Abstract
Background
Kaufman oculo-cerebro-facial syndrome (KOS) is caused by recessive UBE3B mutations and presents with microcephaly, ocular abnormalities, distinctive facial morphology, low cholesterol levels and intellectual disability. We describe a child with microcephaly, brachycephaly, hearing loss, ptosis, blepharophimosis, hypertelorism, cleft palate, multiple renal cysts, absent nails, small or absent terminal phalanges, absent speech and intellectual disability. Syndromes which were initially considered include DOORS syndrome, Coffin-Siris syndrome and Dubowitz syndrome.
Methods
Clinical investigations coupled with, karyotype analysis, array-CGH, exome and Sanger sequencing were performed to characterize the condition in this child.
Results
Sanger sequencing was negative for the DOORS syndrome gene TBC1D24 but exome sequencing identified a homozygous deletion in UBE3B (NM_183415:c.3139_3141del, p.1047_1047del) located within the terminal portion of the HECT domain. This finding coupled with the presence of characteristic features such as brachycephaly, ptosis, blepharophimosis, hypertelorism, short palpebral fissures, cleft palate and developmental delay, allowed us to make a diagnosis of KOS.
Conclusion
Our findings highlight the importance of considering KOS as a differential diagnosis for patients under evaluation for DOORS syndrome and expand the phenotype of KOS to include small or absent terminal phalanges, nails, and the presence of hallux varus and multicystic dysplastic kidneys.
Keywords: Kaufman oculo-cerebro-facial syndrome, DOORS, UBE3B, whole exome sequencing, small phalanges
Introduction
Kaufman oculo-cerebro-facial syndrome (KOS) (MIM 244450) is an infrequently described autosomal recessive disease characterized by severe psychomotor retardation, microcephaly, hypotonia, ocular abnormalities, low cholesterol levels and distinctive facial dysmorphisms.1–4 Facial features include hypertelorism, epicanthus, upslanted palpebral fissures, short palpebral fissures, low-set, posteriorly angulated and round ears with narrow external meati. Variable features include seizures, hypoplasia/aplasia of corpus callosum, and renal anomalies. Basel-Vanagaite et al. identified biallelic UBE3B mutations as the genetic cause of KOS.2 In that report, the patients were initially reported as blephariphimosis-ptosis-intellectual disability syndrome (previously MIM 615057). However, when Flex et al.4 described UBE3B mutations in two KOS patients, re-evaluation of the Basel-Vanagaite patients allowed to re-classify them as having KOS.
To date, 14 molecularly-confirmed patients from 11 families have been reported.1–5 There is marked variability in the phenotype. The variability in the phenotype has caused some patients to be initially diagnosed as having other entities such as Toriello-Carey syndrome or a “new syndrome”.6, 7 Other syndromes with blepharophimosis and intellectual disability such as Ohdo syndrome (MIM249620) or the SBBYS variant of Ohdo syndrome (MIM603736), del(3p) syndrome, Maat-Kievit-Brunner syndrome (MIM 300895) and Dubowitz syndrome can be considered in the differential diagnosis of KOS.3 Herein, we present the clinical details of a boy with an unusual phenotype who was subsequently diagnosed having KOS following identification of a novel non frameshift deletion in UBE3B by exome and Sanger sequencing.
Materials and Methods
Clinical characterisation
This study was approved by the ethics committee of the Baylor College of Medicine. Written informed consent was obtained from the patient’s parents. Clinical records of physical examinations of both patients were reviewed. Prior to sequencing analysis, individual 1 underwent echocardiography and brain CT examination while individual 2 went through a series of clinical assessment including echocardiography, karyotype analysis, array-CGH, renal ultrasound, DMSA renal scan and cortical renal scintigraphy. Individual 2 presented with features consistent with DOORS syndrome including microcephaly, hearing loss, absent nail, small or absent terminal phalanges and intellectual disability, hence prompting initial targeted Sanger sequencing of all coding exons in TBC1D24.
