placebo controls or MGBG treated animals, consistent with previous findings.23 Viral loads in all animals peaked at 8 dpi and remained elevated in MGBG treated or placebo controls (data not shown). There were no statistically significant differences in plasma viral load in the MGBG treated versus placebo treated animals (data not shown).
DISCUSSION
With effective combination anti-retroviral therapy (cART) mortality due to AIDS related causes has decreased,2,48 however secondary co-morbidities have increased.3 While cART decreases plasma virus to low or undetectable levels, chronic immune activation that potentially drives the progression of cardiovascular disease persists.49 Statins, that are likely to have a mild immune suppressive effect on myeloid cells,50 have been used successfully to decrease CVD with HIV infection51–53 Two studies used rosuvastatin in uninfected individuals51 and HIV+ individuals on cART with a moderate cardiovascular risk52 found a reduced rate of cIMT progression. Additionally atorvastatin reduced non-calcified plaque volume and high-risk coronary plaque features (positive remodeling and low attenuation of plaques) among HIV+ individuals with arterial inflammation.53 Statins do not directly target macrophages that are key players in HIV- and SIV-associated CVD54–56 and myocarditis57,58 and to date few therapies target them.
We and others have shown that markers of immune activation, sCD14 and sCD16359 and not traditional risk factors of CVD are associated with atherosclerotic plaques with HIV suggesting that chronic immune activation with HIV infection plays a role in the development of HIV-associated CVD. Increased sCD1460 and monocyte chemoattractant protein 1 (MCP-1) and tumor necrosis factor alpha (TNF-α)61 also associated with increased coronary artery calcium (CAC), a marker of coronary atherosclerosis. FDG-PET imaging showed that with HIV infection there is increased arterial inflammation in the ascending aorta that correlated with levels of sCD163,16 and an increase in high-risk plaque morphology.62 Soluble CD163 made by activated monocytes and macrophages also was associated with non-calcified plaques in HIV+ individuals.59 High-risk, non-calcified plaques tend to have large necrotic lipid rich cores and increased macrophage numbers compared to stable plaques.54 HIV elite controllers, who control viremia, have elevated myeloid markers of immune activation8,63 and an increased prevalence of non-calcified coronary plaques further suggesting a link between monocyte/macrophage activation and HIV-associated CVD.10
Previously we have shown that the SIV-infected, CD8+ T-lymphocyte depletion model of rapid AIDS in rhesus macaques can be used to study the effects of SIV on the development of cardiac fibrosis.23 Using this model we found that directly blocking macrophage traffic to the heart, using an anti-α4 antibody, decreased overall cardiac pathology, macrophage inflammation, and cardiac fibrosis.24 The anti-alpha 4 antibody is less useful long term in HIV+ individuals because of the potential emergence of JC virus infection with long term usage seen in patients with MS and Crohns.64,65 In the current study, we examined the effects of an oral formulation of methylglyoxal-bis-guanylhydrazone (MGBG), that is specifically taken up by and concentrated in monocytes/macrophages40 and not T-lymphocytes. We have recently demonstrated that MGBG in vitro inhibits HIV integration and replication.40 Importantly in this study, MGBG was selectively taken up by monocyte/macrophages and not T lymphocytes. We have also found that MGBG treatment results in the down regulation of CD16 on CD14+CD16+ monocytes in vitro and decreased osteopontin expression by macrophages (using gene array and immunohistochemistry (data not shown). In the current study we investigated the effects of oral administration of MGBG on SIV-associated cardiovascular inflammation in the carotid artery and heart, cIMT, and cardiac fibrosis. Animal groups consisting of placebo and MGBG treated animals were sacrificed early, when placebo controls developed AIDS, or study endpoint.
We found a trend of decreased inflammation in carotid arteries of MGBG treated animals compared to placebo controls, as well as a significant decrease in the cIMT. Carotid artery intima-media thickness, a marker of atherosclerosis and subclinical cardiovascular disease66–68 is associated with myocardial infarction and stroke.69,70 cIMT increases more rapidly in HIV+ individuals compared to age-matched control individuals71,72 and is associated with a low CD4+ T-lymphocyte count (<200cells/mm3)73. A limitation of the current study is not having carotid arteries from uninfected animals to compare to MGBG treated animals. It is interesting to note in the current study that 7 of the 11 MGBG treated animals did not develop AIDS. This is of significance especially considering that MGBG is not taken up by CD4+ T lymphocytes and therefore likely has its effect more directly on macrophages underscoring their importance, in this model, on the development of AIDS. We further note, that CVD with HIV or SIV infection does not seem only to be dependent on the development of AIDS. Lastly, while we have found in preliminary studies that MGBG treatment can result in decreased CD16 expression on CD14+ CD16+ monocytes, we did not find differences in the percentage of monocytes expressing CD16 in the animals in the current study until necropsy (data not shown).
