Abstract
Introduction
Our various previous findings have shown the suitability of norfloxacin in the treatment of bacterial infections and burn wounds in alone as well as in combination with Curcuma longa in various topical (ointments, gels, and creams) and transdermal drug delivery systems.
Aims and methods
Keeping these facts in consideration, we have made an another attempt to prepare semisolid formulations containing 1% w/w of norfloxacin and metronidazole with different bases like Carbopol, polyethylene glycol, and hydroxypropylmethyl cellulose for effective treatment of bacterial infections and burn wounds. The prepared formulations were evaluated for physicochemical parameters, in vitro drug release, antimicrobial activity, and burn wound healing properties.
Results
The prepared formulations were compared with Silver Sulfadiazine cream 1%, USP. Antimicrobial activity of norfloxacin semisolid formulations was found to be equally effective against both aerobic and anaerobic bacteria in comparison to a marketed formulation of Silver Sulfadiazine 1% cream, USP. Based on the burn wound healing property, the prepared norfloxacin semisolid formulation was found to be in good agreement with marketed Silver Sulfadiazine 1% cream, USP.
Conclusions
These findings suggest formulations containing norfloxacin and metronidazole may also prove as an effective alternative for existing remedies in the treatment of bacterial infections and burn wounds.
Keywords: transdermal, ointments, norfloxacin, gels, infection, burns
Introduction
Infection is a major complication of burn injury and is responsible for 50–75% of hospital deaths. A moist, thermally coagulated burn wound, with its constantly replenished supply of diffusing serum nutrients and warm surface temperature, provides an environment suitable for rapid microbial growth. As local microbial growth increases, the potential for invasion to subjacent viable tissues and the penetration into circulation increases. Microorganisms that cause burn wound infections have changed over years related to changes made in the treatment of burn patients. Ramakrishanan et al. [1] and Wang et al. [2] reported separately that anaerobic bacteria are the causative organisms for infection in around 15% of burn-infected patients. Huo et al. [3] reported that silver norfloxacin (NF) proved valuable in the treatment of burn wound infection caused by invading organisms, particularly by Silver Sulfadiazine-resistant strain of Pseudomonas.
NF, a broad-spectrum fluoroquinolone antibacterial agent, is commonly employed in the treatment of urinary and genital tract infections [4–6]. Metronidazole (MTZ) is the prototype nitroimidazole used for trichomoniasis and later found to be a highly active amoebicide [7]. It also exhibits antibacterial activity against all anaerobic cocci and bacilli, both gram-positive and gram-negative [8]. Our various previously published researches have shown the suitability of NF in the treatment of bacterial infections and burn wounds in alone [9, 10] as well as in combination with Curcuma longa [11] in various topical dosage forms like ointments, gels, creams [9, 11], and transdermal plasters [10].
Keeping these facts in mind, we have made an attempt to try the combination of NF and MTZ in semisolid dosage forms in the treatment of bacterial infections and burn wounds. The objective of this study was to prepare various topical drug delivery systems such as gels and ointments to evaluate their antimicrobial activity and burn wound-healing efficacy and compare with Silver Sulfadiazine 1% cream, USP.
Materials and Methods
NF and MTZ (Pfiscar India Ltd., Murthal, Haryana, India) and Carbopol (Noveon, Mumbai, India) were used to prepare formulations. All other chemicals used were of analytical grade.
Preparation of topical formulations
Various semisolid formulations containing NF and MTZ were prepared with different bases like Carbopol, polyethylene glycol (PEG), and hydroxypropylmethyl cellulose (HPMC) using standard procedures (Table I). In each of the formulation, NF and MTZ were incorporated at 1% w/w concentration in the base with trituration using geometric dilution procedure to get a homogeneous mass [9, 11–13].
Table I.
Composition of various optimized dermatological bases containing 1% w/w norfloxacin and metronidazole, respectively
| Dermatological base | ||||
|---|---|---|---|---|
| Ingredients | Carbopol base (g) | HPMC base (g) | PEG base (g) | Ointment base (g) |
| Norfloxacin (NF) | 1.0 | 1.0 | 1.0 | 1.0 |
| Metronidazole (MTZ) | 1.0 | 1.0 | 1.0 | 1.0 |
| Carbopol gel base | 98.0 | – | – | – |
| Propylene glycol | – | – | – | – |
| Methyl paraben | – | 0.3 | – | – |
| Ethyl paraben | – | 0.2 | – | 10.0 |
| HPMC gel base | – | 2.0 | – | – |
| PEG 4000 | – | – | 53.0 | – |
| PEG 300 | – | – | 45.0 | – |
| DMSO | – | – | – | 10.5 v/w |
| Isopropyl myristate | – | – | – | 10.0 v/w |
| Mineral oil | – | – | – | 30.0 v/w |
| White petrolatum | – | – | – | 30.7 v/w |
| Bees wax | – | – | – | 5.8 v/w |
| Glycerine | – | 10.0 | – | – |
| Water | – | Quantity sufficient | – | 1.0 v/w |
Carbopol gels
Carbopol 940 was sprinkled slowly into a small quantity of distilled water and continuously stirred to get a uniform dispersion. To this dispersion, a weighed quantity of predissolved methyl paraben and propyl paraben was added to 5 ml of water. This preparation was kept overnight so that Carbopol became uniform in texture and appearance and the air bubbles could escape. When the temperature of the base was about room temperature, NF and MTZ were added to develop the respective formulation by the levigation method [13].
