Summary
Development of multiple drug resistance has been attributed to the overexpression of the ATP‐binding cassette B1 (ABCB1) gene. In this study, the major purpose was to assess the expression and methylation levels of ABCB1 in human lung adenocarcinoma and to reveal the relationship between these processes and acquisition of cisplatin (DDP) resistance in the human cancer cell line A549. Methylation and expression levels of the ABCB1 gene ABCB1 in clinical human lung tissue were assessed using bisulphite sequencing, reverse transcription real‐time PCR (RT 2‐PCR) and Western blot methods. Cell viability, DDP resistance and apoptosis of A549 cells were evaluated using the Cell Counting Kit‐8 and fluorescence‐activated cell sorter analysis. Our results showed that the onset of resistance to the cisplatin analogue, DDP, was associated with hypermethylation of the ABCB1 gene. Expression of the ABCB1 gene was enhanced at both mRNA and protein levels. Treatment with 5‐Aza‐C contributed to the hypomethylation of the ABCB1 gene and decreased ABCB1 protein expression in A549 cells. In conclusion, this in vitro and human tissue study of lung adenocarcinoma cells demonstrated that hypermethylation of the ABCB1 gene correlated with increased gene expression and was associated with the acquisition of resistance to the cisplatin analogue, DDP in human lung adenocarcinoma cells. Taken together, our study highlighted the connection between increased ABCB1 methylation level and upregulated expression of the gene in lung cancer. Moreover, the abnormally high expression of ABCB1 in A549 cells contributed to the development of the DDP resistance.
Keywords: ATP‐binding cassette B1, Biomarker, diamminedichloroplatinum, Hypermethylation, lung cancer
Introduction
Lung cancer, specifically non‐small‐cell lung cancer (NSCLC), is one of the most commonly diagnosed cancer types and causes more than 1.38 million deaths worldwide every year (Ferlay et al. 2010). NSCLC comprises squamous cell carcinoma and adenocarcinoma. Adenocarcinoma of the lung is less amenable to surgical treatment and is more commonly treated with chemotherapy. Chemotherapy is an important component in the range of available treatments for lung cancer. However, chemotherapy can be less effective due to the side effects and the high recurrence rate in lung cancer. The reduced effectiveness of chemotherapy in the treatment of lung cancer and other cancers has been shown to be mainly due to the development of multiple drug resistance (MDR) (Lu et al. 2013).
MDR is one of the major obstacles for cancer chemotherapy. There many pathways involved in the development of MDR, including increased drug efflux, and modifications in DNA repair and apoptotic signalling pathways (Jabr‐Milane et al. 2008). The most widely recognized mechanism for the development of MDR has been attributed to the overexpression of the ATP‐binding cassette B1 (ABCB1) (multidrug resistance 1) MDR1 gene that encodes permeability glycoprotein (P‐gp). P‐gp is a 170‐kDa protein, the function of which is to export molecules, including drugs from the cell (Bosch & Croop 1996). Overexpression of ABCB1 and P‐gp in cancer cells will reduce the accumulation of chemotherapeutic drugs within the cancer cell (Gros et al. 1986; Pastan et al. 1988; Endicott & Ling 1989; Herzog et al. 1993; Ling 1997).
A study by Gervasini and colleagues reported that the gene polymorphism of ABCB1, G2677T/A, was closely associated with increased risk of lung cancer (Gervasini et al. 2006). Wang and colleagues showed that the genetic susceptibility of lung cancer to chemotherapy was related to the common variants in 3′UTR of the ABCB1 gene (Wang et al. 2009). Therefore, we hypothesize that the targeted blockade of transcription of ABCB1, which will further inhibit the expression of P‐gp, may improve the efficacy of existing chemotherapeutic agents in the treatment of lung cancer and other cancers (Ford & Hait 1990).
There have been some recent epigenetic studies that have begun to reveal the mechanisms that drive the upregulation of the ABCB1 gene in cancer cells. The downstream promoter of the ABCB1 gene contains a short interspersed DNA sequence known as a CpG island that makes the ABCB1 gene a possible candidate for epigenetic regulation of its function, including methylation. The hypermethylation of CpG islands within the downstream gene promoter has been shown to contribute to the activation of ABCB1 transcription (Reed et al. 2008). Several studies have supported the role for the ABCB1 gene in which hypomethylation of the upstream promoter in drug‐resistant tumour cells has been shown to be accompanied by increased ABCB1 gene transcription (Desiderato et al. 1997; Chen et al. 2005). Although data from several studies have now supported the key role of the methylation state of the ABCB1 gene in the development of cancer cell drug resistance, few studies have been carried out in lung cancer. Given the prevalence and poor prognosis of lung cancer, the role of hypermethylation in the development of resistance to chemotherapy was believed to be an important area for study, with a view of developing future therapeutic strategies against this important cancer.
Based on the findings of these previous studies, the aim of this preliminary study was to investigate the role of hypermethylation of the ABCB1 gene downstream promoter and the overexpression of the ABCB1 gene in the development of MDR in lung adenocarcinoma. We determine the methylation status and expression levels of the ABCB1 gene in human lung tumours and normal adjacent lung tissues using bisulphite sequencing (BSP) and Western blot. The methylation status and expression level of the ABCB1 gene in the human lung adenocarcinoma cell line, A549, can be downregulated by incubation with the 5‐azacytidine (5‐Aza‐C) as previously described (Guo et al. 2013). We study the drug‐resistant capability of A549 lung adenocarcinoma cells, when treated with different concentrations of the cisplatin analogue, diaminedichloroplatinum (DDP), using techniques including the Cell Counting Kit‐8 cell viability in cell proliferation assay and fluorescence‐activated cell sorting (FACS). We believe that this study revealed the association of the methylation state of the ABCB1 gene with MDR in lung adenocarcinoma cells. This supplements a previous study (Guo et al. 2013) and is valuable for developing future therapeutic strategies against this important cancer.
