Abstract
Objective:
To determine the adverse drug reaction (ADR) profile of risperidone and their association with dopamine (DRD2 − 141 C Ins/Del/rs1799732) and serotonin receptor (5HTR2C −759 C>T/rs3813929) gene polymorphisms in patients with schizophrenia.
Materials and Methods:
The study was conducted among 289 patients who were diagnosed with schizophrenia and were on treatment with risperidone (4–8 mg/day)-based therapy for a minimum of 4 weeks. Genotyping was carried by real-time quantitative polymerase chain reaction. All the patients were observed for the occurrences of ADRs during the study. Changes in prolactin levels and body weight were analyzed for a subgroup of 102 and 97 patients, respectively.
Results:
Risperidone-induced extrapyramidal symptoms (EPSs) were seen in 36.7% of patients. Among them, tremors were the most common symptom 31.8%. Risperidone-induced hyperprolactinemia and weight gain were seen in 87.2% and 53.6% in subgroup patients. Adverse effects such as sedation, gastrointestinal effects, and amenorrhea were seen in 9.7% (28/289), 5.1% (15/289), and 6.1% (7/114), respectively. Occurrence of DRD2 − 141 Ins/Del and Del/Del polymorphisms were significantly associated with increased prolactin levels in response to risperidone (odds ratio [OR] = 10.45; 95% confidence interval = 1.29–84.89, P = 0.004). No such association was observed with 5HTR2C (−759 C>T) polymorphism. Weight gain and EPS were not associated with the above genetic polymorphisms.
Conclusion:
Hyperprolactinemia, weight gain, and EPSs (>36.7%) were common adverse effects of risperidone. DRD2 – 141C Ins/Del and Del/Del polymorphisms were significantly associated with increased prolactin levels (OR = 10.45) in response to risperidone.
Keywords: Adverse drug reactions, hyperprolactinemia, risperidone, rs1799732, rs3813929, schizophrenia
Introduction
It is estimated that at least 26 million people are suffering from schizophrenia worldwide.[1] Second-generation antipsychotics (SGA) have largely replaced conventional antipsychotics for the treatment of behavioral disorders such as schizophrenia, bipolar disorder, and autism due to their modest therapeutic efficacy and better safety profile.[2] Therapeutic efficacy of atypical antipsychotics varies from drug to drug in different individuals.[3] Risperidone is one of the well-established SGA and widely used in the treatment of schizophrenia.[4] It has a combined antagonist effects for dopamine (DRD2) and serotonin (5HTR2A, 2C) receptors. DRD2 and 5HTR2 neurotransmission are the major pathways involved in the disease pathophysiology as well as symptom improvement and drug-induced extrapyramidal symptoms (EPSs) in schizophrenia.[5,6] When compared to conventional antipsychotic drugs, it has greater efficacy in symptom amelioration and less severe adverse effects. Several studies have reported high prevalence of risperidone-associated adverse effects such as hyperprolactinemia, weight gain, EPSs, and other adverse effects in patients with schizophrenia.[7,8,9,10] These adverse effects may lead to other secondary complications such as metabolic syndrome, diabetes mellitus, sexual dysfunction, and cardiac problems in patients suffering from schizophrenia which can reduce their quality of life.[11]
Several studies have suggested that polymorphism in dopamine and serotonin receptors was associated with reduced receptor density which may play an important role in drug efficacy and drug-induced adverse effects.[12,13,14] Recently, a study by Zhang et al. reported the importance of DRD2 − 141 C Ins/Del polymorphism in antipsychotic (any one of the five antipsychotics) response and associated adverse effects in Chinese population (thirty patients received risperidone) and shown a significant association.[15] Similarly, other studies reported the role of serotonin receptor polymorphism 5HTR2C (−759 C>T) in antipsychotic-induced weight gain, prolactin increase, and EPSs.[16,17,18] Due to limited data and involvement of various antipsychotics, none of the studies have established genetic polymorphisms as biomarkers in the clinical setting. Although several studies have reported the adverse effects of risperidone, very few studies reported related adverse effects with genetic variants to dopamine and serotonin receptor in the Indian population.[19,20] Since it is likely that DRD2 polymorphism can affect the EPS and 5HTR2C polymorphism in food intake the present study was aimed to investigate the safety profile of risperidone and the role of genetic variants DRD2 (−141 C Ins/Del/rs1799732) and 5HTR2C (−759 C>T/rs3813929).