HPMC gels
A weighed quantity of HPMC powder was dispersed and hydrated in a portion of hot water (about 1/3 of total volume), heated above 90 °C with vigorous stirring to prevent lumping and the resultant dispersion was cooled. Methyl paraben, propyl paraben, and NF and MTZ were dispersed in another portion of cold water. This dispersion was added to the HPMC dispersion to achieve complete solubilization of HPMC. The resultant solution was mixed thoroughly with trituration [13].
Macrogol gel (PEG) base
A weighed quantity of PEG 4000 was melted. To this melt, PEG 400 was added under continuous stirring. The melt was removed from the heating source and the stirring was continued until the melt started congealing. NF and MTZ were incorporated into the base at room temperature by the levigation method [13].
Simple ointment base
A weighed quantity of hard paraffin, bee wax and white petrolatum were taken and melted together. To this melt, mineral oil, dimethyl sulfoxide (DMSO), and isopropyl myristate were added. The molten mass was removed from the heating source and the stirring was continued until the melt started congealing. When the temperature of the base reached room temperature, NF and MTZ were incorporated by the levigation method [13].
Evaluation of transdermal formulations
Physical appearance
The physical appearance of formulations was checked visually (color, consistency, and texture) while greasiness was assessed by application onto the skin surface [13].
Drug content
Hundred milligrams of formulation was dissolved in 1% v/v acetic acid, filtered, and the volume was made to 100 ml with 1% v/v acetic acid. The resultant solution was suitably diluted with 1% v/v acetic acid and absorbance was measured using simultaneous estimation at 277.6 nm and 305 nm for NF and MTZ, respectively. From this, the drug content was determined using calibration curve for both drugs [13].
pH
Direct measurements were made using digital pH meter [13].
Rheological studies
The prepared formulations were evaluated for the following rheological characteristics [13].
Apparent viscosity of the formulations was determined using Brookfield Synchroelectric Viscometer at 2.5 rpm.
Spreadability of the formulations was measured using spreadability apparatus. About 500 mg of the test formulation was sandwiched between 2 slides of 6 × 2 cm each. The lower slide was fixed on the board of the apparatus and the upper slide was tied to a nonflexible string to which a 20-g load was applied with the help of a simple pulley. The time taken for the upper slide to travel the distance of 6 cm and separate from the lower slide under the influence of weight was noted. Spreadability was calculated using the following equation:
where w is the weight tied to the upper slide (20 g), l is the length of the glass slide (6 cm), and t is the time in seconds.
Extrudability of the formulations was determined using extrudability apparatus. A closed collapsible tube containing formulation was pressed firmly at the crimped end. When the cap was removed, formulation extruded until the pressure dissipated. Weight, in grams, required to extrude 0.5 cm ribbon of the formulation in 10 s was determined. The average extrusion pressure in grams was reported [14].
In vitro drug release studies
In vitro drug release studies were carried out using the classical cylindrical tube [13, 15]. Semisolid formulation (0.5 g) was taken on the cellophane membrane and tied securely to one end of the tube; the other end was kept open to ambient conditions. The cell was inverted and immersed slightly in 100 ml of 1% v/v acetic acid in a beaker at 37 ± 1 °C, and stirred at 100 rpm for 8 hr. Samples (5.0 ml) were withdrawn at 1 hr interval till the 7th hour and assayed spectrophotometrically separately at 277.6 nm and 311 nm for NF and MTZ, respectively, using UV Spectrophotometer (Jasco V-530, Jasco Inc., 8649, Commerce Dr., Easton, MD, USA).
Kinetic analysis of in vitro release data
The in vitro release data of the best three semisolid formulations were subjected to kinetic analysis to establish the drug release mechanism. The release data were fitted to the zero-order, first-order, and matrix (Higuchi model) equations [16, 17].
Skin irritation test
The Draize patch test was used on rabbits to evaluate the irritation potential of the topical formulations.
Animals
White New Zealand rabbits of either sex (2.75 ± 0.25 kg, 8–9 weeks) were housed individually in the animal house with food and water given ad libitum. Rabbits were divided into three groups (n = 3): group 1 – no application (control), group 2 – placebo semisolid topical base without NF–MTZ, and group 3 – formulations treated. The back of the rabbits was clipped free of hair 24 hr prior to the formulation application. The formulation, 0.5 g, was applied on the hair-free skin of rabbits by uniform spreading over an area of 4 cm2. The skin surface was observed for any visible change such as erythema (redness) after 24, 48, and 72 hr of the formulation application. The mean erythemal scores were recorded depending on the degree of erythema: no erythema = 0, slight erythema (barely perceptible – light pink) = 1, moderate erythema (dark pink) = 2, moderate to severe erythema (light red) = 3, and severe erythema (extreme redness) = 4 [18].