MGBG treatment decreased macrophage inflammation in cardiac tissues and cardiac fibrosis compared to placebo controls. Cardiac inflammation and fibrosis in MGBG treated animals was similar to levels seen in uninfected animals. We did not find any effect of MGBG on the number of CD3+ T-lymphocytes present in cardiac tissues consistent with our findings that MGBG is selectively taken up and concentrated in monocytes and macrophages, and not T lymphocytes.40 We have previously shown in our rapid AIDS model that the accumulation of macrophages, and not T-lymphocytes nor viral infected cells, best correlates with cardiac fibrosis and cardiomyocyte damage.23 Plasma viral load for all placebo and MGBG treated animals in this study were similar as these animals were CD8+ T-lymphocyte depleted. We did not find SIV-RNA+ cells in the hearts of our monkeys, a result that is consistent with prior reports of low or scattered SIV- and HIV-infected macrophages in cardiac tissues.74,75 Together, these data underscore the role of macrophages in the development of SIV-associated CVD. Targeting macrophages directly might be a possible way to diminish inflammation in the heart and cardiac fibrosis. While the incidence of cardiac fibrosis has declined in the cART era among HIV+ individuals76,77 when studied recently it is still prevalent among HIV+ individuals.78,79 While some animals in this study that received MGBG developed AIDS, others receiving the drug did not. However, there were no differences in the levels of fibrosis and cIMT in MGBG treated animals with and without AIDS suggesting that the development of AIDS alone is not the sole cause of cardiovascular disease, adding evidence that chronic inflammation with SIV and HIV infection likely plays a more central role.
In this study we examined the effects MGBG has on SIV-associated cardiovascular inflammation and cardiac fibrosis by directly targeting macrophages. Animals receiving daily doses of MGBG showed decreased inflammation in the carotid artery and cardiac tissue when compared to placebo controls. MGBG treatment also prevented an increase in cIMT and cardiac fibrosis that remained at levels similar to SIV negative animals. These data suggest that therapies designed to target chronic inflammation and immune activation seen with HIV infection could be used as adjunctive therapies to cART to alleviate HIV-associated cardiovascular disease.
Acknowledgments
We would like to thank Wedgwood Pharmacy for formulating MGBG and placebo. We thank the veterinary staff at the NEPRC for animal care, pathology residents and staff for assisting with necropsies and tissue collection.
This work was supported by the following grants: R01 NS040237 (Williams), R01 NS082116 (Burdo) and U19MH08183 (McGrath).
Sources of Funding
Michael McGrath is a shareholder and consultant in Pathologica, LLC.
Footnotes
Conflicts of Interest
For the remaining authors there are no conflicts of interests.
Parts of this data were presented at the Conference on Retroviruses and Opportunistic Infections, Boston, MA. 2016.
References
- 1.Coquet I, Pavie J, Palmer P, et al. Survival trends in critically ill HIV-infected patients in the highly active antiretroviral therapy era. Crit Care. 2010;14:R107. doi: 10.1186/cc9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landay A, Miller CJ, Baker JV, et al. Adjudicated Morbidity and Mortality Outcomes by Age among Individuals with HIV Infection on Suppressive Antiretroviral Therapy. PLoS One. 2014;9:e95061. doi: 10.1371/journal.pone.0095061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and Aging in HIV-Infected Persons The Swiss HIV Cohort Study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 4.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JZ, Arnold KB, Lo J, et al. Differential Levels of Soluble Inflammatory Markers by Human Immunodeficiency Virus Controller Status and Demographics. Open Forum Infect Dis. 2014;2:ofu117–ofu117. doi: 10.1093/ofid/ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan S, Wilson EM, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis. 2014;209:931–939. doi: 10.1093/infdis/jit581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereyra F, Lo J, Triant V, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2415. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999–2013. Am J Cardiol. 2016;117:214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petoumenos K, Reiss P, Ryom L, et al. Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: a comparison of the D:A:D CVD risk equation and general population CVD risk equations. HIV Med. 2014;15:595–603. doi: 10.1111/hiv.12162. [DOI] [PubMed] [Google Scholar]
