Abstract
Reactive oxygen species (ROS) are generated by ionizing radiation, and one of the organs commonly affected by ROS is the lung. Radiation-induced lung injury including pneumonia and lung fibrosis is a dose-limiting factor in radiotherapy (RT) of patients with thorax irradiation. Administration of antioxidants has been proved to protect against ROS. The present study was aimed to assess the protective effect of hesperidin (HES) against radiation-induced lung injury of male rats. Fifty rats were divided into three groups. G1: Received no HES and radiation (sham). G2: Underwent γ-irradiation to the thorax. G3: Received HES and underwent γ-irradiation. The rats were exposed to a single dose of 18 Gy using cobalt-60 unit and were administered HES (100 mg/kg) for 7 days before irradiation. Histopathological analysis was performed 24 h and 8 weeks after RT. Histopathological results in 24 h showed radiation-induced inflammation and presence of more inflammatory cells as compared to G1 (P < 0.05). Administration of HES significantly decreased such an effect when compared to G2 (P < 0.05). Histopathological evaluation in 8 weeks showed a significant increase in mast cells, inflammation, inflammatory cells, alveolar thickness, vascular thickness, pulmonary edema, and fibrosis in G2 when compared to G1 (P < 0.05). HES significantly decreased inflammatory response, fibrosis, and mast cells when compared to G2 (P < 0.05). Administration of HES resulted in decreased radiation pneumonitis and radiation fibrosis in the lung tissue. Thus, the present study showed HES to be an efficient radioprotector against radiation-induced damage in the lung of tissue rats.
Keywords: Hesperidin, lung fibrosis, pneumonia, radioprotector
Introduction
Ionizing radiation (IR) generates reactive oxygen species (ROS) and disrupts the structure of chemical bonds. Absorbed energy of IR causes ionization of different atoms and molecules, comprising H2O and essential macromolecules such as DNA and membrane lipids.[1,2] The harmful role of IR is mainly due to ROS, including superoxide radical (O2°−), hydroxyl radical (OH°), and hydrogen peroxide generated by the decomposition of water.[3] ROS play significant role in many physiological and pathological reactions that may cause injury or disease. IR can increase production of ROS through interaction with water molecules, activation of redox system, and inhibition of antioxidant enzymes.[4] The lung is a radiosensitive organ and one of the organs commonly affected by ROS.
Radiotherapy (RT) is commonly applied as a part of cancer treatment, and it is assessed that more than half of all cancer patients will receive RT.[5] The lung is irradiated in patients with thoracic-region tumors that include breasts, lung, esophagus, lymphomas, and other mediastinal neoplasms.[6] Lung injuries are classified into two phases. The first (early) phase is named radiation pneumonitis (acute syndrome). According to histopathological evaluation, acute syndrome evidenced by loss of Type I pneumocytes, alveolar capillary congestion, increased capillary permeability, inflammatory cell accumulation, and interstitial edema in the alveolar space. These injuries reveal at approximately 1–6 months after RT in 10%–15% of patients who received chest region irradiation.[7,8,9,10] According to histopathological changes in this phase, the first symptom of a lung injury is extensive alveolar damage.[11] The second or latent phase (chronic syndrome) is pulmonary fibrosis that occurs months to years after RT. The pathologic findings in pulmonary fibrosis characterized by excessive accumulation of extracellular matrix (ECM) and reshaping of the lung structure.[12] Pulmonary fibrosis is the repair process of pneumonitis radiation that usually develops with the loss of capillaries, thickened alveolar septa, and obliteration of the alveolar space.[10] Mast cells are immune effectors involved in allergic and hypersensitivity reactions that are actively implicated in a number of physiological and pathological situations, varying from normal wound healing and host defense to tissue inflammation and tumor growth.[13,14,15] Pathologically, mast cells contribute to alveolitis development through the secretion of chemokines and cytokines that serve to recruit inflammatory cells.[16,17]
In spite of improving the clinical RT treatment planning and delivery technologies, there is a considerable toxicity of RT for normal tissues.[18] To attain optimum treatment, the normal tissues should be protected against radiation damage. Thus, radioprotective compounds are essential in clinical RT.[19] Among varieties of radioprotectors, most failed because of their toxicity and side effects. The search for radioprotectors with less toxicity has motivated interest in the development of natural products that prevent damaging effects of IR and have some proven therapeutic advantages.[20]
Hesperidin (HES) (hesperetin-7-rhamnoglucoside), a flavanone glycoside, is a member of the flavonoids. Flavonoids are a family of natural polyphenolic structures that are found in fruits and vegetables. HES is the major flavonoids that discovered in sweet orange and lemon. In immature oranges, it has up to 14% of the weight of the fruit.[21,22] HES has been reported to exert a wide range of pharmacological effects, which include anticarcinogenic, antiallergic, vasoprotective, hypolipidemic, antioxidant, and anti-inflammatory actions.[23,24] These biological effects of HES are mainly associated with their antioxidant properties. Histopathologically, several investigations have demonstrated that HES has radioprotective effect against normal tissues exposed to RT.[25,26,27,28] This study was performed to evaluate the protective effect of HES against γ-irradiation-induced acute and chronic lung injury in male Sprague–Dawley rats.