Materials and methods
Patients studied
Twenty patients were studied (12 men: 8 women) (age range, 35–68 years; mean age, 57 years). All patients had undergone lobectomy for peripheral adenocarcinoma of the lung, with confirmed histological diagnosis. In each case, the diagnostic pathologist was able to obtain a sample of tumour for the study and a sample of normal, adjacent lung. All patients had detailed clinical information available, relating to tumour grade, stage and other prognostic indices. Patients who had undergone any lung cancer therapy before surgery were excluded. Tissue sections were prepared from tissue samples and examined histologically by senior pathologists; some tissue was stored frozen at −80°C for further analysis. Matched pairs of lung tumour tissues and normal, adjacent lung samples were used for the detection of methylation and expression levels of ABCB1.
Local ethics committee approval was given for this study, including patient screening, tissue sampling and patient data collection. All patients who participated in the study signed a written informed consent form. All investigations were undertaken according to the provisions of the Declaration of Helsinki.
A549 lung adenocarcinoma cell line and cell culture
The human lung adenocarcinoma cell line A549 was purchased from American Type Culture Collection (ATCC) and cultured in RPMI‐1640 medium supplemented with 10% foetal bovine serum (FBS), 100 U/ml of penicillin and 100 U/ml of streptomycin in an atmosphere of 5% CO2 at 37°C with a saturated humidity. The A549 cells were selected for resistance to DDP (Sigma‐Aldrich, St. Louis, MO, USA) using the CCK8 assay as described previously: in brief, selection began at a drug dose (dose 1) that was 1000‐fold less than the concentration at which 50% of parental A549 cells are killed (the IC50). The dose was then increased 1.5‐ or threefold until the maximally tolerated dose was achieved. Table 1 depicts the DDP concentrations (doses) to which the cells were exposed. The resistance index of A549/DDP lung cancer cells was 11.07. The selected A549/DDP cell lines were cultured routinely for subsequent experiments.
Table 1.
Concentrations of DDP used at each selection dose
| Dose | DDP |
|---|---|
| IC50 | 7.20 μg/ml |
| Dose 1 | 7.20 pg/ml |
| Dose 2 | 21.6 pg/ml |
| Dose 3 | 64.8 pg/ml |
| Dose 4 | 194.4 pg/ml |
| Dose 5 | 583.2 pg/ml |
| Dose 6 | 1.75 μg/ml |
| Dose 7 | 5.25 μg/ml |
| Dose 8 | 15.75 μg/ml |
| Dose 9 | 23.63 μg/ml |
| Dose 10 | 35.44 μg/ml |
| Dose 11 | 53.16 μg/ml |
| Dose 12* | 79.73 μg/ml |
A549 human lung adenocarcinoma cells were exposed to progressively higher concentrations (doses) of DDP drugs. The initial dose was equivalent to 1/1000th of the concentration required to kill or inhibit the growth of 50% of wild‐type A549 cultured human lung adenocarcinoma cells (the IC50). The symbol *denotes the highest concentration of DDP allowing for cell survival (the maximally tolerated dose).
Antibodies, cisplatin analogue diamminedichloroplatinum (DDP) and DNA methyltransferase (DNMT) inhibitor 5‐azacytidine (5‐Aza‐C)
Antibody against the ABCB1/MDR1 (Catl. No. A‐6195‐100) protein was purchased from Epigentek, New York, USA. Antibody against GAPDH (Catl. No. ab8245) was purchased from Abcam, Cambridge, UK. The cisplatin analogue, diaminedichloroplatinum (DDP), and 5‐azacytidine (5‐Aza‐C), which is a DNA methyltransferase (DNMT) inhibitor, were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Plasmid construction
To generate an ABCB1 expression vector, the coding sequence of ABCB1 (NM_000927.4, 3843 bp) was synthesized and subcloned into pCDNA4.1 vector (constructed by Sango Biotech, Shanghai, China). And the plasmids were confirmed by DNA sequencing (Sangon, Shanghai, China).
Experimental design for in vitro assays
A549 and A549 + 5‐Aza cells (A549 cells pre‐incubated with 100 μM 5‐Aza) were subjected to DDP (7.20 μg/ml) for 72 h for subsequent in vitro assays. Thereafter, A549 cells were then transfected with ABCB1 promoter to form A549/ABCB1 cells. Twenty‐four hours after transfection, the cells were collected and cultured routinely, and then, A549 cells and A549/ABCB1 cells were incubated with DDP (22.52 μg/ml) for 72 h for subsequent in vitro assays.
Reverse transcription real‐time polymerase chain reaction (RT2‐PCR)
Whole RNA was extracted from cell lines and lung tissues using the TRIzol method, following the manufacturer's instructions. GAPDH was selected as the reference gene. cDNA templates were achieved by reverse transcription of the RNA using an RT‐PCR kit (Fermentas, St. Leon‐Rot, Germany). The reaction mixture of 20 μL volume consisted of 10 μL of SYBR Premix Ex Taq II (Clontech) with 0.5 μL of each primer, 1 μL of the cDNA template and 8 μL of RNase‐free H2O. The primers were as follows: ABCB1: forward: 5′‐GTCCCAGGAGCCCATCCT‐3′, reverse: 5′‐CCCGGCTGTTGTCTCCATA‐3′; GAPDH: forward: 5′‐TATGATGATATCAAGAGGGTAGT‐3′, reverse, 5′‐TGTATCCAAACTCATTGTCATAC‐3′. Amplification conditions were as follows: a denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 1 min and 72°C for 30 s. Relative expression levels of ABCB1 were analysed with Applied Biosystems (Thermo Fisher Scientific, Waltham, MA, USA) according to the expression of 2−▵▵ct.
