Summary
Pancreatic cancer is the fourth most common cause of cancer‐related mortality. Novel molecular biomarkers need to be identified for personalized medicine and to improve survival. The aim of this study was to examine chloride intracellular channel 4 (CLIC4) and Indian Hedgehog (Ihh) expression in benign and malignant lesions of the pancreas and to examine the eventual association between CLIC4 and Ihh expression, with clinicopathological features and prognosis of pancreatic cancer. A retrospective study of specimens collected from January 2000 to December 2011 at the Department of Pathology of the Second and Third Xiangya Hospitals, Central South University was undertaken to explore this question. Immunohistochemistry of CLIC4 and Ihh was performed with EnVision™ in 106 pancreatic ductal adenocarcinoma specimens, 35 paracancer samples (2 cm away from the tumour, when possible or available), 55 benign lesions and 13 normal tissue samples. CLIC4 and Ihh expression in pancreatic ductal adenocarcinoma were significantly higher than in paracancer tissue and benign lesions (CLIC4: P = 0.009 and Ihh: P < 0.0001; CLIC4: P = 0.0004 and Ihh: P = 0.0001 respectively). CLIC4 and Ihh expression was negative in normal pancreatic tissues. The expression of CLIC4 and Ihh was associated significantly with tumour grade, lymph node metastasis, tumour invasion and poor overall survival. Thus CLIC4 and Ihh could serve as biological markers for the progression, metastasis and/or invasiveness of pancreatic ductal adenocarcinoma.
Keywords: chloride intracellular channel 4, chronic pancreatitis, Indian Hedgehog, pancreatic ductal adenocarcinoma, pancreatic intra‐epithelial neoplasia
Introduction
Pancreatic cancer represents only 3% of all newly diagnosed cancers, but it is the fourth most common cause of cancer death (Siegel et al. 2013). About 40% of the patients have locally advanced or metastatic disease at diagnosis, and only about 15% of the tumours are operable (Stathis & Moore 2010). The prognosis remains unsatisfactory despite recent therapeutic advances. The median survival of pancreatic ductal adenocarcinoma (PDA) is 13.4 months after resection combined with gemcitabine (Zhou et al. 2014). The currently available markers cannot accurately predict survival and identifying novel markers is required for personalized medicine which could lead to better outcomes.
High cell proliferation rate, migration and invasiveness are specific properties of tumorigenesis, and these features involve chloride channels. The chloride intracellular channel (CLIC) is a novel protein family consisting of seven members (Berryman & Bretscher 2000). Among these, CLIC4 (also called mtCLIC) is expressed in the mitochondria and regulates intracellular pH and cell volume (Jentsch et al. 2002). CLIC4 is present in the mitochondrial membrane, cytoplasm, and nucleus as a soluble protein (Harrop et al. 2001). Under normal physiological conditions, CLIC4 localizes in the nucleus through the nuclear localization signal (NLS) (Suh et al. 2004). Littler et al. 2005 showed that the functional activity of CLIC4 was determined by its redox state: oxidant conditions enhance its membrane binding capacity and channel activity. CLIC4 is also involved in multiple cellular processes including apoptosis (Shiio et al. 2006), inflammation (Ogawa et al. 2005; Sukiennicki & Fowell 2006) and endothelial tubulogenesis (Bohman et al. 2005; Ulmasov et al. 2009). Recent studies revealed that CLIC4 is highly expressed in cancer tissues and tumour‐related stromal fibroblasts, but it is almost absent in normal stromal tissues, suggesting that CLIC4 could be a potential target against some cancers (Shukla et al. 2014; Peretti et al. 2015).
Hammerschmidt et al. 1997 discovered that in drosophila the Hedgehog signalling pathway plays a critical role during embryonic development. It is also involved in tissue regeneration and carcinogenesis in various adult tissues, as well as in the maintenance of both cancer stem cells and adult stem cells (Azoulay et al. 2008). The three mammalian homologs of the Hedgehog ligand (Hh) gene are Sonic Hedgehog (Shh), Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh) (Echelard et al. 1993; Marigo et al. 1995). The Hh signal transduction is mediated by two receptors: patched (PTCH) and GPCR‐like protein smoothened (Smo) (Ingham & McMahon 2001; Lum & Beachy 2004). The activation of Hedgehog signalling is involved in the growth of various tumours such as basal cell carcinoma of the skin (Daya‐Grosjean & Couve‐Privat 2005), medulloblastoma (Dahmane et al. 2001; Berman et al. 2002), lung cancer (Watkins et al. 2003), gastrointestinal cancer (Berman et al. 2003) and prostate cancer (Thayer et al. 2003; Karhadkar et al. 2004). Ihh expression in some malignant tumours is higher than in benign lesions, and Ihh expression is related to the incidence, development, biological behaviours, and prognosis of breast (Xuan & Lin 2009), gastric (Xu et al. 2012), pancreatic (Xu et al. 2013), colorectal (Fu et al. 2010), ovarian (Ray et al. 2011), cervical (Chaudary et al. 2012) and kidney (Jager et al. 2014) cancers.
Nevertheless, the relationship between CLIC4 and Ihh in pancreatic cancer is poorly understood. Therefore, the aim of this study was to use immunohistochemistry to examine CLIC4 and Ihh expressions in benign and malignant lesions of the pancreas and to examine the eventual associations between CLIC4 and Ihh expression with clinicopathological features and prognosis of pancreatic cancer.