Sanger Sequencing of TBC1D24
Sanger sequencing of Genomic DNA from the proband involving all coding exons of TBC1D24 was performed by Beckman Coulter genomics, USA. The reads were mapped to human TBC1D24 (NC_000016.10) and analysed using Geneious version R8.
Exome sequencing, analysis and Sanger Sequencing of UBE3B
Exome sequencing of the proband’s genomic DNA was conducted as described previously.8 Exomes were captured on Nimblegen’s Baylor VCRome library (Roche NimbleGen) and sequencing was performed on the Illumina HiSeq 2000 platform (Illumina). Sequencing reads were aligned to the human genome reference sequence (build GRCh37) with the Burrows-Wheeler Aligner (v.0.6.2) and potential duplicate paired-end reads were marked with Picard (v.1.58). Variants were called using Unified Genotyper algorithm from the Broad Institute Genome Analysis Toolkit (GATK v.2.6.4) and annotated with ANNOVAR.9 Called variants were filtered against dbSNP132, the 1000 Genomes project (February 2012 data release), and the NHLBI Exome Variant Server (EVS, January 2013 data release). An analysis of homozygous variants (considering consanguinity) demonstrated a novel deletion within exon 28 of the ubiquitin protein ligase E3B (UBE3B) gene. This variant was absent from the ExAC database. Primers spanning the deleted region were used to amplify a segment of exon 28 of UBE3B. Amplified genomic DNA from the proband and his parents, were sequenced by the Mcgill University and Génome Québec Innovation Centre. The Sanger sequencing reads were mapped to UBE3B (NC_000012.12) and analysed using Geneious version R8.
Results
Clinical Phenotypes
Individual 1 is the first child of first cousin Iranian parents. Pregnancy was uneventful and delivery at 9 months was by normal vaginal delivery. Birth weight, length and head circumference were 2550 grams (5th centile), 49 cm (50th centile) and 31 cm (<3rd centile), respectively. She was hospitalized at birth for ten days due to meconium aspiration pneumonia and respiratory distress. She had poor feeding, failure to thrive, hypotonia and history of frequent infections. She had three febrile seizures. Facial features included brachycephaly, thin sparse hair, prominent forehead, long face, blepharophimosis, ptosis, short palpebral fissures, hypertelorism, short nose, small mouth, and thin upper and lower vermillion (Figure 1a). There was no cupid bow. The hands were clenched and the wrists were held in a flexed position. No teeth had erupted at 18 months. She had a prominent clitoris giving the impression of ambiguous genitalia. Milestones were delayed. She passed away at the age of 18 months due to pneumonia. Echocardiography and brain CT scan were performed at 7 months of age and yielded both normal results. No exome or sanger sequencing was performed.
Figure 1.
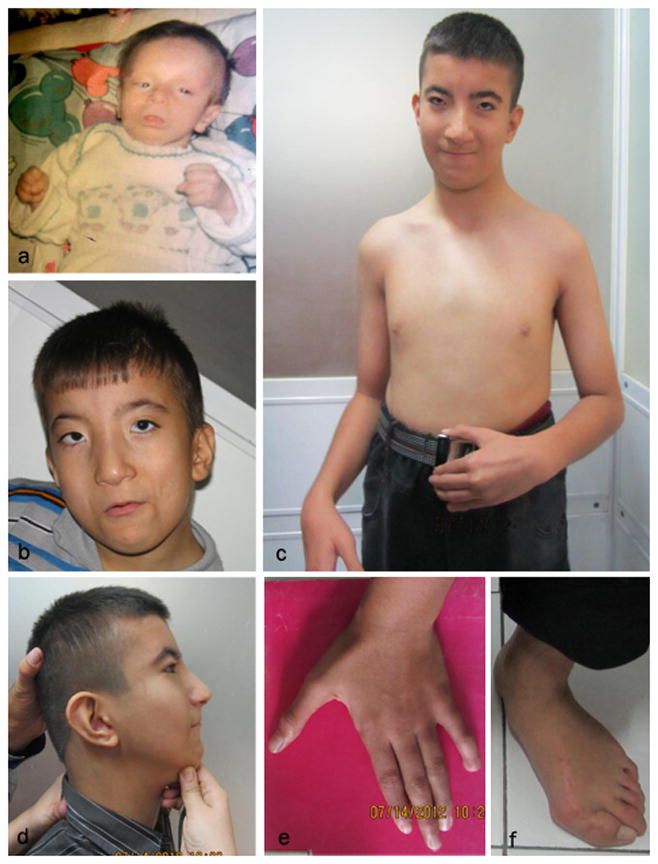
Individual 1 and 2. (a) Individual 1 at 9 months, (b) frontal view of individual 2 at age ten, (c) individual 2 at age 16 years, (d) lateral view of individual 2 at 16 years, (e) note the absent terminal phalanx and nail of the fifth finger of left hand and terminal hypoplasia of other fingers, (f) hallux valgus developed after surgery to repair severe hallux varus.