Microbiological studies
The antibacterial activity of various semisolid formulations of NF against various strains of aerobic and anaerobic microorganisms was evaluated by standard cup-plate method and the inhibition zone diameters were measured with the help of zone reader. Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa (aerobic organisms), and Bacteroides fragilis (anaerobic organism) were used for testing the antibacterial activity. Nutrient agar medium was used for aerobic bacterial cultures and blood agar medium was used for Bacteroides fragilis. The aerobic organisms were incubated at a temperature of 37 ± 0.2 °C for 24 hr in an incubator under aerobic conditions, while Bacteroides fragilis cultures were incubated under hydrogen/carbon dioxide atmosphere in an anaerobic jar at 37 ± 0.2 °C for 48 hr [9–11, 19].
Burn wound healing property
Animals
Healthy Wistar albino rats weighing between 150 and 180 g were used. Animals were divided into various groups, with each group containing 5 animals.
Inflicting burn wound
The experiments were carried out as per the guidelines of Animal Ethics Committee. The dorsum of each rat was shaved. Burn wounds were inflicted on overnight-starved animals under pentobarbitone sodium (6 mg/100 g, i.p.), anesthesia. A 2 × 2 cm metal cylinder was placed on the shaven back of the animals. Melted wax at 80 °C was poured into the metal cylinder and the wax was allowed to solidify. Eight minutes after this, until the wax was completely solidified, the metal cylinder containing wax adhering to the skin was gently removed to inflict a distinctly demarked burn wound [9–11, 20].
Assessment of burn wound healing
NF semisolid formulations and the marketed Silver Sulfadiazine 1% cream, USP (500 mg each) were applied to the wound inflicted areas of animals every day from day 1. Animals were observed for wound healing by measuring the wound contraction (tracing the raw wound area on a transparent polythene paper which was retraced on graph paper to assess the area) up to the 12th day post wounding. The wound contraction was calculated as percentage of original wound size for each animal of the group [9–11, 20, 21].
Assessment of epithelialization
NF–MTZ topical formulations and the marketed Silver Sulfadiazine 1% cream, USP (500 mg) were applied to the wound inflicted areas of animals from day 1 to day 12. The epithelialization period was monitored by recording the number of days required for the eschar to fall off from the burn wound surface without leaving a raw wound behind [21].
Histopathological studies
The rats were anesthetized and the skin tissue samples with burn wounds were collected from rats (days 1, 4, 8, and 12) for histopathological examinations. The samples were fixed in 10% neutral buffered formalin, and were cut into 4 μm sections and stained with hematoxylin and eosin (H&E), and examined by light microscopy for morphological changes. A histopathologist, blinded to the study, was assigned to analyze and grade the histological changes of each group [21, 22].
Statistical analysis
Data pertaining to burn wound healing activity is expressed as mean ± SD (n = 3) and was analyzed by the one-way analysis of variance followed by the post hoc Tukey’s test for multiple comparisons using the Jandel Sigma Stat statistical software, version 2.0.
Results and Discussion
All the formulations were white in color. Carbopol gels appeared to be translucent and glossy while all other formulations were opaque and greasy on application. All the formulations were found to be smooth, free from grittiness and nongreasy on application.
The results obtained revealed that the drug content of all the formulations was found to be in good agreement with the theoretical value indicating the stability of the drug in the formulations (Table II). pH of all the formulations was found to be between 5.7 and 7.2, thus, indicating suitability of the formulations for application on the skin. Rheological properties (spreadability and extrudability) of the NF semisolid formulations were found to be equivalent to Silver Sulfadiazine cream, USP (Table III).
Table II.
Results of drug content analysis of topical formulations
| Drug content (%)a | ||
|---|---|---|
| Formulation base | Norfloxacin (NF) | Metronidazole (MTZ) |
| Carbopol | 95.4 ± 2.7 | 96.1 ± 1.3 |
| PEG | 93.1 ± 2.7 | 95.1 ± 1.4 |
| HPMC | 95.6 ± 3.6 | 97.6 ± 2.3 |
| Ointment | 92.3 ± 4.1 | 91.2 ± 4.1 |
Average of three determinations
Table III.
