Abstract
Background
The aim of this study was to characterize the expression and secretion of hepatitis B surface-antigen (HBsAg) in the hepatocytes of hepatitis B virus (HBV)-infected patients at different phases of infection; as such, the association of intrahepatic HBsAg expression with virological markers and the histological characteristics were analyzed.
Material/Methods
302 chronic HBV infection patients who had not received antiviral therapy were stratified by HBeAg status. The proportion of HBsAg-positive cells was used as an indicator for HBsAg expression level.
Results
In HBeAg-positive patients, there was a significant correlation between serum HBsAg and serum HBV DNA levels (r=0.569, p<0.001). Intrahepatic HBsAg expression and serum HBsAg level in HBeAg-positive patients were higher than those in HBeAg-negative patients (p=0.002 and p<0.001, respectively). A significant correlation between serum HBsAg level and intrahepatic HBsAg expression was found in HBeAg-negative patients (r=0.377, p<0.001), but not in HBeAg-positive patients (r=0.051, p=0.557). Very interestingly, the correlation between serum HBsAg level and HBsAg expression in hepatocytes gradually increased along with disease progression through the immune-tolerant, immune-clearance, inactive, and recovery phases of HBV infection (r=−0.184, 0.068, 0.492, and 0.575; and p=0,238, 0,722, 0.012, and 0.002, respectively).
Conclusions
Different mechanisms may be involved in HBsAg synthesis and secretion in different phases of chronic HBV infection.
MeSH Keywords: Hepatitis B Antigens, Hepatitis B Virus, Protein Biosynthesis, Secretory Pathway
Background
Hepatitis B virus (HBV) infection is a health problem worldwide. Globally there are ~240 million cases of chronic HBV infection, which can lead to complications such as cirrhosis and liver carcinoma, resulting in ~1 million deaths annually [1–3]. HBsAg is a gold-standard marker for diagnosis of HBV infection [4–6]. Recently, HBV surface antigen (HBsAg) level in serum has been proposed to reflect the liver content of covalently closed circular DNA (cccDNA), which serves as a template for the transcription of HBV genes [7,8]. Mathematical modeling of the decline in HBsAg level following treatment has been developed and used to guide PEGylated-interferon alpha-2a and nucleotide/nucleoside analog-based therapies required for HBsAg seroconversion [9–12].
Previous studies have suggested that the dynamic course of chronic HBV infection stemmed from interactions involving viral replication and the host immune system. Among different phases of chronic HBV infection, the immune-clearance phase positive for hepatitis B e antigen (HBeAg)- exhibited less severe histological activities and harbored fewer mutations than that negative for HBeAg [10,13–14]. HBsAg accumulation in the liver was associated with specific viral mutations related to disease activities [15,16]. Recently, several studies reported that serum HBsAg level was associated with that of serum HBV DNA [17]. However, the important question whether HBsAg expression in hepatocytes is related to overall HBsAg level, serum HBV DNA level, and histological activity in different phases of chronic HBV infection remains unanswered. It has been suggested that HBsAg is synthesized from three different types of templates and secreted into the serum: 1) transcription from integrated S gene and then translation, 2) transcription from HBV cccDNA and then translation, and 3) transcription from HBV genomic DNA and then translation. Nevertheless, differences in HBsAg synthesis and secretion that occur in different phases of chronic HBV infection, as well as other influencing factors, remain unknown.
Chronic HBV infection can be divided into a few different phases distinguishable by virological and serological markers. In this study, HBsAg expression in hepatocytes was evaluated in comparison with serum HBsAg level by phase (immune tolerance, immune clearance, inactive, and recovery) of chronic HBV infection in order to discern the complex relationship between HBsAg expression in hepatocytes and serum HBsAg levels. Understanding this relationship will provide insights into the mechanisms of HBsAg synthesis and secretion in different phases of chronic HBV infection.
Material and Methods
Study population
Patients were recruited from January 2014 to January 2015 at the Department of Infectious Diseases of The First Affiliated Hospital of Anhui Medical University, and those who met the inclusion criteria were retrospectively enrolled in this study. The inclusion criteria included positive serum HBsAg for more than six months and no history of anti-HBV treatment or immune-modulatory therapy. The following patients were excluded: 1) patients with coinfection with hepatitis C virus, human immunodeficiency virus, or hepatitis D virus; 2) patients with an autoimmune disease or cirrhosis of the liver; and 3) patients with Wilson’s disease. This study was approved by the ethics committee of the University, and all patients gave informed consent for the liver biopsy procedure.