Materials and Methods
Animals
In this study, chemical materials including HES (CAS registry number: 520-26-3) and phosphate-buffered saline tablet (PBS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Healthy adult male Sprague–Dawley rats were purchased from the Center for Comparative and Experimental Medicine, Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. The rats weighed around 220 ± 5 g and were housed in accordance with particular practices described in “The Guide for The Care and Use of Laboratory Animals” arranged by SUMS in the university animal house. The practices involved some specific measures which include as follows: characteristics of animal natural life in captivity situation, using spacious cages, preparing appropriate ventilation and light, handling with care, giving standard pellet diet and water ad libitum, etc. All the animals were housed in certain circumstances including temperature (23 ± 2°C), humidity (55% ±5%), and light (12 h of light and dark cycle). Besides, four animals were separately housed together in polypropylene cages containing sterile husk bedding during the experiments, and this study was conducted based on the instructions issued by the SUMS’ Ethical Committee.
Animal exposure
Rats were anesthetized before exposing to radiation using ketamine at a dose of 80 mg/kg and xylazine at a dose of 5 mg/kg with an intraperitoneal injection. Accordingly, the rats were immobilized in the supine position by taping the extremities on a well-ventilated plexiglass container. A cobalt-60 γ-radiation source (Theratron Phoenix, Canada) was used to irradiate the animals in the Department of RT, Nemazee Teaching Hospital, Shiraz, Iran. In three groups, simultaneously, the anesthetized rats were irradiated locally on the thorax. The source-to-skin distance was 55 cm with a dose rate of 300 mGy/min at room temperature. The rats were irradiated with a single dose of 18 Gy γ-rays. In fact, this dose was selected according to the results published by Tahamtan et al., in which they declared that a single dose of 18 Gy develops considerable radiation lung injury.[29]
HES was dissolved in PBS (pH 7.6) and then administered orally using a ball-tipped needle for seven consecutive days before exposure to γ-irradiation. In addition, the drug was freshly prepared every day. Dose of 100 mg/kg was selected for this study based on the previous reports by Rezaeyan et al., Hosseinimehr and Nemati, and Pradeep et al. They have shown that 100 mg/kg has protective effect against radiation-induced damage in lung, heart, liver, kidney and bone marrow.[30,31] To prepare this dose, 22 mg of HES was dissolved in 2 ml of PBS.
Experimental design
In this course of action, 50 male rats were randomly separated into three groups. Group 1 (sham): Fourteen rats were earmarked as controls which only received PBS for 7 days. Group 2 (RT): Eighteen rats received PBS for 7 days and exposed to γ-irradiation 1 h after the last dose of PBS. Group 3 (HES + RT): Eighteen rats were treated with HES for 7 days and exposed to γ-irradiation 1 h after the last dose of HES. After the last administration of PBS at the 7th day, like rats in RT and HES + RT groups, the rats of sham group were anesthetized. In each group, eight rats were sacrificed 24 h after RT for acute histopathological evaluation. Moreover, 26 animals remained (sham = 6, RT = 10, and HES + RT = 10) were sacrificed 8 weeks after RT for chronic histopathological evaluation.
Histopathological evaluation
Rats were anesthetized with ketamine and xylazine. After extracting the lungs from the chest, tissue was fixed, infused with 10% neutral-buffered formalin introduced through the airways, and then laid into paraffin. Whole-mount sections of the lungs were cut (5 µm), processed, and stained with H and E, Masson's trichrome (MTC), and acid-fast (AF). All the histological works were performed at the Unit of Pathology, Faghihee Teaching Hospital, Shiraz, Iran. The blinded histopathological evaluation was carried out using the light microscope (Olympus BX41TF, Japan). Semi-quantitative scoring of each variable was performed by a histopathologist using the following scales: (1) No change, (2) mild, (3) moderate, and (4) severe injury. In the acute phase, the descriptive items for radiation-induced lung injuries were as follows: Presence of neutrophils, macrophages, lymphocytes and incidence of inflammation, erythrocytes (red blood cell [RBC]), hyaline arteriosclerosis, vascular thickness, alveolar thickness, and collapse. On the other hand, those of chronic phase included the presence of macrophages, neutrophils, and lymphocytes; incidence of inflammation, alveolar thickness, vascular thickness, pulmonary edema, thrombosis and pulmonary fibrosis; and presence of mast cells. Sections were stained using H and E for general tissue characterization.[32] Moreover, collagen accumulation was assessed by preparing tissue sections with MTC stain.[33,34] The AF stain was used to evaluate mast cells.[35,36] The lung index was calculated as lung index = (lung weight/body weight) ×100.