Physicochemical properties of topical formulations
| Formulation base | pHa | Apparent viscositya (cPs) | Spreadabilitya (s) | Extrudabilitya (g) |
|---|---|---|---|---|
| Carbopol | 7.1 ± 1.2 | 11 × 106 | 27 ± 1.5 | 505 ± 1.3 |
| PEG | 7.0 ± 0.7 | 29 × 106 | 35 ± 0.6 | 515 ± 0.4 |
| HPMC | 6.0 ± 1.5 | 47 × 106 | 49 ± 0.4 | 572 ± 0.7 |
| Ointment | 6.8 ± 0.9 | 28 × 106 | 37 ± 0.2 | 592 ± 0.5 |
Average of three determinations
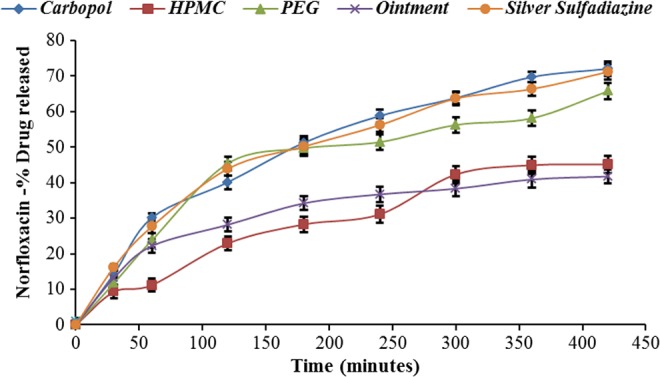
Formulations containing PEG and Carbopol gel base showed better in vitro release profile (Table IV) and gave larger zones of inhibition in comparison with creams and ointment base formulations indicating the better activity of the drug (Table V, Figs 1 and 2). Enhanced drug diffusion from the Carbopol gel base may be attributed to the presence of pores in the gel and lack of over-solubilization of the lipophilic drug in the aqueous vehicle, resulting in relatively free diffusion of the drug to the vehicle and hence leading to faster release [13, 23]. Owing to the biphasic nature of creams and various oleaginous bases, partitioning of the drug occurs in aqueous and oil phases, which results in the slower drug release. In case of gels, drug diffusion occurs through the aqueous phase and hence provides greater drug diffusion and release [13, 23].
Table IV.
In vitro drug release profile of topical formulations
| Percent drug release after 7 hra | |||
|---|---|---|---|
| S. No | Formulation base | Norfloxacin (NF) | Metronidazole (MTZ) |
| 1. | Carbopol | 72.03 ± 2.1.11 | 63.04 ± 1.12 |
| 2. | PEG | 65.74 ± 1.15 | 52.31 ± 1.75 |
| 3. | HPMC | 45.13 ± 2.48 | 47.02 ± 1.23 |
| 4. | Ointment | 41.00 ± 2.59 | 44.98 ± 1.13 |
| 5. | Silver Sulfadiazine cream, USP | 71.17 ± 3.33 | |
Average of three determinations
Table V.
Antimicrobial activity of topical formulations
| Inhibition zone diameter (mm)a | |||||
|---|---|---|---|---|---|
| Formulation base | B. subtilis | S. aereus | E. coli | P. aeruginosa | B. fragilis |
| Carbopol | 50.01 ± 2.1 | 53.01 ± 0.9 | 50.01 ± 1.5 | 49.00 ± 1.5 | 52.01 ± 1.0 |
| PEG | 47.12 ± 1.1 | 45.12 ± 1.1 | 46.13 ± 1.3 | 44.23 ± 1.6 | 50.00 ± 1.3 |
| HPMC | 39.13 ± 1.3 | 37.12 ± 1.3 | 35.21 ± 1.1 | 38.34 ± 1.1 | 40.02 ± 1.5 |
| Ointment | 37.00 ± 2.1 | 38.13 ± 0.9 | 33.01 ± 2.9 | 32.01 ± 1.0 | 33.72 ± 1.7 |
| Silver Sulfadiazine cream, USP | 45.33 ± 0.9 | 51.22 ± 1.4 | 48.31 ± 2.1 | 44.72 ± 1.5 | 47.12 ± 1.3 |
Average of three determinations
Fig. 1.
Comparative in vitro release profile of NF from various semisolid formulations with marketed Silver Sulfadiazine (mean ± SD, n = 3)
Fig. 2.
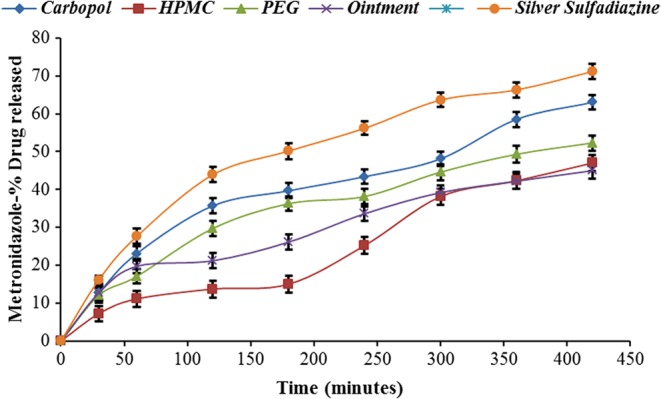
Comparative in vitro release profile of MTZ from various semisolid formulations with marketed Silver Sulfadiazine (mean ± SD, n = 3)
Also, we have previously shown that the in vitro release characteristics of the prepared topical formulations of MTZ and NF separately were in agreement with marketed Silver Sulfadiazine 1% cream, USP using the application of model independent approach [24], which proves the selection of MTZ and NF as appropriate candidates for topical drug delivery systems in combination with an aim to have synergistic antibacterial and burn wound healing effects [25, 26].