Individual 2 is the second child of this family. Pregnancy and delivery at 9 months were uneventful. At birth, it was noticed that the umbilical cord had a single umbilical artery. Birthweight was 2500 grams (5th centile), length was 49 cm (25th–50th centile) and head circumference was 33 cm (10th–25thcentile). He was hospitalized for two days because of respiratory distress. He had poor feeding during the first two years of life due to a cleft palate. Surgery to repair the cleft palate was performed at two years and his food intake improved thereafter. He had a history of frequent respiratory infections and three seizures following fever. He was placed on anticonvulsant treatment for two years. He had two operations at the age of seven, one for repair of bilateral inguinal hernia and correction of severe hallux varus (Figure 2a). The corrected hallux varus developed into hallux valgus after the surgery (Figure 1f)
Figure 2.
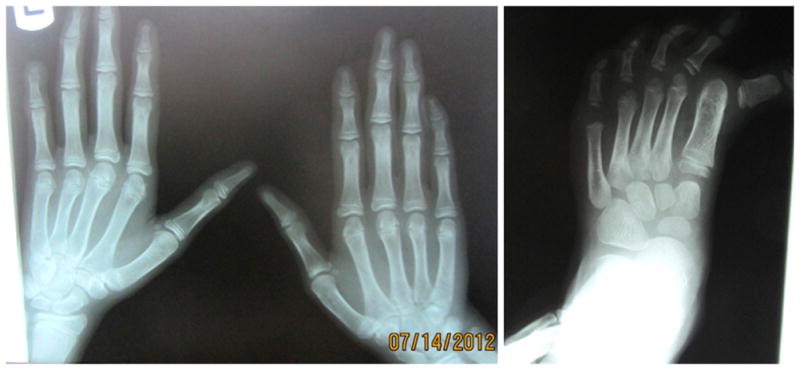
X-rays of left hand and foot. (a) Note hypoplasia of terminal phalanges and absence of terminal phalanx of fifth finger. (b) Note severe hallux varus before surgery.
Milestones were delayed; he held his head at 6 months, sat at 1 year and walked at the age of two years. He never acquired the ability to speak, but can communicate with parents with body language. He understands simple commands. He can perform daily activities such as eating, washing up, and dressing. At examination at ten years of age, his height was 135 cm (25th centile), weight was 28 kg (10th–25th centile) and head circumference was 49.5 cm (2nd centile). He also had brachycephaly, an elongated facies, epicanthal folds, blepharophimosis, ptosis, short palpebral fissures, microcornea, small mouth, thin upper and lower vermillions, no cupid’s bow, narrow ear canals and low-set posteriorly rotated ears (Figure 1b). In the limbs, he had small terminal phalanges of all fingers, absent left distal phalanx of the fifth finger and absent nail, and hallux valgus (Figure 1e). The surgical scars for hallux valgus correction on the feet were seen. Upon re-examination at 16 years of age, his height was 175 cm (50th centile), weight was 57 kg (25th–50th centile) and head circumference was 54 cm (25th–50th centile). The facial features had not changed (Figure 1c,d). He had a cheerful and friendly disposition. Even though he did not speak, he was cooperative during the examination and understood simple commands.