Statistical analysis
In this study, data were analyzed using a commercially available statistical software package (SPSS® for Windows v. 19, Chicago, IL, USA). Histopathological evaluations were analyzed by the Pearson Chi-square test and a pair-wise comparison with Mann–Whitney. Furthermore, to evaluate the body weight, lung weight, and lung index, a one-way ANOVA test with post hoc Tukey honestly significant difference was performed. The survival rate was evaluated with Kaplan–Meier method. The results are presented as mean ± standard deviation. We considered P < 0.05 as statistically significant difference.
Results
Physical examination and survival rate
Figure 1 and Table 1 illustrate the results of the survival rate, body weights, lung weights, and lung indexes of control versus experimental rats evaluated 8 weeks after local-thorax irradiation. Before irradiation, rats were preadministered with 100 mg/kg/d of HES for seven consecutive days, and then, monitoring was done to observe their reaction daily and bimonthly. The results showed that the rats received 18 Gy radiation exhibited symptoms of discomfort which was characterized by reduction not only in physical activity but also in uptake of food and water. Besides, the radiation-exposed groups showed symptoms of radiation sickness such as irritability, ruffling of hair, weight loss, emaciation, and epilation. On the contrary, those rats which were preadministered with HES had reduced symptoms of radiation sickness and significantly enhanced physical activity, body weight, and survival rate.
Figure 1.
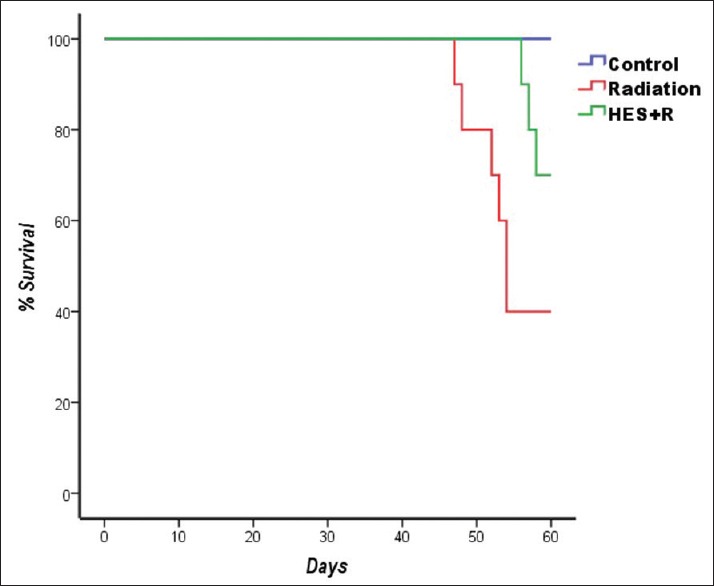
Dose-dependent effect of hesperidin on the survival rate of rats observed for an experimental duration of 60 days. Sham group: Six rats out of six were survived. Radiotherapy group: Four rats out of ten survived. Hesperidin + radiotherapy group: Seven rats out of ten survived.
Table 1.
Effect of hesperidin treatment on body weight, lung weight, and lung index of rats exposed to γ-irradiation

Histopathological examination
Acute phase
At 24 h postirradiation, the sham, RT, and HES + RT groups were evaluated through histopathological observation of the lung sections [Figure 2]. Meanwhile, the descriptive factors examined include the presence of neutrophils, macrophages, and lymphocytes and incidence of inflammation, erythrocytes (RBC), hyaline arteriosclerosis, vascular thickness, alveolar thickness, and collapse. According to the results, between-group analysis demonstrated only a significant difference in the inflammation, lymphocyte, macrophage, and neutrophil (P = 0.001, P = 0.009, P = 0.001, P = 0.001, respectively). Between-group analysis was performed by a pair-wise comparison with Mann–Whitney test and P < 0.05, which indicates a significant difference [Table 2]. The results indicated significant increases in inflammation, lymphocyte, macrophage, and neutrophil frequency in RT group when compared with sham group (P = 0.001). In the HES + RT group, treatment with HES contributed a decrease in these factors to near normal values when compared to RT group (P = 0.003, P = 0.017, P = 0.001, P = 0.001, respectively).
Figure 2.