Western blot
The total protein extracted from cultured cells and tissues were used for the Western blot assay. GAPDH protein was used as a reference. The protein concentration of cell samples was determined according to the bicinchoninic acid (BCA) assay method; 40 μg of protein was subject to a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS‐PAGE). After transferring proteins onto polyvinylidene difluoride (PVDF) sheets, the membranes were washed with a mixture of Tris‐buffered saline and Tween‐20 (TTBS) for 5 min and then incubated with skim milk powder solution for 1 hour. Primary antibodies against the ABCB1/MDR1 protein (1:800) or GAPDH (1:2000) were incubated with PVDF membranes at 4°C overnight. After four additional washes using TTBS, secondary horseradish peroxidase (HRP)‐conjugated IgG antibodies (1:2000) were incubated with the membranes for 45 min at 37°C. Following a further six washes using TTBS, the blots were developed using BeyoECL Plus reagent (Beyotime Biotechnology, China), and the results were recorded in the Gel Imaging System. The relative expression levels of ABCB1/MDR1 or GAPDH in different samples were calculated using the Gel‐Pro Analyzer (Media Cybernetics, Rockville, MD, USA).
Bisulphite sequencing
The methylation status of CpG sites within the ABCB1 promoter in cultured cells and lung tissue samples was examined by bisulphite sequencing as previously described (Bromer et al. 2009). Briefly, genomic DNA was extracted using a DNeasy Blood & Tissue Kit (Qiagen, Mississauga, ON, CA, USA). Bisulphite conversion was performed on 2 μg of DNA using an EpiTect Bisulfite Kit (Qiagen, Mississauga, ON, CA). Converted DNA was amplified with two sequential PCR using the following nested primers: PCR1: 5′‐TATTAAATAAAGG ATGAATAGATGTAATTTAG‐3′ and 5′‐AAAACAAACTAATTACCTTTTATTATTCAAT‐3′; PCR2: 5′‐T TGTTAAGTATGTTGAAGAAAGATTATTGT‐3′ and 5′‐AAAACAAACTAATTACCTTTTATTATTCAAT‐3′. For each PCR, 100 ng of converted DNA, 1.5 mM MgCl2, 200 μM dNTP, 1 μM of primers, 2.5 units of Platinum Taq (Invitrogen, Burlington, ON, CA) and 1 × Platinum Taq buffer (Invitrogen, Burlington, ON, CA) were used in a 50‐μL reaction volume. 5 μL of PCR1 product was used as the template for the second PCR. The PCR cycles were as follows: 94°C for 2 min, 5 cycles of 94°C for 1 min, 50°C for 2 min, 72°C for 3 min followed by 35 cycles of 94°C for 30 s, 50°C for 2 min, 72°C for 1.5 min and a final extension of 72°C for 10 min. The reaction products were purified by gel extraction from a 2% agarose gel. The purified PCR products were sequenced by Sangon (Shanghai, China), and data analysis was performed using BiQ Analyzer software (Bock et al. 2005).
Immunohistochemical detection
For immunohistochemical assay, paraffin sections from patients were placed at 60°C for 2 h before incubation with dimethylbenzene for dewaxing. The sections then were hydrated with different concentrations of alcohol (95% for 2 min, 85% for 2 min and 75% for 5 min.) and washed with ddH2O for 2 min. Afterwards, sections were fixed using 3% H2O2 for 15 min and washed with PBS for three times. They were then incubated with primary antibody (1:50) at 37°C for 30 min before incubation at 4°C overnight. After three cycles of 0.01 M PBS wash, 5 min for each cycle, secondary antibody (1:200) was added to the sections and placed at 37°C for 30 min before another five cycles of PBS wash. HRP‐labelled avidin was then added and incubated with the sections at 37°C for 30 min before addition of DAB. The reaction was carried out for 3–10 min and then stopped by adding ddH2O. Slides were restained using haematoxylin and dehydrated. Scores of the immunohistochemical assay were determined by scanning the slides using an Aperio ScanScope GL (Aperio Technologies, Vista, CA, USA) at 200 × magnification.
Cell proliferation assay
Cell Counting Kit‐8 (CCK‐8) was used to detect cell proliferation. 1 × 104 cells were seeded into a 96‐well plate. Cell viability was evaluated using Cell Counting Kit‐8 at daily intervals from the next 24, 48 and 72 h after seeding. After treated with CCK‐8 at 37°C for 1 h, A549 cells were used to measure the absorbency at 450 nm using a microplate reader Thermo Plate (Rayto Life and Analytical Science Co. Ltd, Germany). Moreover, the cell viabilities of cells sampled at 48 h were further estimated by EdU test using Cell‐Light™ EdU Cell Proliferation Detection (Catl. No. C10338‐3, RiboBio, Guangzhou, China) according to the manufacturers’ instruction. The cell viability of different treatments was measured using a FACS flow cytometer (Accuri C6, BD, USA) as described previously.
Flow cytometry
Cell apoptosis was determined by flow cytometry using an annexin V‐FITC/PI apoptosis detection kit (BestBio, China) in accordance with the manufacturer's instruction. Briefly, cultured cells from different time points were incubated with 5 μL annexin V for 10 min at room temperature and then resuspended with binding buffer supplemented with 5 μL propidium iodide (PI). The cell apoptosis rates were detected using a flow cytometer. Following staining, the cells were gated into quadrants. The cell apoptotic rate was calculated as: upper right (UR) quadrant + lower right (LR) quadrant and as the percentage of apoptotic cells and was equal to the sum of the late apoptotic rate (UR) and the early apoptotic rate (LR).