Laboratory assays
Serum HBV DNA level was determined by using a TaqMan real-time PCR assay (Shanghai ZJ BioTech, Shanghai, China) with a lower detection limit of 1,000 copies/mL. Serum HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were analyzed using commercially available kits (Abbott, Chicago, IL, USA). Serum alanine aminotransferase (ALT) level was determined at the time of sampling on an automatic biochemical analyzer (Roche, Basel, Switzerland). Liver stiffness was evaluated by Fibroscan (FS; EchoSens, Paris, France). FS >9.4 was classified as significant fibrosis, and FS <6.0 was classified as no significant fibrosis [18].
Histological examination and immunohistochemical staining
All liver biopsies were performed by clinical doctors using Surecut needles (Gainesville, Florida, USA) under the guidance of B-mode ultrasound, with the length of the biopsy specimens being 1.6 to 2.2 cm. Liver biopsy specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections with a thickness of 4 μm were made and stained with hematoxylin and eosin and Masson’s trichrome. Histological data were assessed (blinded) by two pathologists.
Pathological analysis of liver tissue was conducted according to a modified histologic activity standard scoring system [19]. The hepatic pathological inflammation grade of chronic hepatitis was evaluated as 0 to 4 (G0–4). Hepatitis activity including lobular activity and periportal activity was classified as none, minimal, moderate, or severe. The stage of fibrosis was also graded on a scale of 0 to 4 (S0–4), and classified as no fibrosis, portal fibrosis, periportal fibrosis, septal fibrosis, and cirrhosis.
Hepatocyte expression of HBsAg was studied by using a streptavidin peroxidase method (mouse anti-HBsAg from ZSGB-BIO, Beijing, China). Negative-control experiments were performed by substituting the primary antibody with phosphate-buffered saline. HBsAg expression in hepatocytes was evaluated by quantitative analysis. Briefly, five fields were selected at random, with 100 liver cells selected from each field, and the number of positive cells was counted. Intracellular HBsAg localization was classified as cytoplasmic, membrane, or cytoplasmic plus membrane following evaluation of at least 500 cells.
HBV infection phase
Patients were divided into four dynamic groups based on their phase of infection: immune tolerant phase, immune clearance phase, residual inactive phase, and reactive immune clearance phase [20,21]. Immune tolerance phase is associated with young, HBeAg seropositive patients with high viral loads, but with a normal serum alanine aminotransferase (ALT) and near-normal liver histology. Immune clearance phase is associated with HBeAg and HBV DNA positivity, elevated or flare ALT, and moderate or severe necro-inflammatory histological changes. Residual inactive phase is associated with sustained normal serum ALT, low serum HBV DNA and no or minimal necro-inflammatory histological changes, although some patients may develop advanced fibrosis or cirrhosis. Reactive immune clearance phase is associated with negative HBeAg, positive HBV DNA, elevated or flare ALT, and moderate or severe necro-inflammatory histological changes.
Statistical analysis
The SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables with normal distributions were compared by t test, and data with non-normal distributions were analyzed by a nonparametric test (Mann-Whitney U test). Correlation between different continuous variables with skewed distribution was determined by Spearman’s rank correlation. Two-tailed values of p<0.05 were considered statistically significant.
Results
Characteristics of enrolled patients
A total of 302 patients who met the inclusion criteria were retrospectively enrolled in this study. The demographics, liver biochemistry, HBsAg expression, and FS scores of all patients are listed in Table 1. Most of the patients were male (71.9%), with a median age of 40 years (31–47 years). HBeAg-positive patients had significantly higher medians (interquartile range) of HBV DNA, HBsAg, ALT, and HBsAg expression levels as compared to those of HBeAg-negative patients (p<0.05) (Table 1).
Table 1.