Materials and methods
Study design and specimens
This was a retrospective study of specimens collected from January 2000 to December 2011 at the Department of Pathology of the Second and Third Xiangya Hospitals, Central South University.
Exclusion criteria were as follows: (i) pre‐operative radiotherapy or chemotherapy; (ii) presence of any tumour not originating from the pancreatic duct (e.g. pancreatic acinar cell carcinoma or neuroendocrine carcinoma); or 3) any treatment complication that could affect survival (e.g. pancreatic fistula). This study included 106 PDA specimens, 35 paracancer samples (2 cm away from the tumour, when possible or available, from the 106 tumour specimens), 55 benign pancreatic lesions (from 55 patients) and 13 normal pancreatic tissue samples (from patients that underwent pancreaticoduodenectomy due to duodenal papilla tumour). All specimens were consecutively and prospectively collected. All diagnoses were reviewed for the present study.
Immunohistochemistry
All specimens were routinely fixed in 4% formaldehyde, embedded in paraffin and sectioned at 4 μm, which were mounted on poly‐L‐lysine‐coated slides. The rabbit‐anti‐human CLIC4 (catalog #AP7564a) and the rabbit‐anti‐human Ihh (catalog #AP2704a) polyclonal antibodies were purchased from Abgent (San Diego, California, USA). Immunohistochemistry of CLIC4 and Ihh was carried out using the EnVision™ system (ChemMate™ EnVison+/HRP/DAB, rabbit/mouse two‐step staining method), according to the manufacturer's protocol (DAKO laboratories, California, USA). Briefly, slides were dried overnight at 65°C, deparaffinized in xylene and dehydrated using a series of graded alcohols. Heat‐induced epitope retrieval was conducted with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween‐20, pH 6.0) at 96°C for 30 min. Endogenous peroxidase activity was inhibited by incubating the sections in 3% hydrogen peroxide for 15 min at room temperature. Non‐specific binding sites were blocked with 10% normal goat serum for 10 min. The slides were incubated with 50 μl of rabbit‐anti‐human CLIC4 or Ihh polyclonal antibodies (1:50 dilution) at 37°C for 1 h. Slides were washed in PBS three times for 5 min. The sections were incubated with 50 μl of solution A (ChemMate™ EnVison+/HRP) at 37°C for 30 min and washed in PBS three times for 5 min. DAB (DAKO Real DAB+Chromogen, K5007) was applied for about 2 min and removed by rinsing with distilled water. Slides were counterstained with haematoxylin. The sections were dehydrated, soaked in xylene and mounted with neutral resin. The positive control for CLIC4 was human breast carcinoma tissue sections provided by Abgent. The positive control for Ihh was human lung carcinoma tissue sections provided by Abgent. The 0.01 mol/L PBS liquid (pH 7.4) was used instead of CLIC4 and Ihh antibodies as negative control.
Staining positivity was determined by two senior pathologists blind to clinical information. Disagreements were solved by discussion. Ten high‐power and non‐overlapping fields were randomly selected to examine 400 cancer cells. Cases with ≥25% positive cells were determined as positive and those with <25% were negative (Leppilampi et al. 2003; Span et al. 2003; Madan et al. 2006; Liao et al. 2010).
Follow‐up
Because of the poor prognosis of pancreatic cancer (Siegel et al. 2013), most patients do not survive over 2 years. Three patients survived >2 years and their survival data were censored at 2 years.
Statistical analysis
Data were tested for normality using the Kolmogorov–Smirnov test. Continuous data were presented as mean ± standard deviation. Categorical data were presented as n (%). Data were analysed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The association of CLIC4 and Ihh expressions with clinicopathological features was analysed using the chi‐square or the Fisher's exact test, as appropriate. The Kaplan–Meier survival analysis and log‐rank tests were used for univariate survival analysis. The Cox proportional hazards model (LR method; P < 0.05 for inclusion) was used for multivariate survival analysis and to determine the 95% confidence interval (95% CI) of odds ratios (ORs). P < 0.05 indicated statistical significance.
Ethical approval statement
This study was approved by the ethics committee for human studies of Central South University, China. It complied with the Code of Ethics of the World Medical Association (Helsinki Declaration of 1964, as revised in 2002).
Results
Characteristics of the subjects
Among the included patients, 61 were male (57.5%) and 45 were female (42.5%); mean age was 54.5 ± 11.5 years. There were 38 (35.8%) well‐differentiated PDA, 35 (33.0%) moderately differentiated PDA and 33 (31.1%) poorly differentiated PDA; 29 patients (27.3%) had positive regional lymph nodes; and 64 patients (60.4%) had surrounding organ invasion. When considering the TNM classification, 11 patients (10.4%) were of stage I, 42 (39.6%) were of stage II, 37 (34.9%) were of stage III and 16 (15.1%) were of stage IV. Paracancer tissue was available for 35 patients: tissue was normal in 12 patients, ten showed mild atypical hyperplasia, eight showed moderate atypical hyperplasia, and five showed severe atypical hyperplasia.