Chromosomal studies by karyotype and array-CGH failed to show chromosomal imbalances. Echocardiography and brain MRI yielded normal results. Ultrasound examination of the kidneys at the age of 10 years showed dilatation of the calyces of the left kidney and a distended renal pelvis and ureter. The right kidney was small and had multiple cystic lesions with loss of normal renal architecture. DMSA renal scan showed mild dilatation of left kidney with non-homogenous radiotracer uptake. The right kidney was not visualized showing no uptake. Cortical renal scintigraphy also did not show a right kidney suggesting a non-functioning right kidney.
Auditory brainstem response audiometry demonstrated a moderate sensorineural hearing loss. Total cholesterol levels measured at 16 years of age fell within the normal range: 127 mg/dL (normal <200mg/dL). HDL and LDL cholesterol levels were 25 mg/dL (normal >34mg/dL, 25–34 moderate risk, <25 high risk) and 88 mg/dL (normal<130, 130–160 moderate risk, >160 high risk), respectively.
Mutation Analysis
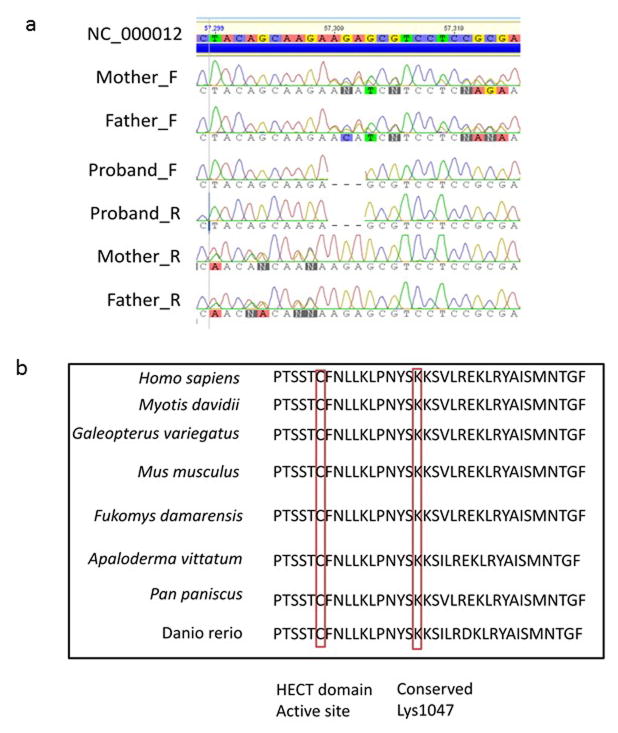
Clinical findings of our individual and all molecularly confirmed individuals are found in Table 1. Because of the abnormal nail and digits, the deafness, intellectual disability and seizures, our patient was included in a DOORS syndrome study. However, Sanger sequencing of all coding exons of the DOORS syndrome gene TBC1D24 failed to identify any mutation. Genomic DNA of individual 2 (proband) was then submitted for exome sequencing, which led to the identification of a novel non frame shift deletion in exon 28 of UBE3B (NM_183415:c.3139_3141del). This mutation results in the deletion of lysine at position 1047 of UBE3B; a few amino acid downstream of the catalytic HECT domain active site (Cys1036). Further assessment using bioinformatics software such as the Ensemble variant effect predictor (http://useast.ensembl.org/info/docs/tools/vep/index.html) and SIFT (http://sift.bii.a-star.edu.sg/www/SIFT_indels2.html), demonstrated the p.1047_1047del variant could be deleterious or damaging. Alignment of a segment of the human UBE3B HECT domain with other HECT domain-containing proteins in mammals, fish and birds showed excellent conservation of amino acids that are in close proximity to the HECT domain catalytic site including Lys1047 and Lys1048. Subsequent Sanger sequencing of DNA from the consanguineous parents alongside the proband, demonstrated that both parents were heterozygous state for the deletion (Figure 3a,b).