The in vitro diffusion data of the formulations of NF–MTZ were subjected to kinetic analysis. The release of the drug from all formulations was found to follow the first-order release kinetics. The results are in agreement with the previous investigation performed by Rafiee and Mehramizi [27]. Higher correlation was observed for the Higuchi matrix release kinetics in all the selected formulations, suggesting diffusion as a probable prominent mechanism of drug release. In diffusion, the rate of drug dissolution within the matrix must be much higher than that of the diffusion rate of the drug leaving the matrix.
Skin irritation placebo and the NF–MTZ formulations were devoid of any irritation potential and no edema formation was observed in any case. Irritation score (primary skin irritation index) for all the formulations was zero, which indicated its safety and acceptability for topical administration.
Majority of the physicochemical characteristics and in vitro drug release findings with NF–MTZ topical formulations were in agreement with our previous topical formulations of aceclofenac [13]. This reason attributes include the application of similar dermatological base compositions and procedures. The present NF–MTZ formulations still need a detailed investigation with the inclusion of various penetration enhancers especially ethanol and DMSO, which may further enhance the drug release either by attacking the dense barrier structure of the skin [28] or by augmenting the solubility and partitioning of the drug in stratum corneum [24], as we have shown previously that ethanol advances the drug release from the dermatological bases, which may help in improving the efficacy of the drug at the site of infection and burn wound.
The difference between mean percentage burn wound contractions of the formulation-treated animals as compared with control was found to be statistically significant. All the prepared formulations, including Silver Sulfadiazine 1% cream, USP, had statistically shown wound healing activity (p = 0.001) in healthy male Wistar albino rats. Burn wound healing studies revealed a maximum percentage wound healing of 88.11 ± 0.63% with Carbopol formulations within 12 days, which is in good agreement with marketed Silver Sulfadiazine formulation showing 84.34 ± 0.49% of wound healing (Table VI).
Table VI.
Percent burn wound contraction of topical formulations
| % Wound contraction | |||
|---|---|---|---|
| Formulation base | 4th day | 8th day | 12th day |
| Control % | 27.61 ± 1.33a | 53.31 ± 1.22a | 67.03 ± 0.92a |
| Carbopol % | 52.17 ± 1.56a | 75.10 ± 1.73a | 88.11 ± 0.63a |
| Carbopol # % | 31.03 ± 1.84a | 58.13 ± 0.37a | 70.10 ± 0.92a |
| PEG % | 49.14 ± 1.67a | 70.13 ± 0.12a | 81.10 ± 0.47a |
| PEG # % | 30.02 ± 1.30v | 56.34 ± 0.24a | 65.31 ± 0.73a |
| HPMC % | 47.23 ± 1.73a | 63.46 ± 1.17a | 72.15 ± 0.76a |
| HPMC # % | 30.10 ± 0.72a | 48.01 ± 0.29a | 58.03 ± 0.29a |
| Ointment % | 44.15 ± 1.73a | 60.01 ± 0.32a | 67.45 ± 0.15a |
| Ointment # % | 29.01 ± 0.93a | 57.13 ± 0.29a | 61.00 ± 0.27a |
| Silver Sulfadiazine 1% cream, USP | 52.00 ± 1.45a | 71.87 ± 0.98a | 84.34 ± 0.49a |
Represents the group treated with base alone
p < 0.001 vs. Control
No mortality was observed in the animals during the study period. The mean period of epithelialization was found to decrease significantly in comparison with the control (54.1 ± 1.6) (Table VII).
Table VII.
Period of epithelialization of topical formulations
| Formulation base | Period of epithelializationa (days) |
|---|---|
| Control | 54.1 ± 1.6 |
| Carbopol | 28.9 ± 1.2 |
| HPMC | 38.0 ± 1.5 |
| PEG | 32.2 ± 1.4 |
| Ointment | 40.1 ± 1.6 |
| Silver Sulfadiazine cream, USP | 25.9 ± 1.3 |
Average of three determinations
No mortality was observed in the animals during the study period. The mean period of epithelialization was found to decrease with all the formulations in comparison with control (54.1 ± 1.6). However, not much difference was observed in the mean values of Carbopol formulations (28.9 ± 1.2) and Silver Sulfadiazine 1% cream, USP (25.9 ± 1.3). Various histopathological changes observed during the study period (days 1, 4, 8, and 12) are tabulated in Table VIII and illustrated in Figs 3–5.
Table VIII.