Regarding the 55 specimens of pancreatic benign lesions, they were from 29 males (52.7%) and 26 females (47.3%). Among them, there were 20 cases of chronic pancreatitis (36.4%), 20 of adenoma (36.4%) and 15 of intra‐epithelial neoplasia (27.3%). Among the patients with chronic pancreatitis, there were 10 cases of mild pancreatitis, six of moderate pancreatitis and four of severe pancreatitis. Among these patients with chronic pancreatitis, the glandular epithelium of three of them showed mild atypical hyperplasia, two had moderate atypical hyperplasia, and one had severe atypical hyperplasia. Among the patients with adenoma, 15 were serous adenomas, while five were mucous adenomas. Among these cases, the glandular epithelium of four patients showed mild atypical hyperplasia, three had moderate atypical hyperplasia and two had severe atypical hyperplasia. For the patients with pancreatic intra‐epithelial neoplasia (PIN), six were of grade I, five were of grade II and four were of grade III. Normal pancreatic tissues of 13 patients were also collected as controls.
CLIC4 and Ihh protein expression
Immunohistochemistry revealed that CLIC4 was localized in the cytoplasm of epithelial and tumour stromal cells (including fibroblasts), while Ihh was localized in the cytoplasm of the epithelial cells only (Figures 1 and 2). As shown in Table 1, among the 106 patients with PDA, CLIC4 and Ihh were positive in 54 (50.9%) and 63 (59.4%) patients respectively. Among the 35 patients with available paracancer tissue, CLIC4 and Ihh were positive in nine (25.7%) and 10 (28.6%) patients respectively. Among the 55 patients with benign lesions of the pancreas, 12 (21.8%) were CLIC4 positive and 10 (18.2%) were Ihh positive. Among the 13 normal pancreatic tissues, CLIC4 and Ihh were negative in all samples. The rate of CLIC4 and Ihh positivity in PDA was significantly higher than in the paracancer samples (P = 0.009 and P < 0.0001), benign lesions (P = 0.0004 and P = 0.0001) and normal pancreatic tissue (P = 0.0005 and P = 0.002). Interestingly, the ductal epithelium of paracancer and benign samples with positive CLIC4 and Ihh expressions displayed mild to severe atypical hyperplasia or intra‐epithelial neoplasia of grades II‐III.
Figure 1.
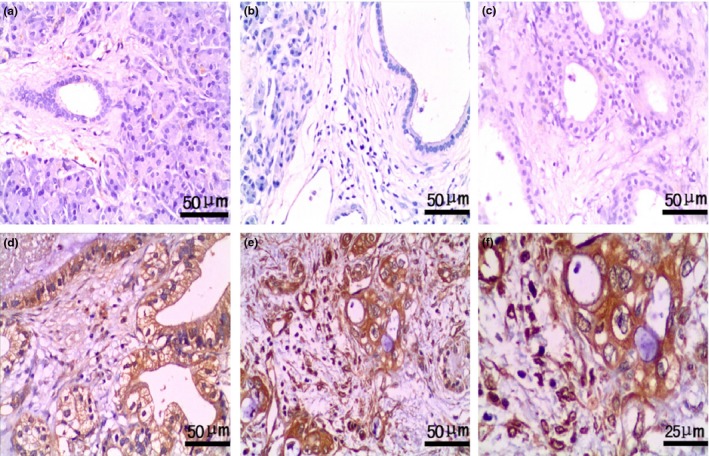
Expression of CLIC4 in pancreatic with benign and malignant lesions. EnVision™ immunohistochemistry: (a) negative CLIC4 expression in pancreatic normal tissues (×200); (b) negative CLIC4 expression in chronic pancreatitis tissues (×200); (c) negative CLIC4 expression in pancreatic serous adenoma tissues (×200); (d) positive CLIC4 expression in well‐differentiated PDA (×200), tumour size was 2.5 cm and TNM stage II; (e) positive CLIC4 expression in poorly differentiated PDA (×200), tumour size was 4 cm and TNM stage IV; (f) magnified image from e, CLIC4 staining was localized in the cytoplasm of epithelial cells and in the nucleus of fibroblasts in poorly differentiated PDA (×400).
Figure 2.
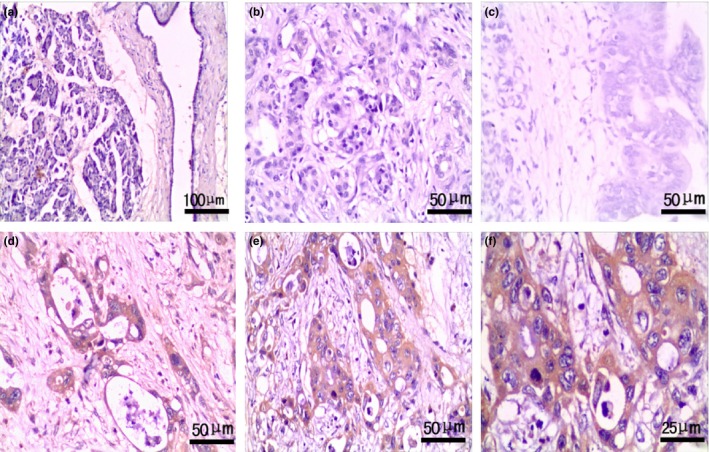
Expression of Ihh in pancreas with benign and malignant lesions. EnVision™ immunohistochemistry: (a) negative Ihh expression in pancreatic normal tissues (×100); (b) negative Ihh expression in chronic pancreatitis tissues (×200); (c) negative Ihh expression in PIN II tissues (×200); (d) positive Ihh expression in well‐differentiated PDA (×200), tumour size of 3 cm and TNM stage I; (e) positive Ihh expression in poorly differentiated PDA (×200), tumour size of 4 cm and TNM stage IV; (f) magnified image from e, Ihh staining was localized in the cytoplasm of epithelial cells in poorly differentiated PDA (×400).