Table I.
Clinical features of molecularly confirmed Kaufman oculocerebrofacial syndrome
| C1741+2G>C | C545-2 A>G | C2180A>C | C61G>T | C556C>T | C1166G>A | C1445T>A | C2990G>C | C2335G>A | C2098C>T | C1A>G | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 Basel-Vanagaite et al. 2012 | Family 2 Basel-Vanagaite et al. 2012 | Family 3 Basel-Vanagaite et al. 2012 | Family 4 Basel-Vanagaite et al. 2012 | Family 5 Flex et al. 2013 | Family 6 Flex et al. 2013 | Family 7 Basel-Vanagaite et al. 2014 | Family 8 Basel-Vanagaite et al. 2014 | Family 9 Basel-Vanagaite et al. 2014 | Family 10 Basel-Vanagaite et al. 2014 | Family 11 Pedurupillay et al. 2014 | Family 12 Present case | |||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | ||
| Sex | F | F | M | F | M | F | F | M | F | M | F | M | F | F | M | |
| Age at examination | 3 years | 7 years | 3 years | 1 year | 33 years | NR | NR | 7 years | 2 years | 16 years | 10 years | 25 years | 8 years | 4 years | 10 y | |
| Birthweight in centile | 3rd | 3rd | 3rd | NR | normal | 25th | 3rd | normal | <3rd | <3rd | <3rd | NR | 3rd | 3rd | 5th | |
| OFC at birth in centile | −2SD | <3.5 | <3rd | NR | NR | NR | NR | NR | NR | NR | NR | <3rd | <3rd | <3rd | 25th | |
| Microcephaly <10th centile | + | + | + | + | − | + | + | + | + | + | + | + | + | + | − | 13/15 |
| Sparse thin scalp hair | + | + | + | + | − | − | − | − | − | + | + | − | − | − | + | 7/15 |
| Long face | − | + | + | + | + | + | − | − | − | + | − | − | + | − | + | 8/15 |
| Arched/sparse laterally broad eyebrows | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | 14/15 |
| Upslanting palpebral fissures | − | + | + | − | + | + | + | − | − | + | + | + | + | + | − | 10/15 |
| Epicanthal folds | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 15/15 |
| Ptosis | + | + | − | + | NR | − | + | NR | NR | NR | NR | NR | + | + | + | 7/15 |
| Blepharophimosis | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | 15/15 |
| Microcornea | − | − | − | − | + | + | + | − | − | − | − | − | − | − | + | 2/15 |
| Microphthalmia | − | − | − | − | + | + | + | − | − | − | − | − | + | + | − | 5/15 |
| Anteverted nares/short nose | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | 13/15 |
| Long philtrum | + | + | + | + | NR | + | + | NR | NR | NR | NR | NR | + | + | + | 9/9 |
| Small mouth | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | 14/15 |
| Thin upper lip | − | + | + | + | + | + | − | − | + | + | + | + | − | − | + | 10/15 |
| Small/frail teeth | − | − | − | NR | NR | + | + | NR | NR | NR | NR | NR | + | + | − | 4/8 |
| Micro/retrognathia | + | + | + | + | − | + | + | + | + | + | + | + | − | − | − | 11/15 |
| Dysplastic ears | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | 14/15 |
| Nail dysplasia | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | + | + | − | 2/3 |
| Hypoplastic/absent terminal phanlanges | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 1/15 |
| Pes talus varus/valgus | − | − | − | − | − | + | + | − | + | − | + | − | − | − | − | 4/15 |
| Hallux varus/valgus | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 1/15 |
| Absent nail | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 1/15 |
| Seizures | - | - | - | + | - | + | + | - | - | + | + | + | + | + | + | 9/15 |
| Hypotonia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 15/15 |
| Abnormal brain imaging | + | + | + | + | + | NR | NR | + | + | + | + | + | + | - | - | 11/13 |
| Renal anomalies | - | - | - | - | - | + | + | - | + | - | - | + | - | + | + | 6/15 |
| Respiratory distress | + | + | - | + | - | + | + | - | - | + | + | - | + | + | - | 9/15 |
| Feeding difficulties | + | + | + | + | NR | + | + | + | + | + | + | + | + | + | + | 14/14 |
| Genital anomalies | - | - | - | + | + | + | + | - | - | + | + | - | + | + | - | 8/15 |
| Hearing impairment | + | - | - | - | + | + | + | + | + | + | - | + | - | + | + | 10/15 |
| Developmental Disability/Intellectual disability | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 15/15 |
| Absent speech | + | + | + | + | + | + | + | - | + | + | + | - | + | + | + | 13/15 |