Hoechst staining
Additionally, the morphological changes in the nuclei of apoptotic cells in different groups were also detected using Hoechst staining kit according to the manufacturer's instruction (Beyotime Biotechnology, Shanghai, China), and the results were observed under a fluorescence microscope at a magnification of 400 ×.
Statistical analysis
Data were expressed in the form of mean ± SD. Student's t‐test was used, and a P‐value < 0.05 was considered to be statistically significant. The statistical analysis was conducted using IBM SPSS Statistics for Windows, version 19.0 (IBM, Armonk, NY, USA).
Ethical approval statement
This study had been approved by the Ethical and Scientific Committees of the 2nd Affiliated Hospital of Guilin Medical University and in accordance with Declaration of Helsinki.
Results
Expression and methylation levels of the ABCB1 gene were enhanced in lung tumour tissues
The results of reverse transcription real‐time PCR (RT‐PCR) and Western blotting analysis showed that the expression of the ABCB1 gene was enhanced at both mRNA and protein levels, and the differences in gene and protein levels between tumour tissues and normal adjacent lung tissues were statistically significant (Figure 1a–c) (P < 0.001). The results were further validated by immunochemical detection, in which ABCB1‐positive cells were stained brown (Figure 1d) showing the association of methylation status with the upregulation of ABCB1 expression; the distribution of ABCB1‐positive cells was expanded. The results of bisulphite sequencing analysis of all the samples are shown in Figure 1e. Aberrant upregulation of DNA methylation in the ABCB1 downstream promoter was observed in the lung tumour samples (P < 0.01). Methylation sites are shown in Figure 1f.
Figure 1.
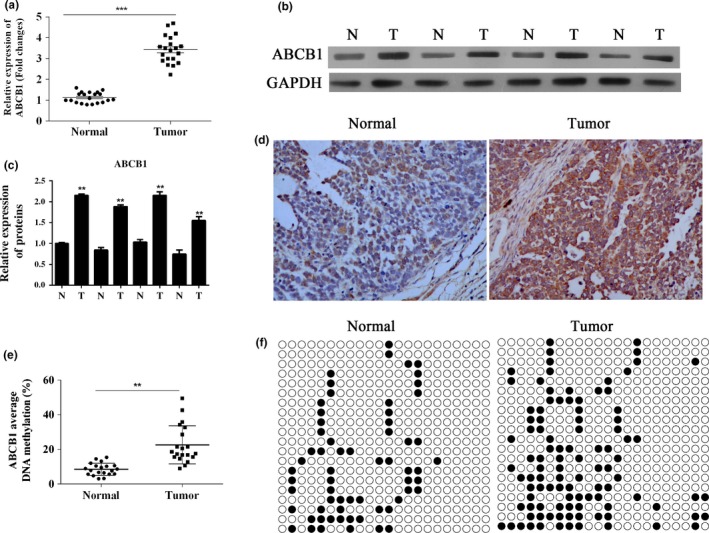
Upregulation of the expression and methylation levels of the ABCB1 gene in clinical lung adenocarcinoma samples. (a) RT‐PCR analysis of ABCB1 expression in human lung adenocarcinoma samples (tumour) and normal lung tissues (normal). ***P < 0.001. (b) Western blot analysis of ABCB1 expression in different tissues, including human lung adenocarcinoma tissues (T) and normal lung tissues (N). GAPDH is included as loading control. (c) Quantitative analysis results of Western blot. (d) Immunochemical detection results of ABCB1 in normal and tumour tissues. (e) The methylation levels of ABCB1 downstream promoter in human lung adenocarcinoma tissues and normal tissues were detected using bisulphite sequencing. (f) Methylation sites of ABCB1 gene. **P < 0.01.
Enhanced expression and methylation levels of ABCB1 were detected in A549/DDP cells
To demonstrate the possible connection between the cisplatin analogue DDP resistance of the A549 cell and upregulation of the ABCB1 gene, the gene methylation state and production of ABCB1 protein were quantified in both A549 and A549/DDP cell lines. The targeted parameters were enhanced in A549/DDP cells (Figure 2a), and the differences in ABCB1 expression and methylation levels between the two cell lines were statistically significant (Figure 2b,c) (P < 0.01). Methylation sites are shown in Figure 2d. These results may reflect the association between acquisition of DDP resistance and upregulation of the ABCB1 gene.
Figure 2.
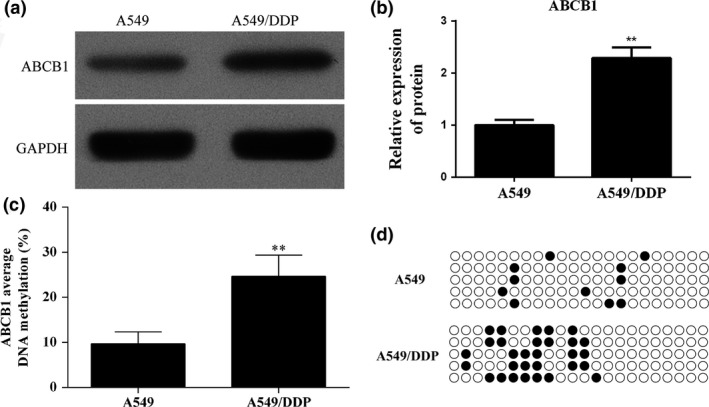
Enhanced expression and methylation levels of ABCB1 were detected in A549/DDP cells. (a) The expression of ABCB1 in A549 and DDP resistance A549 cells was detected using Western blot. (b) Quantitative analysis results of Western blot. (c) Bisulphite sequencing was performed to detect the methylation levels of ABCB1 downstream promoter in A549 and DDP resistance A549 cells. (d) Methylation sites of ABCB1 gene. **P < 0.01.