- 13.Stein JH, Hsue PY. Inflammation, Immune Activation, and CVD Risk in Individuals With HIV Infection. JAMA. 2012;308:405–406. doi: 10.1001/jama.2012.8488. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Tawakol A, Abbara S, et al. Arterial Inflammation in Patients With HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated Levels of Monocyte Activation Markers Are Associated With Subclinical Atherosclerosis in Men With and Those Without HIV Infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 19.Jeziorska M, McCollum C, Woolley DE. Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. J Pathol. 1998;185:10–17. doi: 10.1002/(SICI)1096-9896(199805)185:1<10::AID-PATH71>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Abdelbaky A, Corsini E, Figueroa AL, et al. Early aortic valve inflammation precedes calcification: A longitudinal FDG-PET/CT study. Atherosclerosis. 2015;238:165–172. doi: 10.1016/j.atherosclerosis.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Abdelbaky A, Corsini E, Figueroa AL, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6:747–754. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 22.Timonen P, Magga J, Risteli J, et al. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Walker JA, Sulciner ML, Nowicki KD, Miller AD, Burdo TH, Williams KC. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res Hum Retroviruses. 2014;30:685–694. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker JA, Beck GA, Campbell JH, Miller AD, Burdo TH, Williams KC. Anti-alpha4 Integrin Antibody Blocks Monocyte/Macrophage Traffic to the Heart and Decreases Cardiac Pathology in a SIV Infection Model of AIDS. J Am Heart Assoc. 2015;4:e001932. doi: 10.1161/JAHA.115.001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moniuszko M, Liyanage NPM, Doster MN, et al. Glucocorticoid Treatment at Moderate Doses of SIVmac251-Infected Rhesus Macaques Decreases the Frequency of Circulating CD14+CD16++Monocytes But Does Not Alter the Tissue Virus Reservoir. AIDS Res Hum Retroviruses. 2015;31:115–126. doi: 10.1089/aid.2013.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratai EM, Bombardier JP, Joo CG, et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- 28.Campbell JH, Burdo TH, Autissier P, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho EL, Spudich SS, Lee E, Fuchs D, Sinclair E, Price RW. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res Ther. 2011;8:17. doi: 10.1186/1742-6405-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakasujja N, Miyahara S, Evans S, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80:196–202. doi: 10.1212/WNL.0b013e31827b9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacktor N, Miyahara S, Deng L, et al. Minocycline treatment for HIV-associated cognitive impairment: Results from a randomized trial. Neurology. 2011;77:1135–1142. doi: 10.1212/WNL.0b013e31822f0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly KM, Tocchetti CG, Lyashkov A, et al. CCR5 Inhibition Prevents Cardiac Dysfunction in the SIV/Macaque Model of HIV. J Am Heart Assoc. 2014;3:e000874. doi: 10.1161/JAHA.114.000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francisci D, Falcinelli E, Baroncelli S, et al. Potential anti-inflammatory effects of maraviroc in HIV-positive patients: a pilot study of inflammation, endothelial dysfunction, and coagulation markers. Scand J Infect Dis. 2014;46:466–470. doi: 10.3109/00365548.2014.898332. [DOI] [PubMed] [Google Scholar]
- 34.Corti A, Dave C, Williams-Ashman HG, Mihich E, Schenone A. Specific inhibition of the enzymic decarboxylation of S-adenosylmethionine by methylglyoxal bis(guanylhydrazone) and related substances. Biochem J. 1974;139:351–357. doi: 10.1042/bj1390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams-Ashman HG, Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972;46:288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- 36.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janne J, Holtta E, Kallio A, Kapyaho K. Role of polyamines and their antimetabolites in clinical medicine. Spec Top Endocrinol Metab. 1983;5:227–293. [PubMed] [Google Scholar]
- 38.Tulpule A, Espina B, Pedro Santabarbara AB, et al. Treatment of AIDS related non-Hodgkin’s lymphoma with combination mitoguazone dihydrochloride and low dose CHOP chemotherapy: results of a phase II study. Investigational New Drugs. 2004;22:63–68. doi: 10.1023/b:drug.0000006175.32100.2c. [DOI] [PubMed] [Google Scholar]
- 39.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, McGrath MS, Xu H. Inhibition of HIV Expression and Integration in Macrophages by Methylglyoxal-Bis-Guanylhydrazone. J Virol. 2015;89:11176–11189. doi: 10.1128/JVI.01692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavegnano C, Detorio MA, Bassit L, Hurwitz SJ, North TW, Schinazi RF. Cellular pharmacology and potency of HIV-1 nucleoside analogs in primary human macrophages. Antimicrob Agents Chemother. 2013;57:1262–1269. doi: 10.1128/AAC.02012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soulas C, Conerly C, Kim WK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deopukari R, Dixit A. The study of age related changes in coronary arteries and its relevance to the atherosclerosis. J Anat Soc India. 2010;59:192–196. [Google Scholar]