Materials and Methods
The study was conducted among 289 schizophrenic patients diagnosed as per Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision criteria during December 2013–August 2015. Patients were either prescribed risperidone (subgroup) or on risperidone for 4 weeks or more. The study was approved by the Institute Ethics Committee, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. Patients were recruited from Psychiatry Outpatient Department, JIPMER. After explaining the study procedure, informed consent was obtained from all the patients or their legally acceptable representatives. The study participants were on treatment with risperidone (4–8 mg/day) for a minimum of 4 or more weeks. Patients who had a history of medical illness or substance abuse, pregnant women, nursing women, and with age of <18 years were excluded from the study.
Five milliliter of blood was collected from each patient to study the genetic polymorphisms DRD2 (−141 C Ins/Del) and 5HTR2C (−769 C>T). DNA was extracted from leukocyte by standard phenol-chloroform method. Genotyping for DRD2 (−141 C Ins/Del) and 5HTR2C (−759 C>T) genes were performed on real-time thermocycler (ABI Prism 7300) using Taqman SNP probe (rs1799732, Catalog No. 4331349, Assay ID: 0150250174; rs3813929 Catalog No. 4351379 Assay ID: C_27488117_10 Applied Biosystems, Foster City, CA, USA). All the patients were evaluated for adverse drug reactions (ADRs) after 4 or more weeks of the study period. Changes in prolactin levels and body weight were analyzed for a subgroup of patients for whom the baseline values before start of risperidone was available. Serum prolactin levels were measured by using Advia Centaur® Chemiluminescence Immunoassay Kit code 10309975 (Advia-Centaur CP, Siemens healthcare diagnostics, Germany).[21] After 4 weeks of risperidone therapy, patients showing >20% increase in prolactin levels from baseline were considered to have developed hyperprolactinemia.[22] Weight assessment was done at baseline and after taking risperidone (4–8 mg/day) for 6 weeks. An increase of >5% weight gain in 6 weeks was considered significant.[23,24] The ADRs were classified as per Naranjo algorithm score into four categories, i.e., definite, probable, possible, and doubtful ADRs.[25]
Statistical analysis
Demographic parameters were expressed as mean ± standard deviation. ADRs were analyzed descriptively. Associations between genetic variants, prolactin levels, weight gain, and EPS were analyzed using SNP Stats software (Institut Català d’Oncologia, Barcelona, Spain). Genotype frequencies were assessed for Hardy–Weinberg equilibrium. Correlation between prolactin levels and weight gain were analyzed using Spearman Rank correlation SPSS version 19.0 (IBM – SPSS, Inc., 2009, Chicago, IL, USA) Fisher's test was used to see the association between prolactin levels and EPS using GraphPad InStat software (version 3.06, 32 bit for Windows, September 11, 2003, GraphPad Software, San Diego California USA).
Results
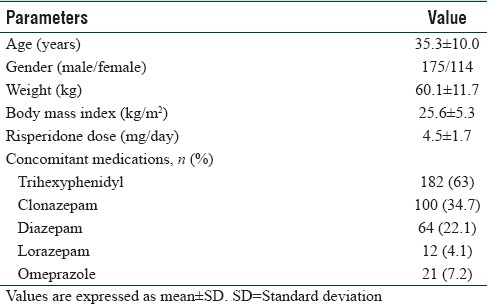
Demographic characteristics of schizophrenic patients receiving a mean dose of risperidone 4.5 mg/day (4–8 mg/day) are given in Table 1.
Table 1.