Pretreatment of DNA methyltransferase (DNMT) inhibitor 5‐azacytidine (5‐Aza‐C)‐induced hypomethylation of the ABCB1 gene and decreased expression of ABCB1 protein in A549 cells
To investigate the role of 5‐Aza‐C on ABCB1 downstream promoter methylation and its protein expression, A549 cells were pretreated with 100 μM 5‐Aza‐C for 24 h. Pretreatment with 100 μM 5‐Aza‐C led to a decreased level of methylation of the ABCB1 gene downstream promoter (Figure 3a). Methylation sites are shown in Figure 3b. The decrease in ABCB1 gene downstream promoter methylation was associated with the downregulation of ABCB1 at the protein level (Figure 3c), and the difference between A549 cells and 5‐Aza‐C cells was statistically significant (Figure 3d) (P < 0.01).
Figure 3.
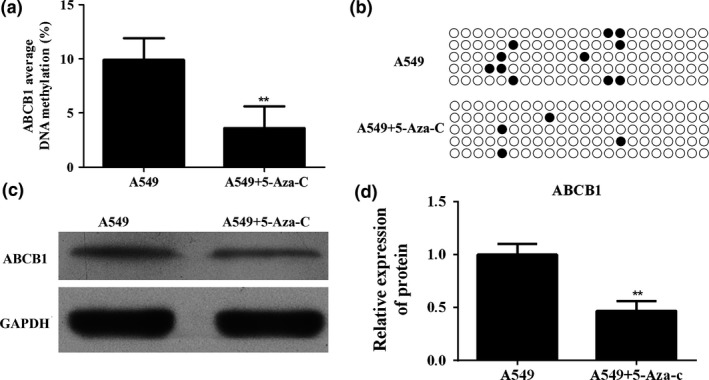
Pretreatment of DNA methyltransferase (DNMT) inhibitor 5‐azacytidine (5‐Aza‐C)‐induced hypomethylation of the ABCB1 gene and decreased expression of ABCB1 protein in A549 cells. (a) Bisulphite sequencing was performed to detect the methylation levels of ABCB1 downstream promoter in A549 cell after 5‐Aza‐C treatment. (b) Methylation sites of ABCB1 gene. (c) Western bolt analysed the expression of ABCB1 in A549 cells after 5‐Aza‐C treatment. (d) Quantitative analysis results of Western blot. **P < 0.01.
Downregulation of methylation of the ABCB1 gene decreased the DDP resistance in A549 lung adenocarcinoma cell lines
Based on the Cell Counting Kit‐8 (CCK‐8) cell viability in cell proliferation assay, the IC50 of DDP used for cell experiments was 7.20 μg/ml. The inhibition of cell viability after DDP treatment (7.20 μg/ml) at 24 h and 48 h was induced by 5‐Aza‐C pretreatment (Figure 4a) (P < 0.01). For cell proliferation analysis, the OD450 values of A549 cells pretreated with 5‐Aza‐C were significantly lower than those of A549 cells at 24 h, 48 h, and 72 h after DDP treatment (Figure 4b) (P < 0.05). The results were further validated by EdU detection: administration of 5‐Aza‐C dramatically decreased cell viability at 48 h (Figure 4c).
Figure 4.
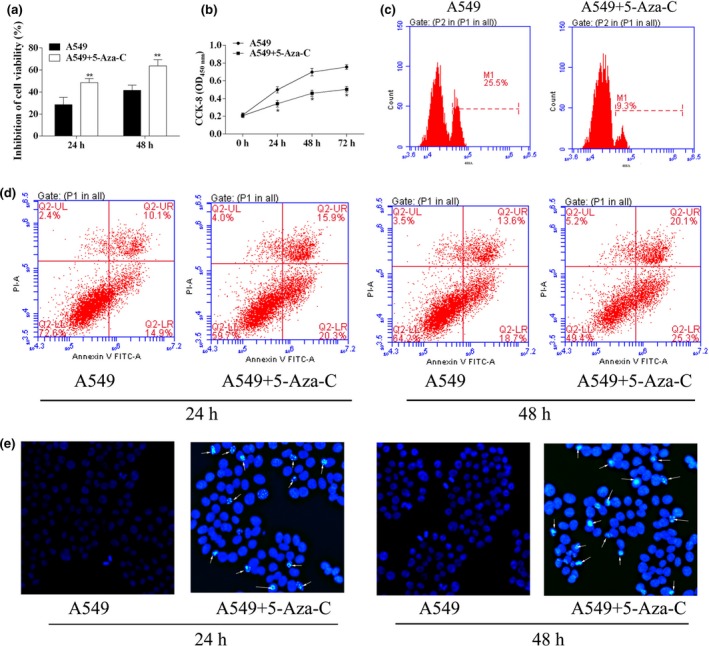
Downregulation of methylation of the ABCB1 gene decreased the DDP resistance in A549 lung adenocarcinoma cell lines. (a) After 5‐Aza‐C treatment for 24 and 48 h, inhibition of A549 cell viability was analysed using CCK‐8 assay. (b) CCK‐8 assay was used to detect A549 cell proliferation after 5‐Aza‐C treatment. (c) EdU assay was used to detect A549 cell proliferation after 5‐Aza‐C treatment. (d) Representative apoptosis assay using flow cytometry, A549 and A549/DDP cells. (e) Hoechst staining of apoptosis. *P < 0.05. **P < 0.01.