Baseline characteristics of patients in the study.
| Characteristics | Total (n=302) | HBeAg− (n=166) | HBeAg+ (n= 136) | P |
|---|---|---|---|---|
| Age (years)* | 40 (31–47) | 43 (18–69) | 34 (14–76) | <0.001 |
| Gender (male/female) | 217/85 | 116/50 | 101/35 | 0.400 |
| ALT (×ULN)* | 1.04 (0.63–1.56) | 0.85 (0.50–1.37) | 1.08 (0.75–1.61) | <0.001 |
| HBV DNA (log10 copies/mL)* | 4.87 (3.36–6.96) | 3.89 (3.00–5.02) | 6.80 (5.30–7.76) | <0.001 |
| HBsAg (log10 IU/mL)* | 3.45 (2.85–4.17) | 3.00 (2.50–3.53) | 4.18 (3.53–4.67) | <0.001 |
| HBeAg (log10 S/CO)* | −056 (−1.01–2.55) | −1.09 (−1.12– −1.036) | 2.8 (1.8–3.1) | <0.001 |
| AFP (IU/ml)a | 2.62 (1.80–4.49) | 2.50 (1.80–3.90) | 3.04 (1.90–4.77) | 0.069 |
| FibroScan score (kPa)* | 6.50 (5.00–8.80) | 6.75 (4.98–8.70) | 6.25 (4.93–9.05) | 0.573 |
| HBsAg expression* | 34.0 (9.00–73.5) | 23.5 (6.00–61.75) | 43.5 (13.25–83.25) | 0.002 |
Median (interquatile range);
ALT – alanine aminotransferase; HBV – hepatitis B virus; AFP – alpha fetoprotein; HBsAg – hepatitis B surface antigen; HBeAg – hepatitis B e antigen.
Clinical, virological, and histological characteristics of the patients
In HBeAg-positive patients, positive correlations existed between HBsAg and HBV DNA or serum HBeAg (r=0.569, p<0.001; and r=0.565, p<0.001, respectively). A similar positive correlation was observed between HBV DNA and serum HBeAg (r=0.636, p<0.001). There was also a significant correlation between ALT and alpha-fetoprotein levels (r=0.365, p<0.001). However, there was a negative correlation between HBeAg and age or fibrosis (r=−2.78, p=0.001 and r=−0.240, p=0.005, respectively), while serum HBsAg levels did not correlate with HBsAg expression in the liver. In HBeAg-negative patients, however, serum HBsAg levels significantly correlated with HBsAg expression in the liver (r=0.377, p<0.001). There were also significant correlations between fibrosis and ALT or serum HBV DNA titer (r=0.349, p<0.001; and r=0.310, p<0.001, respectively) (Table 2).
Table 2.
Relationships among age, HBV DNA, ALT, HBsAg, HBeAg in serum and intrahepatic HBsAg expression.
| + | HBsAg expression | HBsAg | HBV DNA | HBeAg | ALT | Age | AFP | E | |
|---|---|---|---|---|---|---|---|---|---|
| − | |||||||||
| HBsAg expression | r | 0.051 | 0.077 | −0.102 | 0.123 | −0.055 | 0.088 | −0.113 | |
| p | 0.557 | 0.373 | 0.235 | 0.152 | 0.524 | 0.372 | 0.188 | ||
| HBsAg | r | 0.377 | – | 0.569 | 0.565 | 0.063 | −0.210 | −0.151 | −0.271 |
| p | <0.001 | – | <0.001 | <0.001 | 0.468 | 0.014 | 0.122 | 0.001 | |
| HBV DNA | r | 0.130 | 0.110 | – | 0.636 | 0.127 | −0.183 | −0.141 | −0.171 |
| p | 0.095 | 0.157 | – | <0.001 | 0.140 | 0.033 | 0.151 | 0.047 | |
| HBeAg | r | – | – | – | – | 0.037 | −0.278 | −0.266 | −0.240 |
| p | – | – | – | – | 0.671 | 0.001 | 0.006 | 0.005 | |
| ALT | r | 0.144 | 0.077 | 0.358 | – | – | −0.286 | 0.365 | 0.122 |
| p | 0.064 | 0.324 | <0.001 | – | – | <0.001 | <0.001 | 0.158 | |
| Age | r | −0.231 | −0.167 | −0.014 | – | −0.038 | – | 0.140 | 0.206 |
| p | 0.003 | 0.032 | 0.875 | – | 0.628 | – | 0.151 | 0.016 | |
| AFP | r | −0.026 | 0.009 | 0.147 | – | 0.125 | 0.040 | – | 0.251 |
| p | 0.767 | 0.305 | 0.093 | – | 0.155 | 0.653 | – | 0.009 | |
| E | r | −0.027 | 0.012 | 0.310 | – | 0.349 | 0.091 | 0.191 | – |
| p | 0.732 | 0.877 | <0.001 | – | <0.001 | 0.245 | 0.029 | – |
’−’ – HBeAg negative; ‘+’ – HBeAg positive. ALT – alanine aminotransferase; HBV – hepatitis B virus; AFP – alpha fetoprotein; HBsAg – hepatitis B surface antigen; HBeAg – hepatitis B e antigen. E, FibroScan score. HBsAg expression: the degree of expression of HBsAg in hepatocyte. Statistical results were determined by Spearman rank correlation.