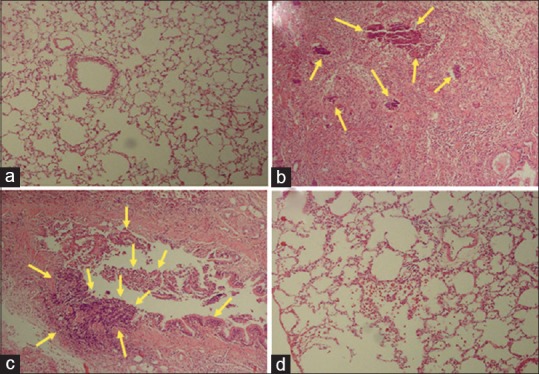
Histopathological investigation of the radioprotective effects of hesperidin and radiation damage in the acute phase (24 h). (a) Sham: Alveolar space, bronchioles, and vascular bed are seen normal. (b) Radiotherapy: Severe interstitial inflammation and pulmonary edema is seen. (c) Radiotherapy: Severe inflammation of bronchial wall is seen with destruction of bronchus. (d) Hesperidin + radiotherapy: Mild inflammation was observed. The arrows indicate an accumulation of lymphocyte, macrophages, and neutrophils in lung tissue (H and E, ×100).
Table 2.
Effect of hesperidin treatment at 24 h postirradiation on histopathological factors in the lung tissue of rats
Chronic phase
With respect to the lung sections in the sham, RT, and HES + RT groups at 8 weeks after radiation, histopathological evaluations were conducted to characterize the chronic changes in lung tissues after RT [Figures 3–5]. The descriptive factors examined were the presence of macrophages, lymphocytes, and neutrophils (inflammatory cells), incidence of inflammation, alveolar thickness, vascular thickness, pulmonary edema, thrombosis, pulmonary fibrosis, and infiltration of mast cells.
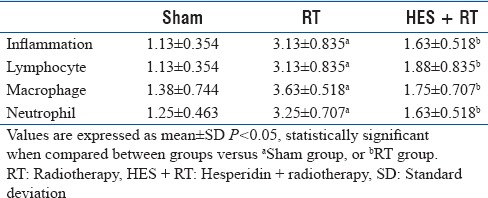
Figure 3.
Histopathological investigation of the radioprotective effects of hesperidin and radiation damage in the chronic phase (8 weeks). (A and a) Sham: Alveolar space, bronchioles, and vascular bed are seen normal. (B and b) Radiotherapy: Acute inflammation in the alveolar space, thickening of alveolar and pulmonary edema is seen with polymorphonuclear leukocyte (neutrophils accumulation). (C and c) Hesperidin + radiotherapy: Mild inflammation was observed. The yellow frame shows a magnification of ×400. (H and E, ABC: Magnification ×100, abc: Magnification ×400).
Figure 5.
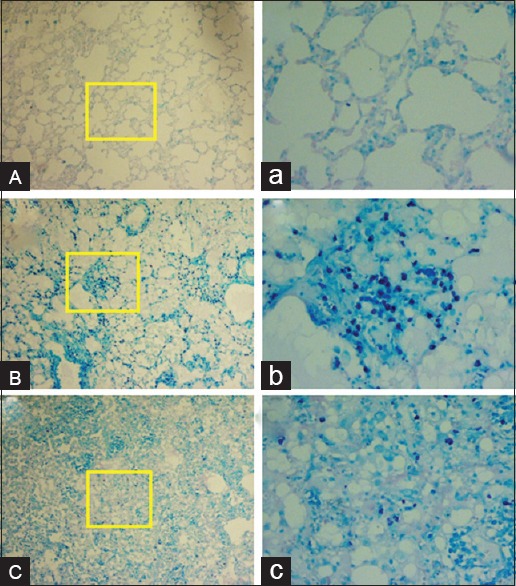
Histopathological investigation of the radioprotective effects of hesperidin and radiation damage in the chronic phase (8 weeks). (A and a) Sham: Alveolar space, bronchioles and vascular bed are seen normal. (B and b) Radiotherapy: Acute mast cells infiltration is seen. (C and c) Hesperidin + radiotherapy: Infiltration of mast cells is seen as mild. Mast cells are seen dark blue points. The yellow frame shows a magnification of ×400. (Acid-fast staining, ABC: Magnification ×100, abc: Magnification ×400).
Figure 4.