Table 1.
CLIC4 and Ihh expression in benign and malignant lesions of pancreas
| Tissue types | N | CLIC4 positive, n (%) | Ihh positive, n (%) |
|---|---|---|---|
| PDA | 106 | 54 (50.9) | 63 (59.4) |
| Paracancer | 35 | 9 (25.7) | 10 (28.6) |
| Benign lesions | 55 | 12 (21.8) | 10 (18.2) |
| Normal tissue | 13 | 0 | 0 |
For CLIC4: PDA vs. paracancer: P = 0.0092; PDA vs. benign lesions: P = 0.0004; PDA vs. benign lesions: P = 0.0004; PDA vs. normal: P = 0.0005.
For Ihh: PDA vs. paracancer: P < 0.0001; PDA vs. benign lesions: P = 0.0001; PDA vs. normal tissues: P = 0.0015.
Among the benign lesions, the rates of CLIC4 positivity in chronic pancreatitis, adenoma and intra‐epithelial neoplasia were 15.0% (3/20), 25.0% (5/20) and 26.7% (4/15), respectively, while those of Ihh were 15.0% (3/20), 20.0% (4/20) and 20.0% (3/15) respectively (all P > 0.05).
Associations between CLIC4 and Ihh expressions and clinical pathological features of PDA
CLIC4 and Ihh positivity was associated with poorly differentiated PDA (P = 0.008 and P < 0.0001), lymph node metastasis (P < 0.0001 and P = 0.001), surrounding organ invasion (P = 0.03 and P < 0.0001) and advanced TNM stage (P = 0.02 and P < 0.0001) (Table 2). CLIC4 and Ihh were not associated with age, gender and tumour size.
Table 2.
Association of CLIC4 and Ihh expression with the clinicopathological characteristics of PDA
| Characteristics | N | CLIC4 | Ihh | ||
|---|---|---|---|---|---|
| Positive, n (%) | P | Positive, n (%) | P | ||
| Age (years) | |||||
| ≤45 | 22 | 15 (68.2) | 0.069 | 16 (72.7) | 0.154 |
| >45 | 84 | 39 (46.4) | 47 (56.0) | ||
| Gender | |||||
| Male | 61 | 31 (50.8) | 0.976 | 34 (55.7) | 0.367 |
| Female | 45 | 23 (51.1) | 29 (64.4) | ||
| Differentiation | |||||
| Well | 38 | 14 (36.8) | 0.008 | 14 (36.8) | <0.0001 |
| Moderate | 35 | 33 (48.5) | 21 (60.0) | ||
| Poor | 33 | 24 (72.7) | 28 (84.8) | ||
| Tumour size (cm) | |||||
| ≤3 cm | 13 | 4 (30.8) | 0.075 | 5 (38.5) | 0.069 |
| 3–5 cm | 68 | 33 (48.5) | 39 (57.4) | ||
| >5 m | 25 | 17 (68.0) | 19 (76.0) | ||
| Lymph node metastasis | |||||
| No | 77 | 30 (39.0) | <0.0001 | 38 (49.4) | 0.001 |
| Yes | 29 | 24 (82.8) | 25 (86.2) | ||
| Invasion | |||||
| No | 42 | 11 (26.2) | 0.032 | 14 (33.3) | <0.0001 |
| Yes | 64 | 43 (67.2) | 49 (76.6) | ||
| T stages | |||||
| I | 11 | 4 (36.4) | 0.018 | 4 (36.3) | <0.0001 |
| II | 42 | 16 (38.1) | 17 (40.5) | ||
| III | 37 | 21 (56.8) | 28 (75.7) | ||
| IV | 16 | 13 (81.3) | 14 (87.5) | ||
Association between CLIC4 and Ihh in PDA
Among the 54 patients with CLIC4 expression, 38 showed Ihh expression. Of the 52 patients without CLIC4 expression, 27 exhibited no Ihh expression. There was an association between the expressions of CLIC4 and Ihh (P = 0.023).
Associations between survival, clinicopathological features and expression of CLIC4 and Ihh in patients with PDA
Follow‐up was carried out in 106 patients with PDA through mail or outpatient visits every 3 months for up to 2 years. Patients surviving (n = 3) over this period were censored. Twenty‐nine patients survived >1 year and 77 patients survived <1 year. Mean survival was 9.4 ± 0.7 months. According to the Kaplan–Meier survival analysis, poor survival of patients PDA was associated with poor differentiation (P < 0.0001), tumour size (P = 0.02), advanced TNM stage (P < 0.0001), lymph node metastasis (P < 0.0001) and surrounding organ invasion (P < 0.0001) (Table 3). The overall survival of patients with positive CLIC4 and Ihh expressions was obviously shorter than that of the CLIC4‐ and Ihh‐negative cases (CLIC4: negative: 12.3 vs. positive: 6.6 months, P < 0.0001; Ihh: negative: 12.5 vs. 7.3 months, P < 0.0001) (Figure 3).