- 44.Brower GL, Gardner JD, Forman MF, et al. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Ruifrok AC, Johnston DA. Quantification of Histochemical Staining by Color Deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 46.Williams K, Schwartz A, Corey S, et al. Proliferating Cellular Nuclear Antigen Expression as a Marker of Perivascular Macrophages in Simian Immunodeficiency Virus Encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lifson JD, Rossio JL, Piatak M, Jr, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312–1321. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beltran LM, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Garcia-Puig J, Moreno JA. Influence of immune activation and inflammatory response on cardiovascular risk associated with the human immunodeficiency virus. Vasc Health Risk Manag. 2015;11:35–48. doi: 10.2147/VHRM.S65885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav A, Betts MR, Collman RG. Statin modulation of monocyte phenotype and function: implications for HIV-1-associated neurocognitive disorders. [published online March 28, 2016] J Neurovirol. doi: 10.1007/s13365-016-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crouse JR, 3rd, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 52.Calza L, Manfredi R, Colangeli V, et al. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Res Hum Retroviruses. 2013;29:547–556. doi: 10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- 53.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tahara N, Mukherjee J, de Haas HJ, et al. 2-deoxy-2-[F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 55.Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218:1376–1384. doi: 10.1016/j.imbio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang M, Zheng J, Miao Y, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
- 58.Falkenham A, de Antueno R, Rosin N, et al. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am J Pathol. 2015;185:927–942. doi: 10.1016/j.ajpath.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 59.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shikuma CM, Barbour JD, Ndhlovu LC, et al. Plasma Monocyte Chemoattractant Protein-1 and Tumor Necrosis Factor-α Levels Predict the Presence of Coronary Artery Calcium in HIV-Infected Individuals Independent of Traditional Cardiovascular Risk Factors. AIDS Res Hum Retroviruses. 2014;30:142–146. doi: 10.1089/aid.2013.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tawakol A, Lo J, Zanni MV, et al. Increased Arterial Inflammation Relates to High-risk Coronary Plaque Morphology in HIV-Infected Patients. J Acquir Immune Defic Syndr. 2014;66:164–171. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Assche G, Van Ranst M, Sciot R, et al. Progressive Multifocal Leukoencephalopathy after Natalizumab Therapy for Crohn’s Disease. N Engl J Med. 2005;353:362–380. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 65.Jilek S, Jaquiéry E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol. 2010;9:264–272. doi: 10.1016/S1474-4422(10)70006-5. [DOI] [PubMed] [Google Scholar]
- 66.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 67.O’Leary DH, Polak JF. Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002;90:18L–21L. doi: 10.1016/s0002-9149(02)02957-0. [DOI] [PubMed] [Google Scholar]
- 68.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RBS. Carotid-Wall Intima–Media Thickness and Cardiovascular Events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 70.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 71.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of Carotid Intima-Media Thickness and Coronary Artery Calcium Over 6 Years in an HIV-Infected Cohort. J Acquir Immune Defic Syndr. 2013;64:51–57. doi: 10.1097/QAI.0b013e31829ed726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsue PY, Ordovas K, Lee T, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol. 2012;109:742–747. doi: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 74.Kelly KM, Tarwater PM, Karper JM, et al. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 75.Yearley JH, Pearson C, Carville A, Shannon RP, Mansfield K. SIV-Associated Myocarditis: Viral and Cellular Correlates of Inflammation Severity. AIDS Res Hum Retroviruses. 2006;22:529–540. doi: 10.1089/aid.2006.22.529. [DOI] [PubMed] [Google Scholar]
- 76.Silwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–874. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khunnawat C, Mukerji S, Havlichek D, Jr, Touma R, Abela GS. Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol. 2008;102:635–642. doi: 10.1016/j.amjcard.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 78.Cheruvu S, Holloway CJ. Cardiovascular disease in human immunodeficiency virus. Intern Med J. 2014;44:315–324. doi: 10.1111/imj.12381. [DOI] [PubMed] [Google Scholar]
- 79.Frustaci A, Petrosillo N, Vizza D, et al. Myocardial and microvascular inflammation/infection in patients with HIV-associated pulmonary artery hypertension. AIDS. 2014;28:2541–2549. doi: 10.1097/QAD.0000000000000426. [DOI] [PubMed] [Google Scholar]