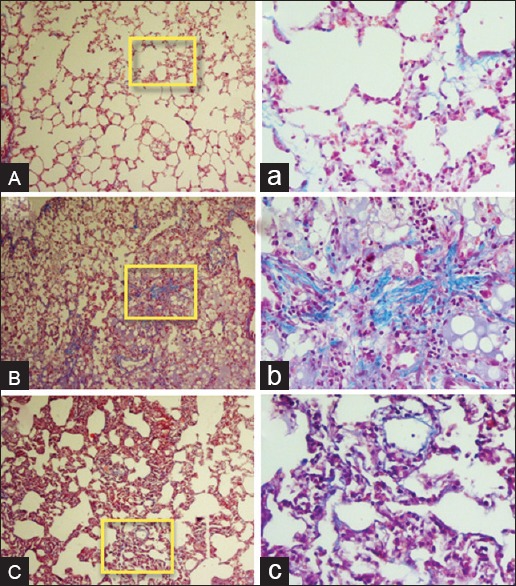
Histopathological investigation of the radioprotective effects of hesperidin and radiation damage in the chronic phase (8 weeks). (A and a) Sham: Alveolar space, bronchioles and vascular bed are seen normal. (B and b) Radiotherapy: Acute collagen deposition is seen. (C and c) Hesperidin + radiotherapy: Deposition of collagen is seen as mild. Collagen deposition is seen light blue. The yellow frame shows a magnification of ×400. (Massonæs trichrome staining, ABC: Magnification ×100, abc: Magnification ×400).
The statistical results of the left lung were found to be as follows: A significant difference between groups was observed in the presence of inflammatory cells, incidence of inflammation, alveolar thickness, vascular thickness, and pulmonary fibrosis, and presence of mast cells, but with the exception of pulmonary edema and thrombosis. The pair-wise comparison was performed in the experimental groups [Table 3]. Compared to the sham group, the radiation-induced lung injury factors were significant in the RT group (P < 0.05). A majority of factors such as frequency of inflammatory cells and mast cells and the incidence of inflammation, pulmonary fibrosis, alveolar thickness, and vascular thickness increased in irradiated lungs as compared to the sham group. Pulmonary edema and thrombosis signs were not found in the left lung of each group. The administration of HES before radiation reduced the vascular damage (P = 0.013) and the incidence of fibrosis (P = 0.012), inflammation (P = 0.006), frequency of mast cell (P = 0.033), macrophage (P = 0.001), lymphocyte (P = 0.026), and neutrophil (P = 0.013) in the HES + RT group when compared to RT group, but no significant difference was observed for vascular thickness (P > 0.05). Besides, except lymphocyte, pulmonary edema, and thrombosis, a significant difference was spotted for these factors to compare between the groups of HES + RT and sham.
Table 3.
Effect of hesperidin treatment at 8 weeks postirradiation on histopathological factors in the lung tissue of rats
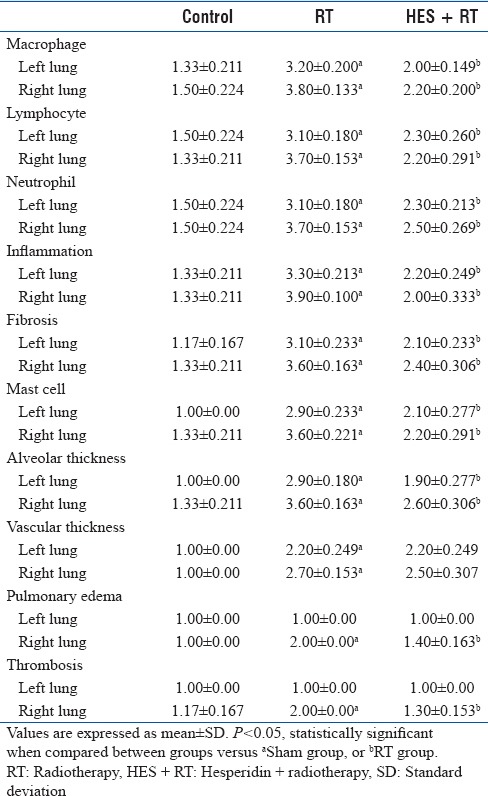
In the right lung, according to the results, a significant difference between groups was found in all of the mentioned histopathological factors (P < 0.05). To pair-wise comparison in the groups of radiation and control, all of these factors showed a significant difference (P < 0.05) [Table 3]. To pair-wise comparison in the groups of HES + R and radiation, the HES administration reduced signs of pulmonary fibrosis (P = 0.005), alveolar thickness (P = 0.016), pulmonary edema (P = 0.004), thrombosis (P = 0.001), mast cells (P = 0.003), inflammation (P < 0.001), and inflammatory cells (P < 0.05). As similar to the left lung, HES cannot reduce vascular thickness (P = 0.698). In addition, comparing the groups of HES + R and control, a significant difference was observed for these factors except for lymphocyte, inflammation, pulmonary edema, and thrombosis (P > 0.05). Overall, in the radiation group, the microscopic results and statistical analysis show histopathological damage in the left lung lesser than the right lung. Further, in the HES + R group, HES treatment reduced lung injury in the left lung more than the right lung, except lymphocyte and inflammation.