Table 3.
Relationship between CLIC4 and Ihh expressions, clinicopathological characteristics and survival of patients with PDA
| Characteristics | N | Median survival [range] (months) | P value |
|---|---|---|---|
| Age (years) | |||
| ≤45 | 22 | 8.2 (3–19) | 0.143 |
| >45 | 84 | 9.7 (2–24) | |
| Gender | |||
| Male | 61 | 10.0 (2–24) | 0.198 |
| Female | 45 | 8.6 (2–21) | |
| Differentiation | |||
| Well | 38 | 11.3 (3–24) | <0.0001 |
| Moderate | 35 | 9.7 (3–21) | |
| Poor | 33 | 6.9 (2–14) | |
| Tumour size (cm) | |||
| ≤3 cm | 13 | 13.5 (5–21) | 0.023 |
| 3–5 cm | 68 | 9.3 (2–22) | |
| >5 m | 25 | 7.4 (3–24) | |
| Lymph node metastasis | |||
| No | 77 | 10.6 (2–24) | <0.0001 |
| Yes | 29 | 6.4 (2–12) | |
| Invasion | |||
| No | 42 | 13.3 (5–24) | <0.0001 |
| Yes | 64 | 6.8 (2–17) | |
| T stages | |||
| I | 11 | 16.5 (11–24) | <0.0001 |
| II | 42 | 11.4 (3–22) | |
| III | 37 | 7.1 (2–17) | |
| IV | 16 | 4.6 (2–8) | |
| CLIC4 | |||
| − | 52 | 12.3 (3–24) | <0.0001 |
| + | 54 | 6.6 (2–16) | |
| Ihh | |||
| − | 43 | 12.5 (5–24) | <0.0001 |
| + | 63 | 7.3 (2–22) | |
Figure 3.
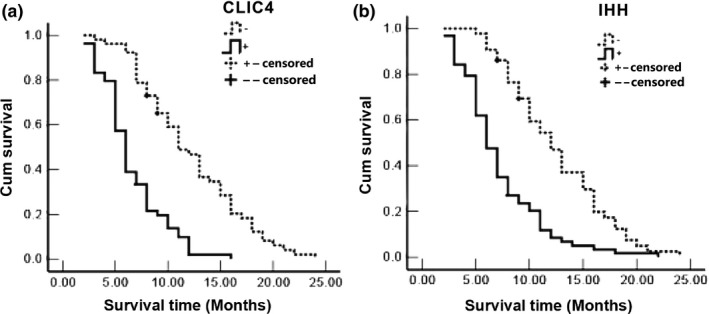
Association between survival and expression of CLIC4 and Ihh in patients with PDA. (a) Survival curves for CLIC4 expression in PDA. Median survival was 12.3 months for negative cases and 6.6 months for positive ones (P < 0.001). (b) Survival curves for Ihh expression in PDA. Median survival was 12.5 months for negative cases and 7.3 months for positive ones (P < 0.001).
Multivariate analysis
The Cox multivariate analysis showed that overall survival was independently and negatively associated with poor differentiation, tumour size >3 cm, TNM stage III or IV, lymph node metastasis, surrounding organ invasion, CLIC4 expression and Ihh expression (Table 4).
Table 4.
Multivariate Cox regression analysis of overall survival in patients with PDA
| Groups | Factors | B | SE | Wald | P | RR | 95% confidence interval | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Differentiation | Well/moderate/poor | 0.689 | 0.244 | 7.970 | 0.005 | 1.992 | 1.234 | 3.213 |
| Tumour mass size | <3 cm/3–5 cm/>5 cm | 1.098 | 0.397 | 7.651 | 0.006 | 2.997 | 1.377 | 6.524 |
| Lymph node metastasis | No/yes | 0.974 | 0.338 | 8.285 | 0.004 | 2.648 | 1.364 | 5.140 |
| Invasion | No/yes | 0.821 | 0.345 | 5.680 | 0.017 | 2.274 | 1.157 | 4.468 |
| TNM stages | I/II/III/IV | 0.777 | 0.245 | 10.085 | 0.001 | 2.175 | 1.346 | 3.513 |
| CLIC4 | −/+ | 0.891 | 0.265 | 11.320 | 0.001 | 2.437 | 1.450 | 4.094 |
| Ihh | −/+ | 0.697 | 0.247 | 7.948 | 0.005 | 2.007 | 1.237 | 3.259 |
B, regression coefficients; SE, standard error; RR, Relative risk.
Discussion
Pancreatic cancer is the fourth most common cause of cancer‐related mortality. There is a need for novel molecular biomarkers to improve personalized medicine and survival. The aim of this study was to examine CLIC4 and Ihh expression in benign and malignant lesions of the pancreas and to attempt to determine associations between CLIC4 and Ihh expressions and clinicopathological features and prognosis of pancreatic cancer. Results showed that both CLIC4 and Ihh expression in PDA was significantly higher than in paracancer tissue and benign lesions. In normal pancreatic tissue, CLIC4 and Ihh expression was negative. The expression of both CLIC4 and Ihh was significantly associated with tumour grade, lymph node metastasis, tumour invasion and poor overall survival.