Demographic characteristics (n=289)
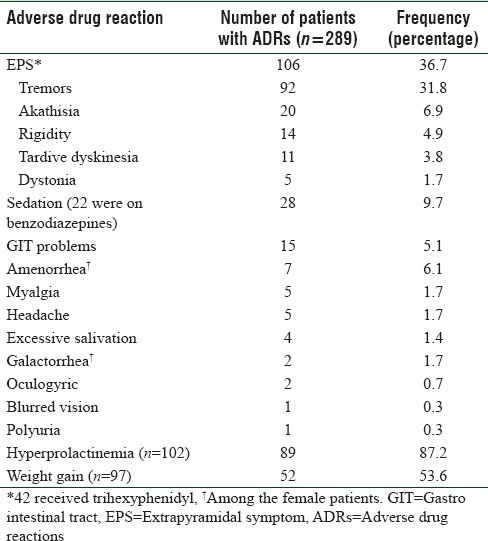
Among 289 patients, 197 experienced at least 1 ADR (68.2%). Fifty patients experienced only 1 ADR whereas others experienced 2 or more ADRs such as tremors, rigidity, akathisia, tardive dyskinesia, sedation, hyperprolactinemia, and weight gain.
We could estimate the changes in prolactin levels in 102 (59 male/43 female) and weight in 97 (58 male/39 female) patients. Changes observed in these two parameters and other ADRs are shown in Table 2.
Table 2.
Adverse drug reactions associated with risperidone-based therapy
Causality analysis by Naranjo's algorithm of all the 353 ADRs to risperidone-based therapy showed that 92.6% (327/353) ADRs were having score of 5–8 and belonged to the probable category and 7.4% (26/353) were having score of 1–4 and belong to the possible category. None belongs to the definite or doubtful category. When compared the genotype frequency of 5HTR2C −759 C>T polymorphism of schizophrenia patients [Supplementary Table 1 (78.6KB, tif) ] and healthy individuals from the South India (n = 96 [71 male/25 female], CC = 0.5, CT = 0.21, TT = 0.23; odds ratio [OR] = 0.52; 95% confidence interval [CI] = 0.30–0.92; P = 0.074) we did not find significant difference in polymorphism distribution.
Genotype frequencies (n=289)
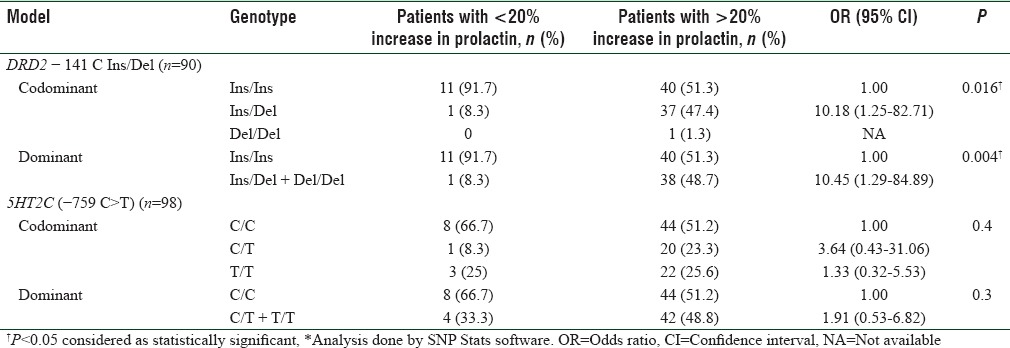
DRD2 gene −141C Ins/del polymorphism did not show any association with risperidone-based therapy induced EPSs (n = 99, OR = 0.68, 95% CI = 0.30–1.52, P = 0.3) and similarly 5HTR2C gene −759 C>T polymorphism was not associated with EPSs (n = 105, OR = 0.57, 95% CI = 0.20–1.58, P = 0.2). Association analysis of genotypes with hyperprolactinemia and weight gain is shown in Table 3 and Supplementary Table 2 (458.8KB, tif) .
Table 3.
