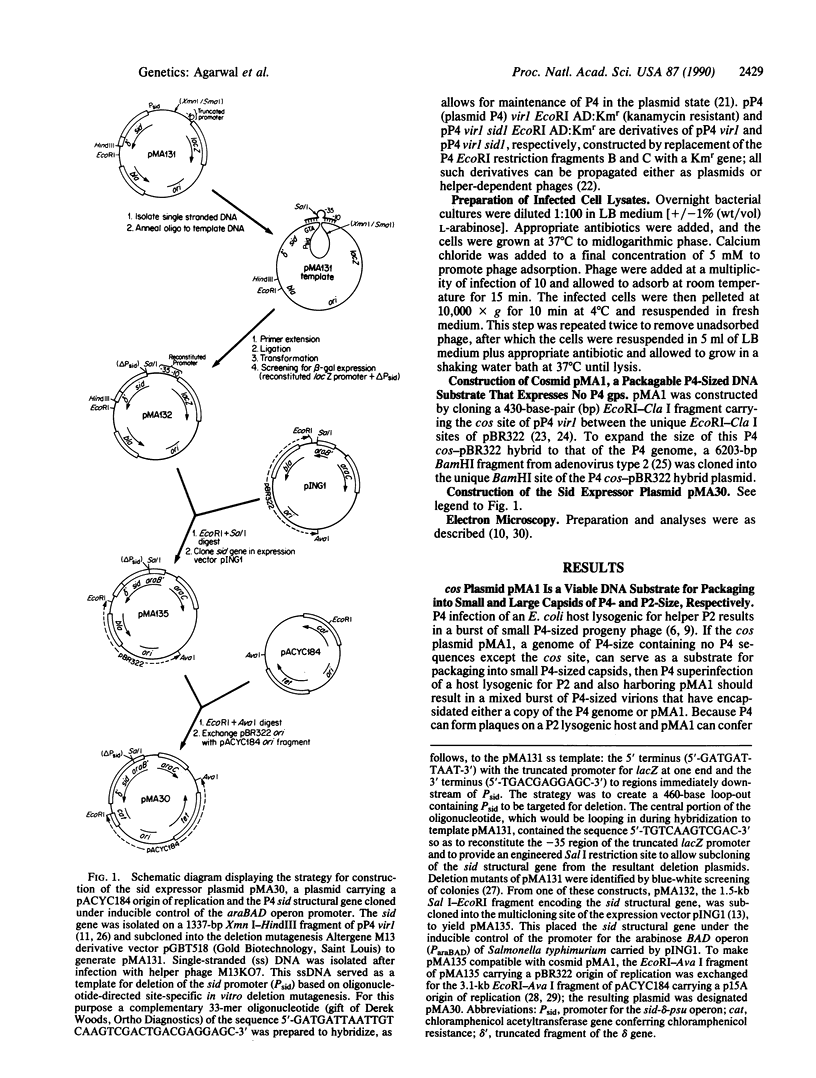
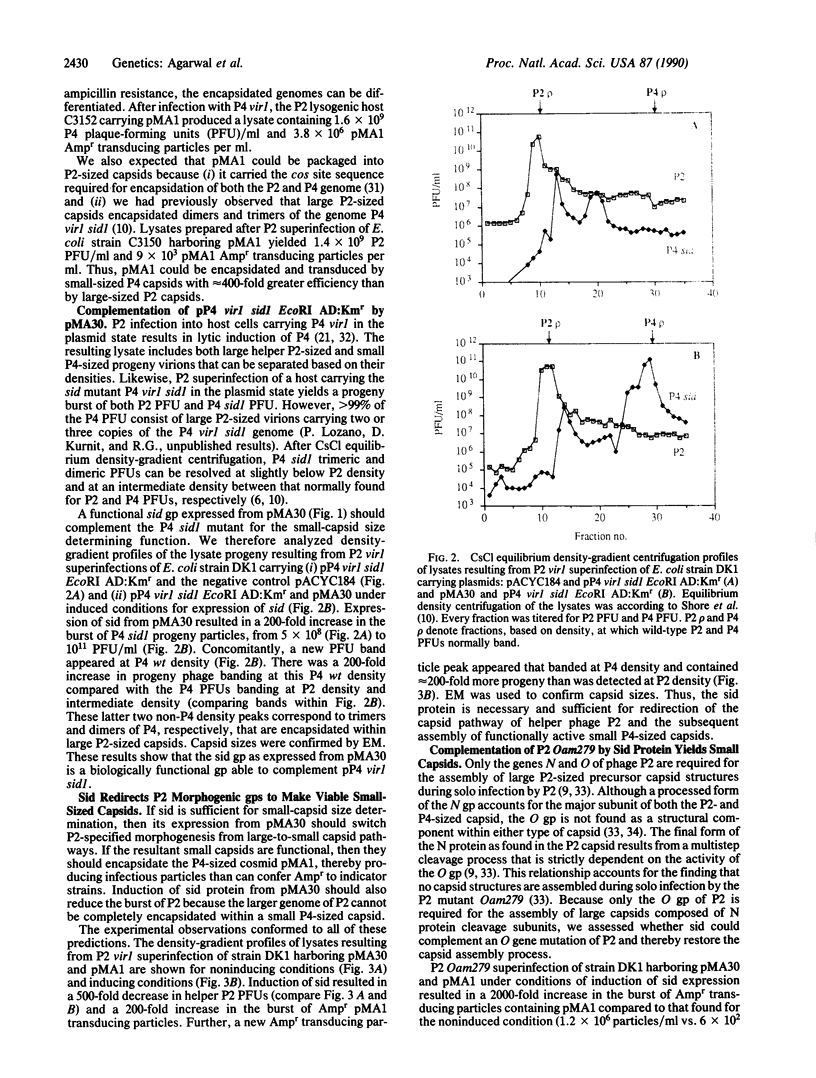
Abstract
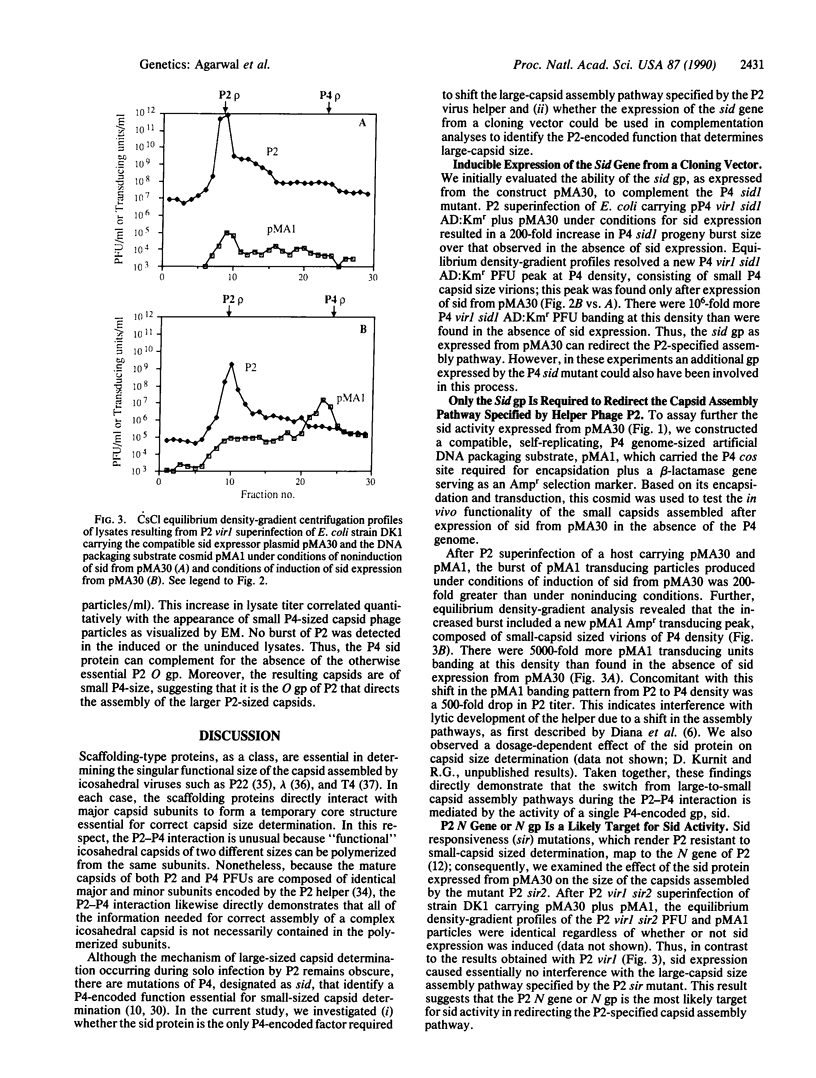
Determination of icosahedral virion capsid size can be directly studied during helper-dependent lytic development of satellite P4 because the assembly pathway specified by the P2 helper virus is altered to yield smaller-sized capsids. Size determination (sid) mutations identify a P4-encoded function regulating this process. To determine whether the sid gene product is necessary and sufficient to redirect the assembly pathway, we (i) cloned the sid structural gene in a plasmid vector (pMA30) under the control of an inducible promoter and (ii) constructed a packaging substrate (pMA1), a P4 genome-sized plasmid containing only that region of P4, the cos site, necessary for encapsidation. Superinfection by P2 of a host carrying pMA30 under induced conditions resulted in a shift from large to small capsid production. P2 superinfection of a host carrying the cos plasmid pMA1 plus pMA30 under induced conditions yielded pMA1-transducing particles of P4 capsid size. These cloning-based analyses directly demonstrate that sid protein is the only P4 gene product required for small-capsid size determination. In the absence of the P2 O gene product no capsids of any size are assembled during solo infection by P2. Nevertheless, P2 Oam mutant superinfection of a host carrying pMA1 and pMA30 under induced conditions yielded small P4-sized transducing particles. We therefore propose that (i) the sid gene product competes with the O gene product to determine the assembly of small vs. large capsid sizes and (ii) both gene products probably function as temporary scaffolding proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertani G., Ljungquist E., Jagusztyn-Krynicka K., Jupp S. Defective particle assembly in wild type P2 bacteriophage and its correction by the lg mutation. J Gen Virol. 