Similar results were observed for the analysis of cell apoptosis. There was a marked increase in apoptotic rates by pretreatment with 5‐Aza‐C after DDP treatment (25 ± 2.6% vs. 36.2 ± 3.5% for A549 cells at 24 h and 32.3 ± 3.6% vs. 45.4 ± 4.8% for A549 cells at 48 h, Figure 4d). Moreover, the apoptosis induced by 5‐Aza‐C was further confirmed by Hoechst staining: cell nuclei in A549 were regularly shaped with dark blue, while cell nuclei in A549 + 5‐Aza‐C group were demolished and the colour of the nuclei was bright blue (Figure 4e). Treatment with 5‐Aza‐C contributed to the hypomethylation of the ABCB1 gene and decreased ABCB1 protein expression in A549 cells. These results supported the view that hypermethylation of the ABCB1 gene contributed to DDP resistance of lung adenocarcinoma cells.
Overexpression of the ABCB1 gene increased DDP resistance of A549 cells
The overexpression of ABCB1 in A549 was achieved by transfection of a plasmid encoding coding sequence of ABCB1 (Figure 5a,b). The IC50 of DDP on A549/ABCB1 (cells transfected with ABCB1 expression vector) was 22.52 μg/ml. To detect the effect of ABCB1 overexpression on DDP resistance in A549 cells, the cell proliferation ability, the cell inhibition rate and cell apoptosis were all analysed. The results showed that overexpression of ABCB1 improved the cell viability (Figure 5c–e). Overexpression of ABCB1 inhibited A549 cell apoptosis after DDP treatment (Figure 5f,g). All the results confirmed the central role of ABCB1 in the acquisition of DDP resistance in A549 cells.
Figure 5.
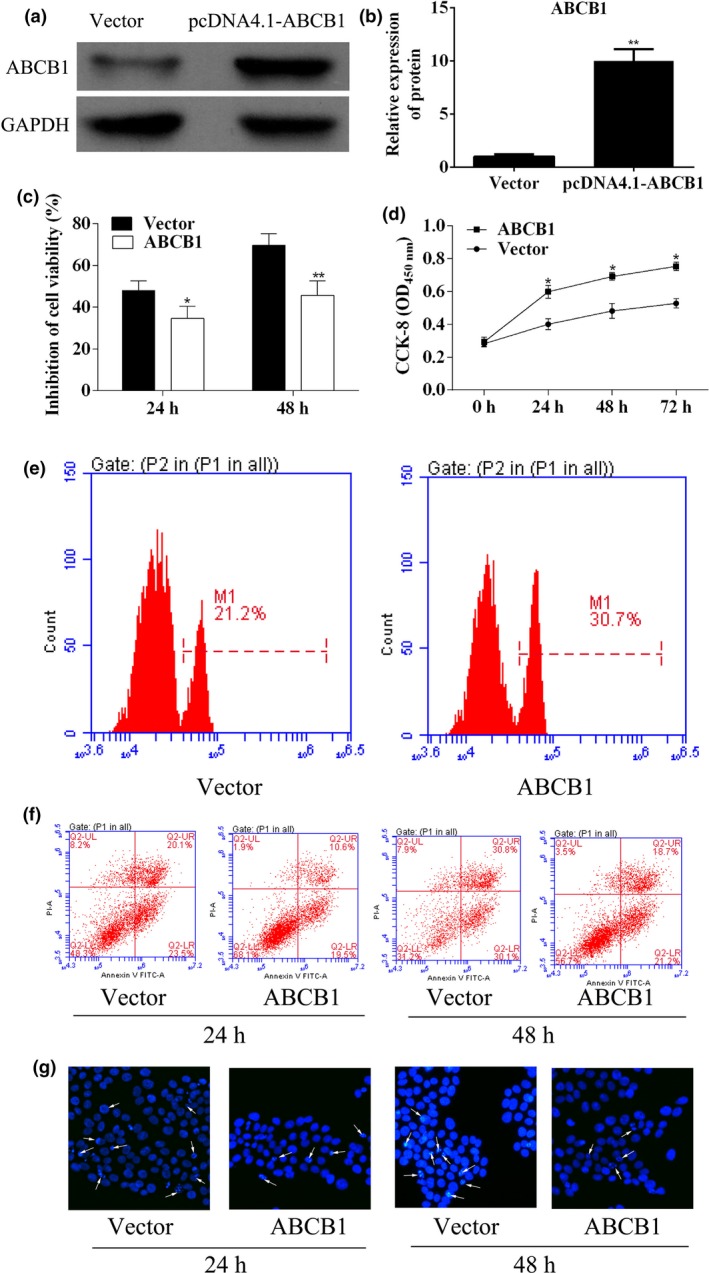
Overexpression of the ABCB1 gene increased DDP resistance of A549 cells. (a) Western blot analysed ABCB1 expression in A549 cells after pcDNA4.1‐ABCB1 transfection. (b) Quantitative analysis results of Western blot. (c) After transfection of pcDNA4.1‐ABCB1 for 24 and 48 h, inhibition of A549 cell viability was analysed using CCK‐8 assay. (d) The cell proliferation of A549 transfected with pcDNA4.1‐ABCB1 was analysed using CCK‐8 assay. (e) EdU assay was used to detect A549 cell proliferation after 5‐Aza‐C treatment. (f) Representative apoptosis assay using flow cytometry in different groups. (g) Hoechst staining of apoptosis. *P < 0.05. **P < 0.01.