Association between DRD2 −141 C Ins/Del and 5HT2C (−759 C>T) genetic variants and risperidone-induced increase in prolactin levels
Association between DRD2 −141 C Ins/Del or 5HT2C (−759 C>T) genetic variants and risperidone induced weight gain
Serum prolactin levels increased from baseline to follow-up (33.21 ± 29.94–71.44 ± 51.99 [ng/ml], n = 102, P < 0.01), and weight change was observed from baseline to follow-up (55.9 ± 12.92–61.8 ± 13.50 [kg], n = 97, P = 0.02). After the follow-up period, we have found the weak positive correlation (n = 92, r = 0.3, P = 0.005) between percentage increase in serum prolactin levels and percentage increase in weight gains. EPSs were seen in 40/102 patients, and we did not find any association with increased prolactin levels (n = 102, OR = 0.96, 95% CI = 0.2–3.1, P = 1.0).
Discussion
In the present study, we have studied the adverse effects of risperidone-based therapy in patients with schizophrenia and the role of DRD2 − 141C Ins/Del and 5HTR2C −759 C>T genetic polymorphisms in the causation of weight gain and hyperprolactinemia, along with EPS.
Although several studies have tried to explain the role of pharmacogenetic factors influencing the adverse effects of other antipsychotic drugs, very few studies are available with respect to risperidone-based therapy. In the present study, risperidone-induced hyperprolactinemia was observed in 87.2% of the patients, and we have found a significant association of −141 C deletion with the risk of increase in prolactin levels. There are few studies which have shown the elevated prolactin levels to be a marker for risperidone response, but our previous study found a nonsignificant difference in prolactin elevation between responders and nonresponders.[22]
Hyperprolactinemia-related adverse effects such as amenorrhea and galactorrhea were observed in 6.1% and 1.7% in female patients, respectively. Available evidence shows that therapeutic doses of first-generation antipsychotics can cause a tenfold increase in prolactin levels when compared to SGA.[26] Our study findings are consistent with the previous study by Zhang et al. They have reported the association of DRD2 (Taq1A; −141 C Ins/Del) gene polymorphism with elevated prolactin levels in Chinese patients on antipsychotic medication.[15] A recent study by Sukasem et al. reported the DRD2 (Taq1A) variants association with elevated prolactin levels, and Zhang et al. reported that DRD2 polymorphism (rs2514218) is associated with positive symptom improvement and prolactin elevation with risperidone therapy in male patients with first-episode psychosis.[27,28] However, Calarge et al. reported no association between DRD2 (−141 C Ins/Del) polymorphism and prolactin levels on risperidone treatment in non-Hispanic Caucasians population.[13] Similarly, other studies by Nagai et al. and Yasui-Furukori et al. also reported no association between DRD2 polymorphism and prolactin levels in the Japanese population.[29,30] These differences may be attributed to other factors responsible for variation of single point prolactin levels such as age, food habits, menstrual cycle, exercise, and stress that can vary with geographical location and ethnicities.[31] Weight gain was observed in 53.6% of the patients. It has been reported that benzodiazepines do not cause weight loss; hence, their concomitant use has not contributed in weight gain.[32,33] Several studies have suggested that 5HTR2C receptor is a candidate gene for antipsychotic drug-induced weight gain which has an important role in feeding behavior. It has been proposed that risperidone-induced central inhibition of 5HTR2C receptors increases food intake in spite of satiety, resulting in weight gain.[34] Genetic variant in the promoter region of 5HT2C receptor gene (−759 C>T) has been commonly studied in association with antipsychotic drug-induced weight gain in different populations. However, there were no studies in the Indian population. A study by Hoekstra et al. in Dutch children and adolescents with autism spectrum disorders demonstrated a reduced risk of antipsychotic drug-induced weight gain among carriers of T allele of 5HTR2C −759 C>T polymorphism.[35] However, the present study did not find any association between 5HTR2C (−759 C>T)/DRD2 (−141 C Ins/Del) gene polymorphisms and weight gain due to risperidone-based therapy. Our study findings are in contrast to previous study Reynolds et al. in Han Chinese patients.[18,36] However, the frequency of weight gain (53.6%) in the study is higher than earlier study by Almoguera et al. (36%).[37] Available literature says that hyperprolactinemia can also induce the weight gain by decreasing insulin sensitivity and by producing an imbalance in gonadal hormones.[36] Early and rapid weight gain in the initial stages of therapy is a predictor of long-term weight gain due to antipsychotics.[38]
Earlier studies have reported a weak positive correlation between prolactin levels and weight gain.[39] However in the present study, we found a weak positive correlation (r = 0.3) between increase in prolactin levels and increase in weight gain. Moreover prospective studies with larger sample size are required to draw a strong conclusion.