Comparative observation of morphological changes in histopathological study of selected norfloxacin–metronidazole topical formulations with Silver Sulfadiazine 1% cream, USP
| Days | NF–MTZ Carbopol formulation | Silver Sulfadiazine 1% cream, USP (SS) |
|---|---|---|
| Day 1 | Prominent ulceration with diffuse burns on the skin and subcutaneous tissue, evidence of coagulative necrosis | Presence of ulcer on surface due to injury, covered with slough and necrotic debris |
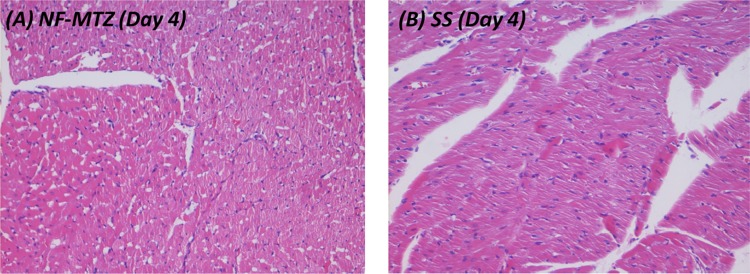
| Day 4 | Beginning of granulation tissue formation with reepithelialization, moderate scab formation, good proliferation, numerous inflammatory macrophages and fibroblasts seen | Adequate scab formed, beneath which a clot composed of fibrin, RBCs and platelets are formed. Inflammatory cells like macrophages and neutrophils migrate to the site |
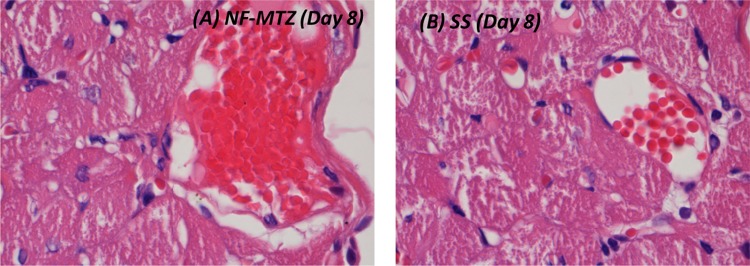
| Day 8 | Proliferation of fibroblasts seen with fresh collagen being laid down, angiogenesis, neovascularization, wound contraction seen | Areas of fibroblast proliferation, new blood vessel formation, wound begin to contract |
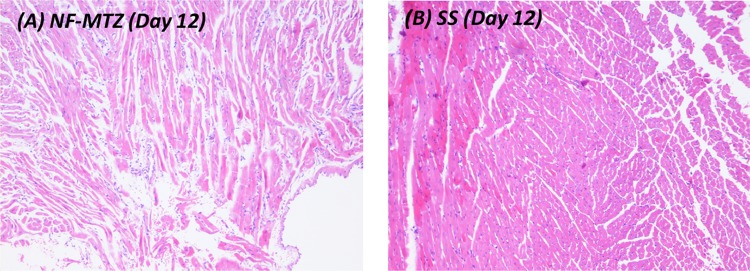
| Day 12 | Prominent wound contraction, collagen and fibrosis are increased prominent scar formation, cellularity reduced, all of which suggest good healing process | Collagen is laid down significantly, inflammatory cells are reduced due to the healing process and presence of fibrous scar is noticed |
Fig. 3.
Photomicrographs of skin and subdermal tissue with burns on the 4th day (H&E, 400×)
Fig. 4.
Photomicrographs of skin and subdermal tissue on the 8th day (H&E, 1000×)
Fig. 5.
Photomicrographs of skin and subdermal tissue on the 12th day (H&E, 100×)
The literature survey has clearly shown that limited attempts have been made on the topical drug delivery systems of NF or MTZ alone or in combination. Pandey et al. [29] have shown that the transdermal formulations containing MTZ and NF in combination from the lanolin petrolatum base with 0.25% w/w DMSO are effective for the treatment of burn wound [29]. In this research, we have employed different dermatological bases and compositions where we have shown the suitability of Carbopol bases which make this different from previous studies.
In case of NF alone, majority of efforts have been made to develop the controlled release in situ gel system for ocular drug delivery [30–32] or imprinted soft contact lenses [33] and with MTZ includes the development of controlled release system using the Natural Rubber Latex [34] and topical vaginal drug delivery system based on superporous hydrogel hybrids [35]. In order to have effective antibacterial local activity, Vijay et al. [36] have formulated topical emulgel of NF and studied the effect of concentration of gelling agent and emulsifiers on the system. Saraf et al. [37] have developed the transdermal drug delivery of NF by mercury casting method using combination of HPMC and EC in the ratio of 10:90 to 50:50 including various concentrations of polyvinyl alcohol. Such investigations have shown the suitability of the topical system. Also, Thakur et al. [38] have prepared and evaluated the composite films of chitosan-montmorillonite containing curcumin in the form of transdermal films. Adhya et al. [39] conducted a randomized controlled trial to compare the effectiveness of topical Silver Sulfadiazine and nano-crystalline silver (AgNP) hydrogel in burn wound management and have shown that AgNP can be an effective and superior alternative to SSD for burn wounds, particularly 2° deep-dermal burns. Healing can be expected, in general, in 6–8 weeks time, depending upon the extent of body surface involvement. All such attempts support this study as an alternative and viability for the effective treatment of bacterial infections and burn wounds in a patient compliant manner.