| Reduced cholesterol values | + | + | - | + | + | - | + | - | + | - | - | + | - | + | - | 8/15 |
| UBE3B mutations | c.1741+2G>C c.1741+2G>C |
c.545-2A>G c.2223-2224delAG |
c.2180A>C c.2180A>C |
c.61G>T c.61G>T |
c.556C>T c.556C>T |
c.1166G>A c.1166G>A |
c.1445T>A c.1616T>A |
c.2990G>C c.2990G>C |
c.2335G>A c.2335G>A |
c.2098C>T c.2990G>C |
c.1A>G c.1773delC |
c.3139_3141del c.3139_3141del |
||||
F, female; M, male; NR, Not reported
Figure 3.
Sanger sequencing analysis and alignment of a segment of UBE3B HECT domain. (a) Sanger sequencing demonstrating heterozygous mutation in parents and homozygous deletion in proband. (b) Conservation of amino acids that are in close proximity to the active site of UBE3B HECT domain, including lys1047.
Discussion
The addition of ubiquitin to substrate proteins, ubiquitination, is essential for the regulation of cellular processes involving protein degradation, cellular localization as well as protein-protein interactions.10 Crucial in the ubiquitination cascade are three types of enzymes namely ubiquitin-activating enzymes, ubiquitin-conjugating enzymes, and ubiquitin ligases. Ubiquitin ligases complete the action of upstream enzymes in the ubiquitination pathway by establishing a covalent isopeptide bond between the C-terminal glycine of ubiquitin and a lysine of the target protein.10, 11 In humans, about 28 proteins have been identified as belonging to the HECT family of ubiquitin ligases.11 Among these is UBE3B, which contains an IQ and a HECT domain.12 The HECT domain mediates the final link between ubiquitin and the substrate protein and mutations within this domain have been described in a few patients with KOS.2, 3
Here we report a family with two children with Kaufman oculo-cerebro-facial syndrome identified by exome sequencing on the second child. Exome sequencing identified a homozygous, non-frameshift deletion of a lysine residue close to the end of UBE3B HECT domain (NM_183415:exon28:c.3139_3141del:p.1047_1047del). The deleted Lys1047 is highly conserved among several species and predicted to have a negative effect on the protein, especially considering its close proximity to the predicted active site (Cys1036) of UBE3B HECT domain. Subsequent targeted Sanger sequencing of exon 28 of UBE3B confirmed the homozygous deletion in the proband and the heterozygous deletion in the parents.
Similarly to the finding of this study, a significant proportion of UBE3B variants described so far occurs within the HECT domain and are predicted to perturb the E3 ligase function of the UBE3B protein.3, 5 It is also worth noting that a subset of the UBE3B variants that occur upstream of the HECT domain are either missense, truncating or frameshift and hence, are perceived to compromise the enzymatic activity of the HECT domain or the function of UBE3B protein. Even though UBE3B is expressed in the brain and craniofacial structures during embryonic development,2 it is unclear how mutations in UBE3B lead to KOS. This is probably due to the fact that the actual substrates of UBE3B remain largely unknown. Nonetheless, since UBE3B belongs to the family of E3 ubiquitin-conjugating enzymes, alterations of conserved residues, especially those affecting the HECT domain, could presumably alter ubiquitination, protein interaction and degradation of key substrates implicated biological processes such as brain development, cholesterol production, reproduction and growth. In fact, UBE3B null-mice have deficiencies in cholesterol and lathosterol production, reduced brain size, diminished reproductive potential, severe growth defects as well as augmented embryonal and/or perinatal lethality.2 Moreover, Venhoranta and colleagues13 recently noted that Ayrshire cattle bearing a mutation that results in deletion of 40 amino acids, 20 of which occurs within the HECT domain of UBE3B, presented with ptosis, reduce size, hypotonia, abnormal upper eye lid, learning deficiencies and required special care; findings that are consistent with those seen in patients with aberration in UBE3B and presenting with clinical features of KOS.