Drug-induced EPS are other important adverse effects of risperidone. Tremors are the most frequent EPS in the present study (86.8%) despite the fact that 63% of patients got trihexyphenidyl. Akathisia and rigidity accounted for 18.9% and 13.2% of EPS, respectively. This was similar to the study by Almoguera et al. (2013) in Spanish patients, where EPS was found to occur at a frequency of 36%. However, the frequency of EPS observed in our study is slightly higher than that observed in a multicenter, randomized controlled trial (32.8%, n = 111) in Chinese patients.[40] A recent study by Mas et al. (2016) reported higher frequency (41%) of risperidone-induced EPS in Spanish patients with a first psychotic episode.[41] Further, based on a systematic review, risperidone was associated with more incidences of EPS than clozapine, olanzapine, quetiapine, and ziprasidone.
This is the first study to report adverse effects of risperidone-based therapy in relation with genetic variants of DRD2 and 5HT2C in South Indian patients with schizophrenia. Limitation of our study is masking of EPS due to simultaneous prescription of trihexyphenidyl.
Conclusion
Hyperprolactinemia, weight gain, and EPSs were the common ADRs among schizophrenic patients treated with risperidone. DRD2 − 141 C Ins/Del genetic variant was significantly associated with increase in prolactin levels. A weak positive correlation was observed between percentage increase in prolactin levels and weight gain. The study could not detect a significant association between 5HTR2C −759 C>T gene polymorphism and risperidone-induced weight gain.
Financial support and sponsorship
The study was supported by Jawaharlal Institute of Postgraduate Medical Education and Research Intramural Research Grant (Grant No. JIP/Res/Intra-PhD/01/2014).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fleischhacker WW, Arango C, Arteel P, Barnes TR, Carpenter W, Duckworth K, et al. Schizophrenia – Time to commit to policy change. Schizophr Bull. 2014;40(Suppl 3):S165–94. doi: 10.1093/schbul/sbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bioque M, Llerena A, Cabrera B, Mezquida G, Lobo A, González-Pinto A, et al. A pharmacovigilance study in first episode of psychosis: Psychopharmacological interventions and safety profiles in the PEPs project. Int J Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyv121. pii: pyv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller R. Mechanisms of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions: Part II. Curr Neuropharmacol. 2009;7:315–30. doi: 10.2174/157015909790031184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komossa K, Rummel-Kluge C, Schwarz S, Schmid F, Hunger H, Kissling W, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011:1. doi: 10.1002/14651858.CD006626.pub2. CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinberg DL, Davis JM, de Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol. 1999;19:57–61. doi: 10.1097/00004714-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Yen YC, Lung FW, Chong MY. Adverse effects of risperidone and haloperidol treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:285–90. doi: 10.1016/j.pnpbp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Kim Y, Lee JH. Antipsychotic-induced sexual dysfunction and its management. World J Mens Health. 2012;30:153–9. doi: 10.5534/wjmh.2012.30.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahra SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CM, et al. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2:1725–34. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uçok A, Gaebel W. Side effects of atypical antipsychotics: A brief overview. World Psychiatry. 2008;7:58–62. doi: 10.1002/j.2051-5545.2008.tb00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.dos Santos Júnior A, Henriques TB, de Mello MP, Ferreira Neto AP, Paes LA, Della Torre OH, et al. Hyperprolactinemia in children and adolescents with use of risperidone: Clinical and molecular genetics aspects. J Child Adolesc Psychopharmacol. 2015;25:738–48. doi: 10.1089/cap.2015.0094. [DOI] [PubMed] [Google Scholar]