Several studies have investigated the expression and regulation of CLIC4 in tumours. Significant alterations in CLIC4 expression have been documented in bladder cancer (Dyrskjot et al. 2004), uterine leiomyoma (Bae et al. 2004), glioma (Zhong et al. 2012) and melanoma (Alonso et al. 2007). In colon cancer, CLIC4 was positive in 67.2% of 421 patients, while only 2.1% of the matching mucosa was positive. The expression of CLIC4 in colorectal cancer was considered to be related to the distribution of cancer stem‐like cells (Deng et al. 2014). This was considered as to be an innate factor contributing to the aggressiveness of the metastatic cancer stem‐like cells of colorectal cancer. CLIC4 expression was reported to be a marker of colon cancer stem cells and to be associated with poor prognosis (Deng et al. 2014). It also enhanced matrix metallopeptidase 9 expressions and invasion in cancer cell lines escaping photodynamic therapy (PDT). Overexpression of CLIC4 in A375 and MDA‐MB‐231 cancer cells constrained the PDT‐induced suppression of invasiveness (Chiang et al. 2013). CLIC4 is present in the exosomes released from human ovarian cancer cells (Liang et al. 2013) and high levels of circulating CLIC4 were identified as a marker of prognosis in these patients (Tang et al. 2013). Furthermore, CLIC4 could be a potential biomarker to monitor tumour progression and recurrence in human cancers, but this is controversial. However, another study revealed loss of CLIC4 in multiple human epithelial tumours including renal, ovarian and breast cancers and reported that CLIC4 expression is increased in stromal cells with the progression of malignancy (Suh et al. 2007). Suh et al. (2005) suggested that decreasing CLIC4 expression by using siRNA inhibited the growth of human osteosarcoma cells, increased apoptosis and decreased cell proliferation. Ronnov‐Jesson et al. (2002) proposed that CLIC4 has an important function in the myofibroblast stroma of breast cancer patients wherein the transcript was upregulated after TGF‐β treatment. Thus, the exact mechanisms by which CLIC4 functions as a tumour suppressor or stromal activator are still open questions.
In 1980, Nusslein‐Vollhard and Wieschaus showed that the Hedgehog signalling pathway was related to growth, organ development and congenital malformations (Nusslein‐Volhard & Wieschaus 1980). Later, other studies showed that the Hedgehog signalling pathway was closely related to the growth and differentiation of cells, playing a pivotal role in embryonic development, homeostasis of mature tissue and tumorigenesis (Dahmane et al. 2001). It has been found in recent years that among common malignant tumours (basal cell carcinoma, small cell lung cancer, upper gastrointestinal cancer, prostate cancer and endometrial cancer), tumour development was closely related to the abnormal activation of the Hedgehog signalling pathway (Pasca di Magliano & Hebrok 2003; Feng et al. 2007). In breast cancer, increased expression of Ihh was associated with increased proliferating index of Ki‐67. Ihh expression was also associated with lymph node metastasis and clinical stage of breast cancer. Hedgehog signalling molecules are essential to the progression of invasive ductal carcinoma of the breast (Thayer et al. 2003). The discovery of cyclopamine made the Hedgehog signalling pathway more clinically significant. These steroid alkaloids mainly block the function of the SMO receptor to inhibit the activation of the Hedgehog signalling pathway, therefore inhibiting the growth of cancer cells (Xuan & Lin 2009). Kayed et al. (Kayed et al. 2004) proposed that Ihh and its receptors PTCH and Smo were expressed in pancreatic cancer and that blocking the Hedgehog signalling pathway could result in the inhibition of pancreatic cancer cell growth, thereby suggesting that aberrant activation of the Ihh pathway contributes to pancreatic tumour development. Recent studies showed that Ihh expression level is closely associated with the incidence, development, biological behaviours and prognosis of some malignant tumours (breast, gastric, pancreatic, colorectal, ovarian, cervical and kidney cancers) (Berman et al. 2003; Thayer et al. 2003; Karhadkar et al. 2004; Xuan & Lin 2009; Fu et al. 2010; Xu et al. 2012, 2013).
Although the expression of CLIC4 and Ihh has been previously reported in some cancers, to our knowledge, this is the first report showing CLIC4 and Ihh expression in benign and malignant lesions of the pancreas. In this study, an extensive collection of PDA and benign pancreatic lesions were used to demonstrate the clinical and pathological significance of CLIC4 and Ihh expressions in PDA. In accordance with results observed in other types of cancers, the present study showed that CLIC4 and Ihh expressions was associated with PDA. Among patients with PDA, CLIC4 and Ihh expression was associated with poor prognosis and poor survival. Therefore, CLIC4 and Ihh could play a critical role in carcinogenesis and progression of PDA.
The present study is not without limitations. The sample size was relatively small and all cases were from the same institution. In addition, the retrospective nature of the study prevented examining factors that were not identified in the charts. It would be interesting to observe the expression of other molecular markers of prognosis in relation to CLIC4 and Ihh. Additional studies are still necessary to grasp a better understanding of these markers in PDA.