Individual 2 was initially examined when he was 10 years old, and based on the clinical findings including low birth weight, microcephaly, hypertelorism, ptosis, short palpebral fissures, cleft palate, delayed tooth eruption (in individual 1), speech delay, and intellectual disability, Dubowitz syndrome was suspected. However, we were aware that our patients had clinical features that were not consistent with Dubowitz syndrome such as microcornea, hearing impairment, renal abnormalities, small and absent terminal phalanges and absent nails.
Syndromes that included small/absent terminal phalanges and absent nails such as DOORS syndrome and Coffin-Siris syndrome were other considerations. Our patient had microcephaly, hearing loss, hypertelorism, ptosis, cleft palate, multiple renal cysts, absent nail, small/absent terminal phalanges, and intellectual disability which are all consistent with DOORS syndrome but the presence of blepharophimosis, microcornea, long face and thin lower lips were not consistent with this diagnosis. The absence of the terminal phalange of the fifth finger and nail made us also consider Coffin-Siris syndrome but the facial gestalt of this condition (coarse face, thick eyebrows, long prominent eyelashes, macrostomia and prominent lips) was not present in our patients.
Our patients have the main characteristic findings of KOS such as brachycephaly, ptosis, blepharophimosis, hypertelorism, short palpebral fissures, cleft palate, intellectual disability and absent speech. However, individual 2 has some features that were not previously reported in KOS.
Mild limb abnormalities including long thin hands, polydactyly, clinodactyly of fifth finger, and small distal phalanges have been reported in KOS.3, 7, 14 While small distal phalanges have been previously reported, absent terminal phalanges and absent nails have not been described before in KOS. Pes talus valgus/varus has been reported but the severe hallux varus seen in our patient has not been reported before.
Renal anomalies such as vesicoureteral reflux up to grade V and duplicated renal pelvis, and abnormal shape of kidneys without functional problems have been reported.3, 15 Our patient has cystic lesions in the left kidney which made it non-functional. This finding has not been previously reported.
Ophthalmological abnormalities have been reported such as microphthalmia, pale optic discs, iris coloboma and microcornea.1, 2, 4, 7 The microcornea present in individual 2 is a relatively rare finding and present in only 1/14 molecularly-confirmed individuals.
In conclusion, we would like to stress the clinical variability of KOS and add absent terminal phalanges and nails, hallux varus and multiple renal cysts as features of this disorder, which increases the clinical variability of the disorder. This being said, patients diagnosed with atypical DOORS or Coffin-Siris syndrome without mutations in genes known to cause these disorders might be affected by KOS and re-evaluation of such patients should be considered.
Acknowledgments
We thank the family for participating in this project, and The Réseau de Médecine Génétique Appliquée du Québec for help with bioinformatics analyses. NFA is funded by the CHU Sainte-Justine Foundation, the Fonds de recherche du Québec - Santé postdoctoral scholarship. PMC is funded by the Canadian Institutes of Health Research Grants CIHR RN315908 and RN324373, and the Fonds de recherche du Québec Santé grant FRQS 30647. This work was supported by the BCM Intellectual and Developmental Disabilities Research Center (HD024064) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the BCM Advanced Technology Cores with funding from the NIH (AI036211, CA125123, and RR024574), the Rolanette and Berdon Lawrence Bone Disease Program of Texas, and the BCM Center for Skeletal Medicine and Biology and NIH grants U54 HG006542 (to RAG) and U54 HG003273-09 (to RAG).