1978 Feb;38(2):251–261. doi: 10.1099/0022-1317-38-2-251. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- Caspar D. L. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980 Oct;32(1):103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E. C., Christie G. E., Calendar R. Organization and expression of the satellite bacteriophage P4 late gene cluster. J Mol Biol. 1986 Dec 20;192(4):793–803. doi: 10.1016/0022-2836(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Diana C., Dehò G., Geisselsoder J., Tinelli L., Goldstein R. Viral interference at the level of capsid size determination by satellite phage P4. J Mol Biol. 1978 Dec 15;126(3):433–445. doi: 10.1016/0022-2836(78)90050-5. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Chidambaram M., Goldstein R. Transcriptional control of capsid size in the P2:P4 bacteriophage system. J Mol Biol. 1978 Dec 15;126(3):447–456. doi: 10.1016/0022-2836(78)90051-7. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Sedivy J. M., Walsh R. B., Goldstein R. Capsid structure of satellite phage P4 and its P2 helper. J Ultrastruct Res. 1982 May;79(2):165–173. doi: 10.1016/s0022-5320(82)90028-4. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Goldstein R., Lengyel J., Pruss G., Barrett K., Calendar R., Six E. Head size determination and the morphogenesis of satellite phage P4. Curr Top Microbiol Immunol. 1974;(68):59–75. doi: 10.1007/978-3-642-66044-3_3. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Sedivy J., Ljungquist E. Propagation of satellite phage P4 as a plasmid. Proc Natl Acad Sci U S A. 1982 Jan;79(2):515–519. doi: 10.1073/pnas.79.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M. Escherichia coli recA deletion strains that are highly competent for transformation and for in vivo phage packaging. Gene. 1989 Oct 30;82(2):313–315. doi: 10.1016/0378-1119(89)90056-5. [DOI] [PubMed] [Google Scholar]