Discussion
The adenosine triphosphate (ATP)‐binding cassette (ABC) genes are ubiquitously expressed throughout the animal gene kingdoms. The ABCB1/multidrug resistance gene (MDR1) encodes transporter and channel proteins possessing multiple membrane‐spanning domains and is responsible for cellular homeostasis (Croop 1993; Rosenberg et al. 1997; Jones & George 2004; Trussardi‐Regnier et al. 2009). The expression and methylation levels of the ABCB1 gene have been studied and shown to be involved in resistance to chemotherapeutic agents in vitro and in a variety of malignant tumours (Ueda et al. 1986; Burger et al. 1999; Ho et al. 2008; Reed et al. 2008). In the present study, we investigated the expression and methylation status of the ABCB1 gene in the human lung adenocarcinoma cell line A549 and found that the onset of resistance to the cisplatin analogue, DDO was associated with ABCB1 hypermethylation.
In the clinical samples studied, it was found that hypermethylation of the ABCB1 gene was associated with upregulation of the ABCB1 gene. It is commonly recognized that methylation of DNA will lead to the blockade of the expression and function of the genes. However, in the previous study, it has been reported that hypermethylation of the ABCB1 downstream gene promoter accompanied increased ABCB1 gene expression and contributed to drug resistance in breast tumour cells (Reed et al. 2008). This process might be initiated through the activation of an upstream promoter via the hypermethylation of the commonly used downstream promoter. Although this study was performed with breast tumour cells, we believed the existence of similar mechanism in lung tumour cells as our clinical data exhibited identical expression and methylation patterns of ABCB1 as those in breast tumour cells.
To further explore the relation between drug resistance acquisition and function of ABCB1 in lung tumour, the expression and methylation levels of the ABCB1 gene in A459 lung adenocarcinoma cells and DDP‐resistant cell lines (A549/DDP) were investigated. DDP can interact with phosphatidylserine to alter the characteristics of the cell membrane and is used as a chemotherapy agent in the treatment of a variety of tumour types (Burger et al. 1999). In this study, levels of expression and methylation of the ABCB1 gene in A549/DDP were markedly increased compared with those in A459 cells. This finding confirmed that acquisition of DDP resistance occurs with upregulation of the ABCB1 gene in lung tumours. The promoter methylation level of ABCB1 in A549 cells was decreased by incubation with DNA methyltransferase (DNMT) inhibitor 5‐azacytidine (5‐Aza‐C). The expression of ABCB1 protein decreased after 5‐Aza‐C treatment, which was associated with the demethylation of ABCB1 gene. These findings reflected the complex relationship between ABCB1 promoter methylation and its expression as validated by previous studies (Reed et al. 2008; Trussardi‐Regnier et al. 2009).
The apoptotic characteristics of tumour cells play an important role in the oncogenesis and development of cancers; changes in plasma membrane phospholipids are associated with the apoptosis of MDR tumour cells treated with chemotherapeutic drugs, which may imply the molecular mechanisms of the MDR phenomena (Huang et al. 2003). The protein production of ABCB1, P‐gp, is expressed in a polarized manner in the plasma membrane of cells in organs associated with drug metabolism and elimination. The protein restricts the permeability of drugs across blood–tissue barriers (Fromm 2004). Therefore, increased P‐gp due to the upregulation of ABCB1 may also protect tumour cells from the damage of chemotherapeutic drugs and is highly relevant to the clinical challenge of MDR.
The findings of this study are in agreement with previous study of Reed and colleagues with breast cancer cells, which showed that hypermethylation of the ABCB1 downstream gene promoter accompanies increased ABCB1 gene expression in resistant breast tumour cells (Reed et al. 2008). Moreover, study by Trussardi‐Regnier and colleagues working on myeloma cells also report that in resistant cells, expression and hypermethylation levels of ABCB1 gene also increased (Trussardi‐Regnier et al. 2009). However, this preliminary study had some limitations: the number of patients was limited to those available at a single centre with those who were able to fulfil strict inclusion criteria. Future larger multicentre studies may address some of these limitations. Although every effort was made to ensure that adenocarcinoma tissue samples were obtained, non‐small‐cell lung cancer (NSCLC) is known to be heterogeneous by histological evaluation. Phenotypically ‘mixed’ lung tumours may have been included in the analysis. The ‘normal’ adjacent lung tissue samples in this study were identified by the pathologists using macroscopic evaluation. In future studies, histological sampling of all such samples would be recommended. In this preliminary study, a single adenocarcinoma cell line was used, which may not reflect the behaviour or responses of lung adenocarcinoma cells in vivo. These limitations, combined with the conflicting reports in the literature on MDR and ABCB1 gene methylation in breast cancer cell lines and myeloma (Reed et al. 2008; Trussardi‐Regnier et al. 2009), highlight the importance of following up our preliminary findings with future studies.
Conclusion
This in vitro and human tissue study of lung adenocarcinoma cells demonstrated that hypermethylation of the ABCB1 gene correlated with increased ABCB1 gene expression and that increased ABCB1 gene expression and methylation were associated with the acquisition of resistance to the cisplatin analogue, DDP in human lung adenocarcinoma cells. This study addresses the connection between ABCB1 gene methylation state and cisplatin chemotherapy resistance in adenocarcinoma of the lung. More comprehensive studies need to be carried out to promote the development and application of ABCB1 as a potential oncological therapeutic target.
Conflict of Interests
The authors declared no potential conflict of interests with respect to the research, authorship and/or publication of this article.
Funding source
This work was supported by National Natural Science Foundation of China (81160296, 81160273) and Natural Science Foundation of Guangxi (2013GXNSFCB019005, 2013JJAA40484).