Discussion
Radiation-induced tissue damage is mediated generally by either direct attack on the genetic material or by generating ROS by the radiolysis of water (activation of redox systems and inhibition of antioxidant system) that assault the cellular macromolecules. Lung tissue damage includes damage to endothelial cells by increasing the permeability, edema, and fibrin accumulation of ECM. These changes are associated with an inflammatory response, including accumulation and activation of macrophage, inflammatory cells, chemokines, cytokines, and endothelial–leukocyte adhesion molecules that are attached to the damaged area or involved in inflammation.[8] Since ROS play the main role in radiation toxicity, administration of antioxidants may be important for patients who have experienced RT to deter normal tissues from damages.
Animals receiving higher doses of radiation are usually reported to exhibit signs of radiation sickness.[37] In this study, rats that received 18 Gy of γ-irradiation showed signs of discomfort, characterized by a decreased physical activity, and decreased uptake of food and water. In contrast, the rats that were treated with HES showed reduced signs of radiation sickness and appeared normal. Treatment with HES showed for 7 days significantly improved their body weight and lung weight. This result is very important because it is an important side effect in lung radiation adversely impacts nutritional intake which accounts for fatigue and weakness of patients. Therefore, it is established beyond doubt that malnutrition reduces treatment probability, survival, and quality of life.[38,39,40]
Radiation-induced inflammatory response is related to various cellular and molecular pathways. Molecular pathways including exudation of several cytokines such as tumor necrosis factor-α, interferon-γ, transforming growth factor-β, interleukin-1 (IL-1), IL-6, and IL-8. These cytokines increase expression of MAPKs, NF-κB, NADPH oxidase, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2. NADPH oxidase is generated by neutrophils and iNOS is induced by macrophages.[41] Inflammatory response in the lung tissue is described by vascular thickness change, leukocytes infiltration, and increased number of macrophages, neutrophils, and lymphocytes. On the other hand, pieces of evidence indicated that mast cells have been involved in inflammation and have been associated with early and delayed radiation damages, including tissue remodeling and fibrosis.[42,43] Preclinical reports on the role of mast cells in radiation injury show that these cells were associated with radiation fibrosis and collagen deposition in the lung tissue of rat.[44,45]
In this investigation, radiation increased the counts of macrophages, neutrophils, and lymphocytes and that resulted in the occurrence of inflammation at 24 h postirradiation [Table 2]. Supplementation with HES before irradiation reduces inflammatory cells including macrophages, neutrophils, and lymphocytes resulting in reduced inflammation [Figure 2]. This reveals HES can decrease acute inflammatory pathways induced by exposure to γ-radiation. In the RT group, histopathological evaluation of the lung sections at 8 weeks after irradiation showed an increase in inflammation, fibrosis, alveolar thickness, vascular thickness, pulmonary edema, and number of mast cells and inflammatory cells [Table 3]. The pulmonary edema and thrombosis signs were not found in the left lung of radiation group. In the left lung of radiation group, histopathological damage is lesser than the right lung. Supplementation with HES before radiation could reduce inflammation, fibrosis, and number of mast cells, inflammatory cells, alveolar thickness, pulmonary edema, and thrombosis significantly [Figures 3–5]. Furthermore, reduction of vascular thickness was not significant. Overall, in the HES + R group, HES treatment reduced lung injury in the left lung more than the right lung, except lymphocyte and inflammation.
According to previous studies, oral administration of HES can reduce γ-radiation-induced damage in the lung tissue of rats. HES acts by scavenging free radicals and by maintaining intracellular superoxide dismutase (SOD) and glutathione levels, thereby preventing lipid peroxidation and tissue damage.[28] In another similar study, the irradiation of the whole thorax of the rats with 18 Gy, a single dose of X-ray resulted in oxidative stress and histopathological changes in the heart tissue. The early oxidative stress is associated with the increased malondialdehyde level and decreased SOD enzyme activity. Long-term changes such as inflammation, fibrosis, increased mast cells and macrophages, plaque, vascular leakage and myocardial degeneration were observed in both right and left ventricles. Preadministration of HES is used to ameliorate oxidative stress, histopathological changes, and subsequent cell death after radiation treatment, which can cause the increased risk of heart diseases during years after RT.[27] Some studies showed HES supplement reduces oxidative and pathologic damages induced by IR in the liver, heart, and kidney.[25,26,31]
These investigations show that HES ameliorates inflammation signs including reduction of macrophages accumulation specially. Macrophages have a major role in acute and chronic inflammation and oxidative stress in lung tissues. Hence, administration of supplements that alleviate of macrophage activity can be advantageous. HES also has been reported to exert a protective effect on lung tissues that include modulated expression of pro-inflammatory cytokines and chemokines, reduction of polymorphonuclear neutrophils secretion in the airway, and improved pulmonary edema and lung morphology. HES effectively reduces pulmonary vascular permeability and leads to ameliorate pulmonary edema.[46] Hence, HES may protect the integrity of the alveolar membrane, resulting in the prevention of the infiltration of mast cells, and significantly improves lung morphology. Thus, HES has anti-inflammatory and specific protective effects against inflammatory disorders which are done through a mechanism involving the antioxidant activity of free radicals.