- 13.Calarge CA, Ellingrod VL, Acion L, Miller DD, Moline J, Tansey MJ, et al. Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics. 2009;19:373–82. doi: 10.1097/FPC.0b013e328329a60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura I, Zhang JP, Hagi K, Lencz T, Kane JM, Yabe H, et al. Variants in the DRD2 locus and antipsychotic-related prolactin levels: A meta-analysis. Psychoneuroendocrinology. 2016;72:1–10. doi: 10.1016/j.psyneuen.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XR, Zhang ZJ, Zhu RX, Yuan YG, Jenkins TA, Reynolds GP. Sexual dysfunction in male schizophrenia: Influence of antipsychotic drugs, prolactin and polymorphisms of the dopamine D2 receptor genes. Pharmacogenomics. 2011;12:1127–36. doi: 10.2217/pgs.11.46. [DOI] [PubMed] [Google Scholar]
- 16.Correia CT, Almeida JP, Santos PE, Sequeira AF, Marques CE, Miguel TS, et al. Pharmacogenetics of risperidone therapy in autism: Association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenomics J. 2010;10:418–30. doi: 10.1038/tpj.2009.63. [DOI] [PubMed] [Google Scholar]
- 17.Gunes A, Dahl ML, Spina E, Scordo MG. Further evidence for the association between 5-HT2C receptor gene polymorphisms and extrapyramidal side effects in male schizophrenic patients. Eur J Clin Pharmacol. 2008;64:477–82. doi: 10.1007/s00228-007-0450-x. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet. 2002;359:2086–7. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal AK, Bashyam VS, Channabasavanna SM, Dhavale HS, Khan MA, Khanna S, et al. Risperidone in Indian patients with schizophrenia. Indian J Psychiatry. 1998;40:247–53. [PMC free article] [PubMed] [Google Scholar]
- 20.Avasthi A, Aggarwal M, Grover S, Khan MK. Research on antipsychotics in India. Indian J Psychiatry. 2010;52(Suppl 1):S317–40. doi: 10.4103/0019-5545.69261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margari L, Matera E, Petruzzelli MG, Simone M, Lamanna AL, Pastore A, et al. Prolactin variations during risperidone therapy in a sample of drug-naive children and adolescents. Int Clin Psychopharmacol. 2015;30:103–8. doi: 10.1097/YIC.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charan A, Shewade DG, Rajkumar RP, Chandrasekaran A. Relation between serum prolactin levels and antipsychotic response to risperidone in patients with schizophrenia. Psychiatry Res. 2016;240:209–13. doi: 10.1016/j.psychres.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Goeb JL, Marco S, Duhamel A, Kechid G, Bordet R, Thomas P, et al. Metabolic side effects of risperidone in early onset schizophrenia. Encephale. 2010;36:242–52. doi: 10.1016/j.encep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, Delacrétaz A, Choong E, Solida-Tozzi A, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76:e1417–23. doi: 10.4088/JCP.14m09358. [DOI] [PubMed] [Google Scholar]
- 25.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Rivera JL, Lal S, Ettigi P, Hontela S, Muller HF, Friesen HG. Effect of acute and chronic neuroleptic therapy on serum prolactin levels in men and women of different age groups. Clin Endocrinol (Oxf) 1976;5:273–82. doi: 10.1111/j.1365-2265.1976.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 27.Sukasem C, Hongkaew Y, Ngamsamut N, Puangpetch A, Vanwong N, Chamnanphon M, et al. Impact of pharmacogenetic markers of CYP2D6 and DRD2 on prolactin response in risperidone-treated thai children and adolescents with autism spectrum disorders. J Clin Psychopharmacol. 2016;36:141–6. doi: 10.1097/JCP.