Conclusion
Topical drug delivery system provides effective and safe approach to deliver NF and MTZ for local bacterial infections and burn wound treatment. The in vitro release characteristics along with the burn wound healing property of prepared topical formulations containing NF and MTZ were quite encouraging and in good agreement with marketed Silver Sulfadiazine 1% cream, USP. Among all the semisolid formulations prepared, Carbopol gel base was found to be the most suitable dermatological base in comparison with various other dermatological bases. It also has aesthetic appeal, which other bases lack, an important aspect from the patient compliance and consumer point of view. The findings suggest formulations containing combination of NF and MTZ may also prove as an effective alternative for existing remedies in the treatment of bacterial infections and burn wounds.
Author’s contribution
The work presented was performed in collaboration with all authors. VRM and KD participated in the study’s conception and design. VRM and KD performed all the formulation development, physico-chemical characterization, and in-vitro experiments. SC performed the histopathological studies. JM, GG, and RA carried out the statistical analysis and figure design. All authors participated in the interpretation of data, preparation, and editing of manuscript for intellectual content. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that there is no conflict of interest involved with this manuscript.
Acknowledgements
The authors are very thankful to M/s Pfiscar India Pvt. Ltd. (Murthal, Haryana, India) and G. C. M. Laboratories (Chandigarh, India) for their generous gift sample of norfloxacin. The authors would also like to thank U. P. Technical University (Lucknow, India) and Nitte Education Trust for their valuable support.
Funding Statement
Funding sources: None declared.
References
- 1.Ramakrishanan KM, Rao DK, Doss CR, Mathicanan T, Manokaran G, Thyagarajan SP: Incidence of burn wound sepsis in 600 burned patients treated in a developing country. Burns 11, 404–407 (1985) [DOI] [PubMed] [Google Scholar]
- 2.Wang WD, Wagao L, Xiao G, Zhan Y-P: The tangential excision of extensive on deep burns. Burns 11, 192–195 (1985) [DOI] [PubMed] [Google Scholar]
- 3.Huo ZL, Chu CS, Chan M, Ouygan BY, Zhang QY: Chemical pulmonary burn injury and chemical intoxication. Burns Incl Therm Inj 9(2), 111–114 (1982) [DOI] [PubMed] [Google Scholar]
- 4.Gennaro AR. (1990): Remington’s Pharmaceutical Sciences (18th ed.) Mack Publishing Company, Philadelphia [Google Scholar]
- 5.Reynolds JEF. (1989): Martindale: The Extra Pharmacopoeia (29th ed.) Pharmaceutical Press, London [Google Scholar]
- 6.Ross DZ, Riley CM: Aqueous solubilities of some variously substituted quinolones antimicrobials. Int J Pharm 63, 237–250 (1990) [Google Scholar]
- 7.Tripathi KD. (2004): Essentials of Pharmacology (5th ed.) Jaypee Brothers, New Delhi, pp. 750–751 [Google Scholar]
- 8.Seth SD. (2004): Text Book of Pharmacology (2nd ed.) Reed Elsevier India Pvt. Ltd., India, pp. 642–643 [Google Scholar]
- 9.Malipeddi VR, Dua K, Sara UVS, Malipeddi H, Agrawal A: Comparative evaluation of transdermal formulations of norfloxacin with silver sulfadiazine cream, USP, for burn wound healing property. J Burns Wounds 5, e4 (2006) [PMC free article] [PubMed] [Google Scholar]
- 10.Dua K, Ramana MV, Sara UVS, Agrawal DK, Pabreja K, Chakravarthi S: Preparation and evaluation of transdermal plasters containing norfloxacin: A novel treatment for burn wound healing. Eplasty 10, e44 (2010) [PMC free article] [PubMed] [Google Scholar]
- 11.Dua K, Chakravarthi S, Kumar D, Sheshala R, Gupta G: Formulation, characterization, in vitro, in vivo, and histopathological evaluation of transdermal drug delivery containing norfloxacin and Curcuma longa. Int J Pharm Investig 3(4), 183–187 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pabreja K, Dua K, Padi SSV: Evaluation of extemporaneously manufactured topical gels containing aceclofenac on inflammation and hyperalgesia in rats. Curr Drug Deliv 7, 324–328 (2010) [DOI] [PubMed] [Google Scholar]
- 13.Dua K, Ramana MV, Pabreja K: Aceclofenac topical dosage forms: In vitro and in vivo characterization. Acta Pharm 60, 467–478 (2010) [DOI] [PubMed] [Google Scholar]
- 14.Chakole CM, Shende MA, Khadatkar SN: Formulation and evaluation of novel combined halobetasol propionate and fusidic acid ointment. Int J Chem Tech Res 1, 103–116 (2009) [Google Scholar]
- 15.Ezzedeen FW, Shihab FA, Husain EJ: Percutaneous diffusion of cefalexin, sulfamethoxazole and diphenhydramine from ointments. Pharmazie 45, 512–514 (1990) [PubMed] [Google Scholar]