Conclusions
It is known that HES has pharmacological effects including antioxidant, anti-inflammatory, and anticarcinogenic actions. The present study indicates that HES significantly diminishes γ-radiation-induced damage in the lung tissues of rats. It seems that HES may be used as a natural radioprotector with anti-inflammatory and antioxidant functions to prevent oxidative stress caused by IR in a short-term after irradiation and acute and chronic inflammation of lung tissue. Based on the results of this study, it is concluded that HES can be a candidate for treating and preventing radiation-induced damage to the tissues, particularly for patients undergoing RT. However, additional investigations are needed to understand the molecular and cellular mechanisms associated with the radioprotective properties of HES in the lungs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lett JT. Damage to cellular DNA from particulate radiations, the efficacy of its processing and the radiosensitivity of mammalian cells. Emphasis on DNA double strand breaks and chromatin breaks. Radiat Environ Biophys. 1992;31:257–77. doi: 10.1007/BF01210207. [DOI] [PubMed] [Google Scholar]
- 2.Dainiak N, Tan BJ. Utility of biological membranes as indicators for radiation exposure: Alterations in membrane structure and function over time. Stem Cells. 1995;13(Suppl 1):142–52. [PubMed] [Google Scholar]
- 3.Ertekin MV, Koçer I, Karslioglu I, Taysi S, Gepdiremen A, Sezen O, et al. Effects of oral Ginkgo biloba supplementation on cataract formation and oxidative stress occurring in lenses of rats exposed to total cranium radiotherapy. Jpn J Ophthalmol. 2004;48:499–502. doi: 10.1007/s10384-004-0101-z. [DOI] [PubMed] [Google Scholar]
- 4.Najafi M, Fardid R, Takhshid MA, Mosleh-Shirazi MA, Rezaeyan AH, Salajegheh A. Radiation-induced oxidative stress at out-of-field lung tissues after pelvis irradiation in rats. Cell J. 2016;18:340–5. doi: 10.22074/cellj.2016.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringborg U, Bergqvist D, Brorsson B, Cavallin-Ståhl E, Ceberg J, Einhorn N, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001 – summary and conclusions. Acta Oncol. 2003;42:357–65. doi: 10.1080/02841860310010826. [DOI] [PubMed] [Google Scholar]
- 6.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 7.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–71. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 9.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–61. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: An update on radiation pneumonitis and fibrosis. Semin Oncol. 2005;32(2 Suppl 3):S42–54. doi: 10.1053/j.seminoncol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Gross NJ. Experimental radiation pneumonitis. IV. Leakage of circulatory proteins onto the alveolar surface. J Lab Clin Med. 1980;95:19–31. [PubMed] [Google Scholar]
- 12.Takishima T, Shimura S. Definition and classification of pulmonary fibrosis. In: Takishima T, editor. Basic and Clinical Aspects of Pulmonary Fibrosis. Boca Raton, FL: CRC Press; 1994. pp. 293–303. [Google Scholar]
- 13.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–8. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 14.Galli SJ, Tsai M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpin D, Perol D, Blay JY, Falchero L, Claude L, Vuillermoz-Blas S, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol. 2005;23:8748–56. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002;12(1 Suppl 1):26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- 18.Greenberger JS. Radioprotection. In Vivo. 2009;23:323–36. [PMC free article] [PubMed] [Google Scholar]
- 19.Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 20.Maurya DK, Devasagayam TP, Nair CK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol. 2006;44:93–114. [PubMed] [Google Scholar]
- 21.Tiwari AK. Imbalance in antioxidant defense and human diseases: Multiple approach of natural antioxidants therapy. Curr Sci. 2001;81:1179–87. [Google Scholar]
- 22.Barthe GA, Jourdan PS, McIntosh CA, Mansell RL. Radioimmunoassay for the quantitative determination of hesperidin and analysis of its distribution in Citrus sinensis. Phytochemistry. 1988;27:249–54. [Google Scholar]