- Lagos R., Goldstein R. Phasmid P4: manipulation of plasmid copy number and induction from the integrated state. J Bacteriol. 1984 Apr;158(1):208–215. doi: 10.1128/jb.158.1.208-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos R., Jiang R. Z., Kim S., Goldstein R. Rho-dependent transcription termination of a bacterial operon is antagonized by an extrachromosomal gene product. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9561–9565. doi: 10.1073/pnas.83.24.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Lin C. S. Nucleotide sequence of the essential region of bacteriophage P4. Nucleic Acids Res. 1984 Nov 26;12(22):8667–8684. doi: 10.1093/nar/12.22.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato L., Stirmer B., Showe M. K. Isolation and characterization of bacteriophage T4 mutant preheads. J Virol. 1978 Aug;27(2):409–426. doi: 10.1128/jvi.27.2.409-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Murialdo H. The role of gene Nu3 in bacteriophage lambda head morphogenesis. Virology. 1975 Mar;64(1):247–263. doi: 10.1016/0042-6822(75)90096-3. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. Constraints on the assembly of spherical virus particles. Virology. 1984 Apr 15;134(1):1–11. doi: 10.1016/0042-6822(84)90267-8. [DOI] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Shore D., Dehò G., Tsipis J., Goldstein R. Determination of capsid size by satellite bacteriophage P4. Proc Natl Acad Sci U S A. 1978 Jan;75(1):400–404. doi: 10.1073/pnas.75.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Six E. W., Klug C. A. Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology. 1973 Feb;51(2):327–344. doi: 10.1016/0042-6822(73)90432-7. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Martin K. V., Calendar R. On the sequence similarity of the cohesive ends of coliphage P4, P2, and 186 deoxyribonucleic acid. Biochemistry. 1973 May 22;12(11):2119–2123. doi: 10.1021/bi00735a016. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]