Footnotes
Authors declare no conflict of interest.
References
- 1.Kaufman RL, Rimoin DL, Prensky AL, Sly WS. An oculocerebrofacial syndrome. Birth defects original article series. 1971;7:135–138. [PubMed] [Google Scholar]
- 2.Basel-Vanagaite L, Dallapiccola B, Ramirez-Solis R, Segref A, Thiele H, Edwards A, et al. Deficiency for the ubiquitin ligase UBE3B in a blepharophimosis-ptosis-intellectual-disability syndrome. American journal of human genetics. 2012;91:998–1010. doi: 10.1016/j.ajhg.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basel-Vanagaite L, Yilmaz R, Tang S, Reuter MS, Rahner N, Grange DK, et al. Expanding the clinical and mutational spectrum of Kaufman oculocerebrofacial syndrome with biallelic UBE3B mutations. Human genetics. 2014;133:939–949. doi: 10.1007/s00439-014-1436-2. [DOI] [PubMed] [Google Scholar]
- 4.Flex E, Ciolfi A, Caputo V, Fodale V, Leoni C, Melis D, et al. Loss of function of the E3 ubiquitin-protein ligase UBE3B causes Kaufman oculocerebrofacial syndrome. Journal of medical genetics. 2013;50:493–499. doi: 10.1136/jmedgenet-2012-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedurupillay CR, Baroy T, Holmgren A, Blomhoff A, Vigeland MD, Sheng Y, et al. Kaufman oculocerebrofacial syndrome in sisters with novel compound heterozygous mutation in UBE3B. American journal of medical genetics. Part A. 2015;167A:657–663. doi: 10.1002/ajmg.a.36944. [DOI] [PubMed] [Google Scholar]
- 6.Toriello HV, Carey JC, Addor MC, Allen W, Burke L, Chun N, et al. Toriello-Carey syndrome: Delineation and review. American journal of medical genetics. 2003;123A:84–90. doi: 10.1002/ajmg.a.20493. [DOI] [PubMed] [Google Scholar]
- 7.Buntinx I, Majewski F. Blepharophimosis, iris coloboma, microgenia, hearing loss, postaxial polydactyly, aplasia of corpus callosum, hydroureter, and developmental delay. American journal of medical genetics. 1990;36:273–274. doi: 10.1002/ajmg.1320360304. [DOI] [PubMed] [Google Scholar]
- 8.Campeau PM, Lu JT, Sule G, Jiang MM, Bae Y, Madan S, et al. Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Human molecular genetics. 2012;21:4904–4909. doi: 10.1093/hmg/dds326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic acids research. 2010;38:e164–e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nature medicine. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 11.Scheffner M, Kumar S. Mammalian HECT ubiquitin-protein ligases: Biological and pathophysiological aspects. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2014;1843:61–74. doi: 10.1016/j.bbamcr.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Gong TWL, Huang L, Warner SJ, Lomax MI. Characterization of the human UBE3B gene: structure, expression, evolution, and alternative splicing. Genomics. 2003;82:143–152. doi: 10.1016/s0888-7543(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 13.Venhoranta H, Pausch H, Flisikowski K, Wurmser C, Taponen J, Rautala H, et al. In frame exon skipping in UBE3B is associated with developmental disorders and increased mortality in cattle. BMC Genomics. 2014;15:1–9. doi: 10.1186/1471-2164-15-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briscioli V, Manoukian S, Selicorni A, Livini E, Lalatta F. Kaufman oculocerebrofacial syndrome in a girl of 15 years. American journal of medical genetics. 1995;58:21–23. doi: 10.1002/ajmg.1320580106. [DOI] [PubMed] [Google Scholar]
- 15.Dentici ML, Mingarelli R, Dallapiccola B. The difficult nosology of blepharophimosis-mental retardation syndromes: report on two siblings. American journal of medical genetics. Part A. 2011;155A:459–465. doi: 10.1002/ajmg.a.33642. [DOI] [PubMed] [Google Scholar]