In conclusion, CLIC4 and Ihh expression was associated with poor survival of patients with PDA. CLIC4 and Ihh could serve as potential biological markers indicating progression, metastasis and/or invasiveness of pancreatic ductal adenocarcinoma.
Conflict of interests
The authors declare that they have no conflict of interests.
Funding source
The study was funded by the Hunan Provincial Department of science and technology plan project (2011SK3241).
References
- Alonso S.R., Tracey L., Ortiz P. et al (2007) A high‐throughput study in melanoma identifies epithelial‐mesenchymal transition as a major determinant of metastasis. Cancer Res. 67, 3450–3460. [DOI] [PubMed] [Google Scholar]
- Azoulay S., Terry S., Chimingqi M. et al (2008) Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J. Pathol. 216, 460–470. [DOI] [PubMed] [Google Scholar]
- Bae S.M., Kim Y.W., Lee J.M., Namkoong S.E., Kim C.K. & Ahn W.S. (2004) Expression profiling of the cellular processes in uterine leiomyomas: omic approaches and IGF‐2 association with leiomyosarcomas. Cancer Res. Treat. 36, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D.M., Karhadkar S.S., Hallahan A.R. et al (2002) Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561. [DOI] [PubMed] [Google Scholar]
- Berman D.M., Karhadkar S.S., Maitra A. et al (2003) Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425, 846–851. [DOI] [PubMed] [Google Scholar]
- Berryman M. & Bretscher A. (2000) Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 11, 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman S., Matsumoto T., Suh K. et al (2005) Proteomic analysis of vascular endothelial growth factor‐induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J. Biol. Chem. 280, 42397–42404. [DOI] [PubMed] [Google Scholar]
- Chaudary N., Pintilie M., Hedley D. et al (2012) Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation. Cancer 118, 3105–3115. [DOI] [PubMed] [Google Scholar]
- Chiang P.C., Chou R.H., Chien H.F., Tsai T. & Chen C.T. (2013) Chloride intracellular channel 4 involves in the reduced invasiveness of cancer cells treated by photodynamic therapy. Lasers Surg. Med. 45, 38–47. [DOI] [PubMed] [Google Scholar]
- Dahmane N., Sanchez P., Gitton Y. et al (2001) The Sonic Hedgehog‐Gli pathway regulates dorsal brain growth and tumorigenesis. Development 128, 5201–5212. [DOI] [PubMed] [Google Scholar]
- Daya‐Grosjean L. & Couve‐Privat S. (2005) Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 225, 181–192. [DOI] [PubMed] [Google Scholar]
- Deng Y.J., Tang N., Liu C. et al (2014) CLIC4, ERp29, and Smac/DIABLO derived from metastatic cancer stem‐like cells stratify prognostic risks of colorectal cancer. Clin. Cancer Res. 20, 3809–3817. [DOI] [PubMed] [Google Scholar]
- Dyrskjot L., Kruhoffer M., Thykjaer T. et al (2004) Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 64, 4040–4048. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D.J., St‐Jacques B. et al (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430. [DOI] [PubMed] [Google Scholar]
- Feng Y.Z., Shiozawa T., Miyamoto T. et al (2007) Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin. Cancer Res. 13, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Fu X., Yang X., Li J., Tian X., Cai J. & Zhang Y. (2010) Opposite expression patterns of Sonic hedgehog and Indian hedgehog are associated with aberrant methylation status of their promoters in colorectal cancers. Pathology 42, 553–559. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M., Brook A. & McMahon A.P. (1997) The world according to hedgehog. Trends Genet. 13, 14–21. [DOI] [PubMed] [Google Scholar]
- Harrop S.J., DeMaere M.Z., Fairlie W.D. et al (2001) Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4‐A resolution. J. Biol. Chem. 276, 44993–45000. [DOI] [PubMed] [Google Scholar]
- Ingham P.W. & McMahon A.P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- Jager W., Thomas C., Fazli L. et al (2014) DHH is an independent prognosticator of oncologic outcome of clear cell renal cell carcinoma. J. Urol. 192, 1842–1848. [DOI] [PubMed] [Google Scholar]
- Jentsch T.J., Stein V., Weinreich F. & Zdebik A.A. (2002) Molecular structure and physiological function of chloride channels. Physiol. Rev. 82, 503–568. [DOI] [PubMed] [Google Scholar]
- Karhadkar S.S., Bova G.S., Abdallah N. et al (2004) Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431, 707–712. [DOI] [PubMed] [Google Scholar]
- Kayed H., Kleeff J., Keleg S. et al (2004) Indian hedgehog signaling pathway: expression and regulation in pancreatic cancer. Int. J. Cancer 110, 668–676. [DOI] [PubMed] [Google Scholar]
- Leppilampi M., Saarnio J., Karttunen T.J. et al (2003) Carbonic anhydrase isozymes IX and XII in gastric tumors. World J. Gastroenterol. 9, 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Peng P., Chen S. et al (2013) Characterization and proteomic analysis of ovarian cancer‐derived exosomes. J. Proteomics. 80, 171–182. [DOI] [PubMed] [Google Scholar]
- Liao S.Y., Darcy K.M., Randall L.M. et al (2010) Prognostic relevance of carbonic anhydrase‐IX in high‐risk, early‐stage cervical cancer: a Gynecologic Oncology Group study. Gynecol. Oncol. 116, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler D.R., Assaad N.N., Harrop S.J. et al (2005) Crystal structure of the soluble form of the redox‐regulated chloride ion channel protein CLIC4. FEBS J. 272, 4996–5007. [DOI] [PubMed] [Google Scholar]