- 16.Higuchi T: Mechanism of sustained action medication, theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52, 1145–1149 (1963) [DOI] [PubMed] [Google Scholar]
- 17.Costa P, Sousa Lobo JM: Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13, 123–133 (2001) [DOI] [PubMed] [Google Scholar]
- 18.Vermeer BJ: Skin irritation and sensitization. J Control Release 15, 261–265 (1991) [Google Scholar]
- 19.Bauer AW, Kirby WM, Sherris JC, Turck M: Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45, 493–496 (1966) [PubMed] [Google Scholar]
- 20.Anil Kumar SJ, Bhise SB, Jarag RJ, Jadhav NR: Preparation of cream containing Tridax procumbens, Curcuma longa and Azadirachta indica and its evaluation for wound healing property. Indian Pharm 41, 107–110 (2005) [Google Scholar]
- 21.Bairy KL, Somyaji SN, Rao CM: An experimental model to produce partial thickness burn wound. Indian J Exp Biol 37, 70–72 (1997) [PubMed] [Google Scholar]
- 22.Srikanth D, Shenoy RR: The effects of topical (gel) astemizole and terfenadine on wound healing. Indian J Pharmacol 40, 170–174 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhavse VV, Amin PD: Formulation and evaluation of topical bases of ketoprofen. East Pharm 40, 133–135 (1997) [Google Scholar]
- 24.Megraban WAC, Barry BW: Oestradiol permeation across human skin, silastic and snake skin membrane: The effect of ethanol: Water cosolvent system. Int J Pharm 116, 101–112 (1995) [Google Scholar]
- 25.Dua K: Application of model independent approach on in vitro release of extemporaneously prepared semisolid formulations containing metronidazole with marketed silver sulfadiazine 1% cream, USP: A comparative investigation. Bull Pharm Res 3, 1–5 (2013) [Google Scholar]
- 26.Kamal D, Pabreja K, Ramana MV: Comparative investigation on in vitro release of extemporaneously prepared norfloxacin semisolid formulations with marketed silver sulfadiazine 1% cream, USP using model independent approach. Ars Pharm 51, 177–185 (2010) [Google Scholar]
- 27.Rafiee TM, Mehramizi A: In vitro release studies of piroxicam from oil-in-water creams and hydroalcoholic gel topical formulations. Drug Dev Ind Pharm 26, 409–414 (2000) [DOI] [PubMed] [Google Scholar]
- 28.Obata Y, Takayama K, Maitano Y, Machida Y, Nagai T: Effect of ethanol on the permeation of ionized and non-ionized diclofenac. Int J Pharm 89, 191–198 (1993) [Google Scholar]
- 29.Pandey S, Basheer M, Roy S, Udupa N: Development and evaluation of transdermal formulations containing metronidazole and norfloxacin for the treatment of burn wound. Indian J Exp Biol 37, 450–454 (1999) [PubMed] [Google Scholar]
- 30.Rathod KB, Patel MB: Controlled release in situ gel of norfloxacin for ocular drug delivery. Int J Pharm Sci Res 5, 2330–2336 (2014) [Google Scholar]
- 31.Giannola L, de Caro V, Giandalia G, Siragusa MG, Cordone L: Ocular gelling microspheres: In vitro precorneal retention time and drug permeation through reconstituted corneal epithelium. J Ocul Pharmacol Ther 24, 186–196 (2008) [DOI] [PubMed] [Google Scholar]
- 32.Donnenfeld ED, Schrier A, Perry HD, Aulicino T, Gombert ME, Snyder R: Penetration of topically applied ciprofloxacin, norfloxacin, and ofloxacin into the aqueous humor. Ophthalmology 101, 902–905 (1994) [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Lorenzo C, Yañez F, Barreiro-Iglesias R, Concheiro A: Imprinted soft contact lenses as norfloxacin delivery systems. J Control Release 113, 236–244 (2006) [DOI] [PubMed] [Google Scholar]
- 34.Herculano RD, Guimarães SAC, Belmonte GC, Duarte MAH, Oliveira Júnior OND, Kinoshita A, Graeff CFDO: Metronidazole release using natural rubber latex as matrix. Mater Res 13, 57–61 (2010) [Google Scholar]
- 35.Chavda H, Chavada G, Patel J, Rangpadiya K, Patel C: Topical vaginal drug delivery system based on superporous hydrogel hybrids. Protein Pept Lett 21, 1176–1184 (2014) [DOI] [PubMed] [Google Scholar]
- 36.Vijay S, Kuchekar BS, Jagdale S: Development and optimization of site targeted topical delivery of norfloxacin emulgel. J Pharm Nanotechnol 3, 10–22 (2015) [Google Scholar]
- 37.Saraf S, Saraf S, Dixit VKS: Transdermal delivery of norfloxacin. Indian Pharm 5, 74–76 (2006) [Google Scholar]
- 38.Thakur G, Singh A, Singh I: Formulation and evaluation of transdermal composite films of chitosan-montmorillonite for the delivery of curcumin. Int J Pharm Investig 6, 23–31 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adhya A, Bain J, Ray O, Hazra A, Adhikari S, Dutta G, Ray S, Majumdar BK: Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nano-crystalline silver. J Basic Clin Pharm 6, 29–34 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]