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JP, Robinson DG, Gallego JA, John M, Yu J, Addington J, et al. Association of a schizophrenia risk variant at the DRD2 locus with antipsychotic treatment response in first-episode psychosis. Schizophr Bull. 2015;41:1248–55. doi: 10.1093/schbul/sbv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai G, Mihara K, Nakamura A, Suzuki T, Nemoto K, Kagawa S, et al. Prolactin concentrations during aripiprazole treatment in relation to sex, plasma drugs concentrations and genetic polymorphisms of dopamine D2 receptor and cytochrome P450 2D6 in Japanese patients with schizophrenia. Psychiatry Clin Neurosci. 2012;66:518–24. doi: 10.1111/j.1440-1819.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 30.Yasui-Furukori N, Saito M, Tsuchimine S, Nakagami T, Sato Y, Sugawara N, et al. Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1491–5. doi: 10.1016/j.pnpbp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS One. 2012;7:e31305. doi: 10.1371/journal.pone.0031305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oswald I, Adam K. Benzodiazepines cause small loss of body weight. Br Med J. 1980;281:1039–40. doi: 10.1136/bmj.281.6247.1039-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shader RI, Greenblatt DJ. The use of benzodiazepines in clinical practice. Br J Clin Pharmacol. 1981;11(Suppl 1):5S–9S. doi: 10.1111/j.1365-2125.1981.tb01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Luca V, Müller DJ, Hwang R, Lieberman JA, Volavka J, Meltzer HY, et al. HTR2C haplotypes and antipsychotics-induced weight gain: X-linked multimarker analysis. Hum Psychopharmacol. 2007;22:463–7. doi: 10.1002/hup.868. [DOI] [PubMed] [Google Scholar]
- 35.Hoekstra PJ, Troost PW, Lahuis BE, Mulder H, Mulder EJ, Franke B, et al. Risperidone-induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5-hydroxytryptamine 2C receptor gene. J Child Adolesc Psychopharmacol. 2010;20:473–7. doi: 10.1089/cap.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane HY, Liu YC, Huang CL, Chang YC, Wu PL, Lu CT, et al. Risperidone-related weight gain: Genetic and nongenetic predictors. J Clin Psychopharmacol. 2006;26:128–34. doi: 10.1097/01.jcp.0000203196.65710.2b. [DOI] [PubMed] [Google Scholar]
- 37.Almoguera B, Riveiro-Alvarez R, Lopez-Castroman J, Dorado P, Vaquero-Lorenzo C, Fernandez-Piqueras J, et al. Association of common genetic variants with risperidone adverse events in a Spanish schizophrenic population. Pharmacogenomics J. 2013;13:197–204. doi: 10.1038/tpj.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rege S. Antipsychotic induced weight gain in schizophrenia: Mechanisms and management. Aust N Z J Psychiatry. 2008;42:369–81. doi: 10.1080/00048670801961123. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Sun J, Shen X, Guo W, Zhi S, Song G, et al. Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first-episode schizophrenia over 12 months of treatment with risperidone or quetiapine. Shanghai Arch Psychiatry. 2014;26:88–94. doi: 10.3969/j.issn.1002-0829.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Yao C, Shi J, Yang F, Qi S, Wang L, et al. Comparative study of the efficacy and safety between blonanserin and risperidone for the treatment of schizophrenia in Chinese patients: A double-blind, parallel-group multicenter randomized trial. J Psychiatr Res. 2015;69:102–9. doi: 10.1016/j.jpsychires.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Mas S, Gassó P, Lafuente A Bioque M, Lobo A, Gonzàlez-Pinto A, et al. Pharmacogenetic study of antipsychotic induced acute extrapyramidal symptoms in a first episode psychosis cohort: Role of dopamine, serotonin and glutamate candidate genes. Pharmacogenomics J. 2016;16:439–45. doi: 10.1038/tpj.2016.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotype frequencies (n=289)
Association between DRD2 −141 C Ins/Del or 5HT2C (−759 C>T) genetic variants and risperidone induced weight gain