- Lum L. & Beachy P.A. (2004) The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759. [DOI] [PubMed] [Google Scholar]
- Madan R., Smolkin M.B., Cocker R., Fayyad R. & Oktay M.H. (2006) Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum. Pathol. 37, 9–15. [DOI] [PubMed] [Google Scholar]
- Marigo V., Roberts D.J., Lee S.M. et al (1995) Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics 28, 44–51. [DOI] [PubMed] [Google Scholar]
- Nusslein‐Volhard C. & Wieschaus E. (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lozach J., Benner C. et al (2005) Molecular determinants of crosstalk between nuclear receptors and toll‐like receptors. Cell 122, 707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca di Magliano M. & Hebrok M. (2003) Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 3, 903–911. [DOI] [PubMed] [Google Scholar]
- Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M (2015) Chloride channels in cancer: Focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim. Biophys. Acta 1848, 2523–2531. [DOI] [PubMed] [Google Scholar]
- Ray A., Meng E., Reed E., Shevde L.A. & Rocconi R.P. (2011) Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int. J. Oncol. 39, 797–804. [DOI] [PubMed] [Google Scholar]
- Ronnov‐Jessen L., Villadsen R., Edwards J.C. & Petersen O.W. (2002) Differential expression of a chloride intracellular channel gene, CLIC4, in transforming growth factor‐beta1‐mediated conversion of fibroblasts to myofibroblasts. Am. J. Pathol. 161, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio Y., Suh K.S., Lee H., Yuspa S.H., Eisenman R.N. & Aebersold R. (2006) Quantitative proteomic analysis of myc‐induced apoptosis: a direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J. Biol. Chem. 281, 2750–2756. [DOI] [PubMed] [Google Scholar]
- Shukla A., Edwards R., Yang Y. et al (2014) CLIC4 regulates TGF‐beta‐dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 33, 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Naishadham D. & Jemal A. (2013) Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30. [DOI] [PubMed] [Google Scholar]
- Span P.N., Bussink J., Manders P., Beex L.V. & Sweep C.G. (2003) Carbonic anhydrase‐9 expression levels and prognosis in human breast cancer: association with treatment outcome. Br. J. Cancer 89, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis A. & Moore M.J. (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin. Oncol. 7, 163–172. [DOI] [PubMed] [Google Scholar]
- Suh K.S., Mutoh M., Nagashima K. et al (2004) The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J. Biol. Chem. 279, 4632–4641. [DOI] [PubMed] [Google Scholar]
- Suh K.S., Mutoh M., Gerdes M. et al (2005) Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor {alpha}‐induced apoptosis, and inhibits tumor growth. Cancer Res. 65, 562–571. [PubMed] [Google Scholar]
- Suh K.S., Crutchley J.M., Koochek A. et al (2007) Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin. Cancer Res. 13, 121–131. [DOI] [PubMed] [Google Scholar]
- Sukiennicki T.L. & Fowell D.J. (2006) Distinct molecular program imposed on CD4 + T cell targets by CD4 + CD25 + regulatory T cells. J. Immunol. 177, 6952–6961. [DOI] [PubMed] [Google Scholar]
- Tang H.Y., Beer L.A., Tanyi J.L., Zhang R., Liu Q. & Speicher D.W. (2013) Protein isoform‐specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancer. J. Proteomics. 89, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S.P., di Magliano M.P., Heiser P.W. et al (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B., Bruno J., Gordon N., Hartnett M.E. & Edwards J.C. (2009) Chloride intracellular channel protein‐4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am. J. Pathol. 174, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins D.N., Berman D.M., Burkholder S.G., Wang B., Beachy P.A. & Baylin S.B. (2003) Hedgehog signalling within airway epithelial progenitors and in small‐cell lung cancer. Nature 422, 313–317. [DOI] [PubMed] [Google Scholar]
- Xu C., Li J., Lu Y. & Jiang Z. (2012) Estrogen receptor alpha and hedgehog signal pathway developmental biology of gastric adenocarcinoma. Hepatogastroenterology 59, 1319–1322. [DOI] [PubMed] [Google Scholar]
- Xu M., Li L., Liu Z. et al (2013) ABCB2 (TAP1) as the downstream target of SHH signaling enhances pancreatic ductal adenocarcinoma drug resistance. Cancer Lett. 333, 152–158. [DOI] [PubMed] [Google Scholar]
- Xuan Y. & Lin Z. (2009) Expression of Indian Hedgehog signaling molecules in breast cancer. J. Cancer Res. Clin. Oncol. 135, 235–240. [DOI] [PubMed] [Google Scholar]
- Zhong J., Kong X., Zhang H. et al (2012) Inhibition of CLIC4 enhances autophagy and triggers mitochondrial and ER stress‐induced apoptosis in human glioma U251 cells under starvation. PLoS ONE 7, e39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.Y., Wang Y., Zhang J. et al (2014) Retrograde vs conventional dissection technique in pancreaticoduodenectomy: a pilot study. JAMA Surg. 149, 604–607. [DOI] [PubMed] [Google Scholar]