References
- Bock C., Reither S., Mikeska T., Paulsen M., Walter J. & Lengauer T. (2005) BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics 21, 4067–4068. [DOI] [PubMed] [Google Scholar]
- Bosch I. & Croop J. (1996) P‐glycoprotein multidrug resistance and cancer. Biochim. Biophys. Acta 1288, F37–F54. [DOI] [PubMed] [Google Scholar]
- Bromer J.G., Wu J., Zhou Y. & Taylor H.S. (2009) Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150, 3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger K.N., Staffhorst R.W. & De Kruijff B. (1999) Interaction of the anti‐cancer drug cisplatin with phosphatidylserine in intact and semi‐intact cells. Biochim. Biophys. Acta 1419, 43–54. [DOI] [PubMed] [Google Scholar]
- Chen K.G., Wang Y.C., Schaner M.E. et al (2005) Genetic and epigenetic modeling of the origins of multidrug‐resistant cells in a human sarcoma cell line. Cancer Res. 65, 9388–9397. [DOI] [PubMed] [Google Scholar]
- Croop J.M. (1993) P‐glycoprotein structure and evolutionary homologies. Cytotechnology 12, 1–32. [PubMed] [Google Scholar]
- Desiderato L., Davey M.W. & Piper A.A. (1997) Demethylation of the humanMDR1 5′ region accompanies activation of P‐glycoprotein expression in a HL60 multidrug resistant subline. Somat. Cell Mol. Genet. 23, 391–400. [DOI] [PubMed] [Google Scholar]
- Endicott J.A. & Ling V. (1989) The biochemistry of P‐glycoprotein‐mediated multidrug resistance. Annu. Rev. Biochem. 58, 137–171. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C. & Parkin D.M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917. [DOI] [PubMed] [Google Scholar]
- Ford J.M. & Hait W.N. (1990) Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev. 42, 155–199. [PubMed] [Google Scholar]
- Fromm M.F. (2004) Importance of P‐glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 25, 423–429. [DOI] [PubMed] [Google Scholar]
- Gervasini G., Carrillo J.A., Garcia M., San Jose C., Cabanillas A. & Benitez J. (2006) Adenosine triphosphate‐binding cassette B1 (ABCB1)(multidrug resistance 1) G2677T/A gene polymorphism is associated with high risk of lung cancer. Cancer 107, 2850–2857. [DOI] [PubMed] [Google Scholar]
- Gros P., Neriah Y.B., Croop J.M. & Housman D.E. (1986) Isolation and expression of a complementary DNA that confers multidrug resistance. Nature 323, 728–731. [DOI] [PubMed] [Google Scholar]
- Guo R., Wu G., Li H. et al (2013) Promoter methylation profiles between human lung adenocarcinoma multidrug resistant A549/cisplatin (A549/DDP) cells and its progenitor A549 cells. Biol. Pharm. Bull. 36, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Herzog C.E., Tsokos M., Bates S.E. & Fojo A.T. (1993) Increased mdr‐1/P‐glycoprotein expression after treatment of human colon carcinoma cells with P‐glycoprotein antagonists. J. Biol. Chem. 268, 2946–2952. [PubMed] [Google Scholar]
- Ho M.M., Hogge D.E. & Ling V. (2008) MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp. Hematol. 36, 433–442. [DOI] [PubMed] [Google Scholar]
- Huang Z., Tong Y., Wang J. & Huang Y. (2003) NMR studies of the relationship between the changes of membrane lipids and the cisplatin‐resistance of A549/DDP cells. Cancer Cell Int. 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabr‐Milane L.S., van Vlerken L.E., Yadav S. & Amiji M.M. (2008) Multi‐functional nanocarriers to overcome tumor drug resistance. Cancer Treat. Rev. 34, 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.M. & George A.M. (2004) The ABC transporter structure and mechanism: perspectives on recent research. Cell. Mol. Life Sci. 61, 682–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V. (1997) Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol. 40(Suppl), S3–S8. [DOI] [PubMed] [Google Scholar]
- Lu H.Y., Wang X.J. & Mao W.M. (2013) Targeted therapies in small cell lung cancer. Oncol. Lett. 5, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Gottesman M.M., Ueda K., Lovelace E., Rutherford A.V. & Willingham M.C. (1988) A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P‐glycoprotein in MDCK cells. Proc. Natl Acad. Sci. USA 85, 4486–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K., Hembruff S.L., Laberge M.L., Villeneuve D.J., Cote G.B. & Parissenti A.M. (2008) Hypermethylation of the ABCB1 downstream gene promoter accompanies ABCB1 gene amplification and increased expression in docetaxel‐resistant MCF‐7 breast tumor cells. Epigenetics 3, 270–280. [DOI] [PubMed] [Google Scholar]
- Rosenberg M.F., Callaghan R., Ford R.C. & Higgins C.F. (1997) Structure of the multidrug resistance P‐glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J. Biol. Chem. 272, 10685–10694. [DOI] [PubMed] [Google Scholar]
- Trussardi‐Regnier A., Lavenus S., Gorisse M.‐C. & Dufer J. (2009) Thalidomide alters nuclear architecture without ABCB1 gene modulation in drug‐resistant myeloma cells. Int. J. Oncol. 35, 641–647. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cornwell M.M., Gottesman M.M. et al (1986) The mdr1 gene, responsible for multidrug‐resistance, codes for P‐glycoprotein. Biochem. Biophys. Res. Commun. 141, 956–962. [DOI] [PubMed] [Google Scholar]
- Wang H., Jin G., Wang H. et al (2009) Genetic susceptibility of lung cancer associated with common variants in the 3′ untranslated regions of the adenosine triphosphate‐binding cassette B1 (ABCB1) and ABCC1 candidate transporter genes for carcinogen export. Cancer 115, 595–607. [DOI] [PubMed] [Google Scholar]