- 23.Tommasini S, Calabrò ML, Stancanelli R, Donato P, Costa C, Catania S, et al. The inclusion complexes of hesperetin and its 7-rhamnoglucoside with (2-hydroxypropyl)-beta-cyclodextrin. J Pharm Biomed Anal. 2005;39:572–80. doi: 10.1016/j.jpba.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Emim JA, Oliveira AB, Lapa AJ. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol. 1994;46:118–22. doi: 10.1111/j.2042-7158.1994.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalpana KB, Devipriya N, Srinivasan M, Vishwanathan P, Thayalan K, Menon VP. Evaluating the radioprotective effect of hesperidin in the liver of Swiss albino mice. Eur J Pharmacol. 2011;658:206–12. doi: 10.1016/j.ejphar.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Pradeep K, Ko KC, Choi MH, Kang JA, Chung YJ, Park SH. Protective effect of hesperidin, a citrus flavanoglycone, against κ-radiation-induced tissue damage in Sprague-Dawley rats. J Med Food. 2012;15:419–27. doi: 10.1089/jmf.2011.1737. [DOI] [PubMed] [Google Scholar]
- 27.Rezaeyan A, Haddadi GH, Hosseinzadeh M, Moradi M, Najafi M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J Med Phys. 2016;41:182–91. doi: 10.4103/0971-6203.189482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaeyan A, Fardid R, Haddadi GH, Takhshid MA, Hosseinzadeh M, Najafi M, et al. Evaluating radioprotective effect of hesperidin on acute radiation damage in the lung tissue of rats. J Biomed Phys Eng. 2016;6:165–174. [PMC free article] [PubMed] [Google Scholar]
- 29.Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, et al. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17:111–20. doi: 10.22074/cellj.2015.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseinimehr SJ, Nemati A. Radioprotective effects of hesperidin against gamma irradiation in mouse bone marrow cells. Br J Radiol. 2006;79:415–8. doi: 10.1259/bjr/40692384. [DOI] [PubMed] [Google Scholar]
- 31.Pradeep K, Park SH, Ko KC. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. Eur J Pharmacol. 2008;587:273–80. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Gamble M. The hematoxyline and eosin. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 6th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008. pp. 121–34. [Google Scholar]
- 33.Jones ML, Bancroft JD, Gamble M. Connective tissues and stains. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 6th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008. pp. 135–60. [Google Scholar]
- 34.Sheehan D, Hrapchak B. Theory and Practice of Histotechnology. 2nd ed. Ohio: Battelle Press; 1980. [Google Scholar]
- 35.Eagle RC. Eye Pathology: An Atlas and Text. 2nd ed. Philadelphia, PA: London Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 36.Loew JM, Macon WR. Lymph nodes. In: Gattuso P, Reddy VB, David O, Spitz DJ, Haber MH, editors. Differential Diagnosis in Surgical Pathology. 2nd ed. Philadelphia, PA: W.B. Saunders; 2010. p. 761. [Google Scholar]
- 37.Meister M. The Health Effects of Low-Level Radiation. New York: American Council on Science and Health; 2005. pp. 2–4. [Google Scholar]
- 38.Platek ME, Reid ME, Wilding GE, Jaggernauth W, Rigual NR, Hicks WL, Jr, et al. Pretreatment nutritional status and locoregional failure of patients with head and neck cancer undergoing definitive concurrent chemoradiation therapy. Head Neck. 2011;33:1561–8. doi: 10.1002/hed.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isenring E, Bauer J, Capra S. The scored patient-generated subjective global assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr. 2003;57:305–9. doi: 10.1038/sj.ejcn.1601552. [DOI] [PubMed] [Google Scholar]
- 40.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–11. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–56. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153(5 Pt 1):533–9. doi: 10.1667/0033-7587(2000)153[0533:romcie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Richter KK, Langberg CW, Sung CC, Hauer-Jensen M. Increased transforming growth factor beta (TGF-beta) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int J Radiat Oncol Biol Phys. 1997;39:187–95. doi: 10.1016/s0360-3016(97)00290-3. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe S, Watanabe K, Oishi T, Aiba M, Kageyama K. Mast cells in the rat alveolar septa undergoing fibrosis after ionizing irradiation. Ultrastructural and histochemical studies. Lab Invest. 1974;31:555–67. [PubMed] [Google Scholar]
- 45.Ward WF, Molteni A, Ts’ao CH, Hinz JM. Captopril reduces collagen and mast cell accumulation in irradiated rat lung. Int J Radiat Oncol Biol Phys. 1990;19:1405–9. doi: 10.1016/0360-3016(90)90351-j. [DOI] [PubMed] [Google Scholar]
- 46.Yeh CC, Kao SJ, Lin CC, Wang SD, Liu CJ, Kao ST. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro . Life Sci. 2007;80:1821–31. doi: 10.1016/j.lfs.2007.01.052. [DOI] [PubMed] [Google Scholar]