HBsAg expression, virological markers, and histologic activity of liver disease
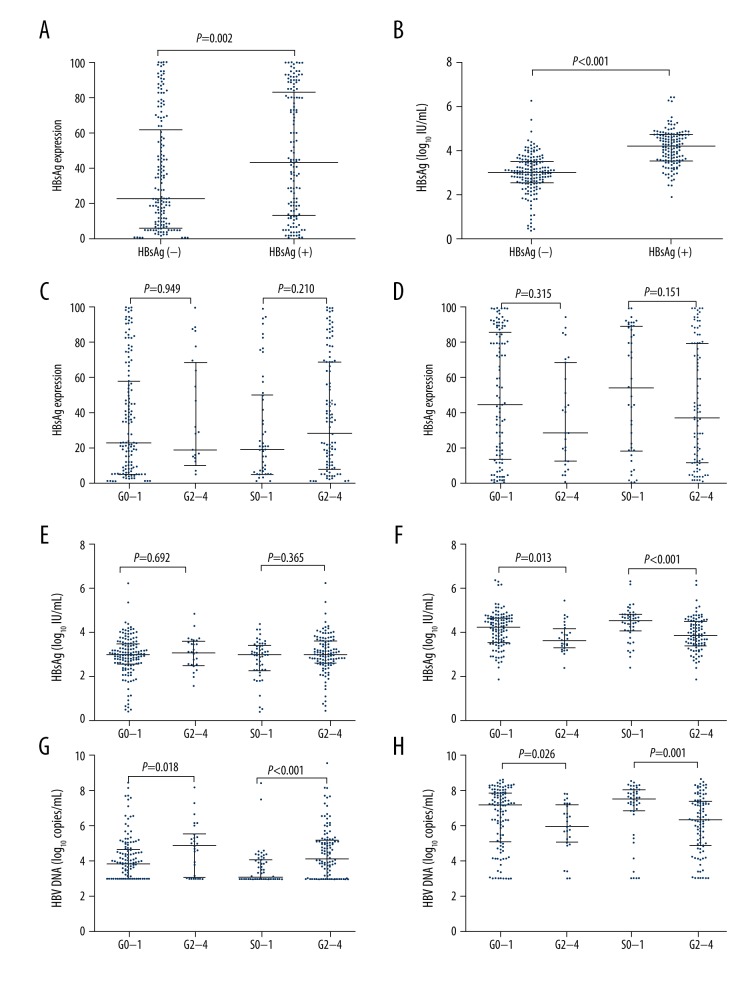
The median intrahepatic HBsAg expression was significantly higher in HBeAg-positive patients compared to HBeAg-negative patients (p=0.002; Figure 1A). Similarly, median serum HBsAg level was also higher in HBeAg-positive patients (p<0.001; Figure 1B). In 302 chronic hepatitis B patients (166 HBeAg-negative and 136 HBeAg-positive), there was no significant correlation between level of histologic activity and stage of fibrosis (S) or inflammation grade (G), or HBsAg expression level in hepatocytes (all p>0.05; Figure 1C, 1D). However, the serum HBV DNA levels correlated with the levels of S or G (all p<0.05; Figure 1G, 1H). In HBeAg-negative patients, there was no correlation between HBsAg serum titer and the level of S or G (all p>0.05; Figure 1E), while S or G level exhibited positive correlation with HBsAg serum titer in HBeAg-positive patients (all p<0.005; Figure 1F).
Figure 1.
HBsAg expression, virological markers and histologic activity of liver disease. The correlation between HBsAg expression in hepatocytes by HBeAg status (A). The correlation between serum HBsAg levels by HBeAg status (B). The correlation between inflammation grade (G), stages of fibrosis (S), and HBsAg expression in hepatocytes by HBeAg status (C: negative, D: positive). The relationship between G and S and serum HBsAg levels by HBeAg status (E: negative, F: positive). The relationship between serum HBV DNA levels and G and S by HBeAg status (G: negative, H: positive).
Correlation between HBsAg titers in hepatocytes and serum, and serum HBV DNA according to HBeAg status
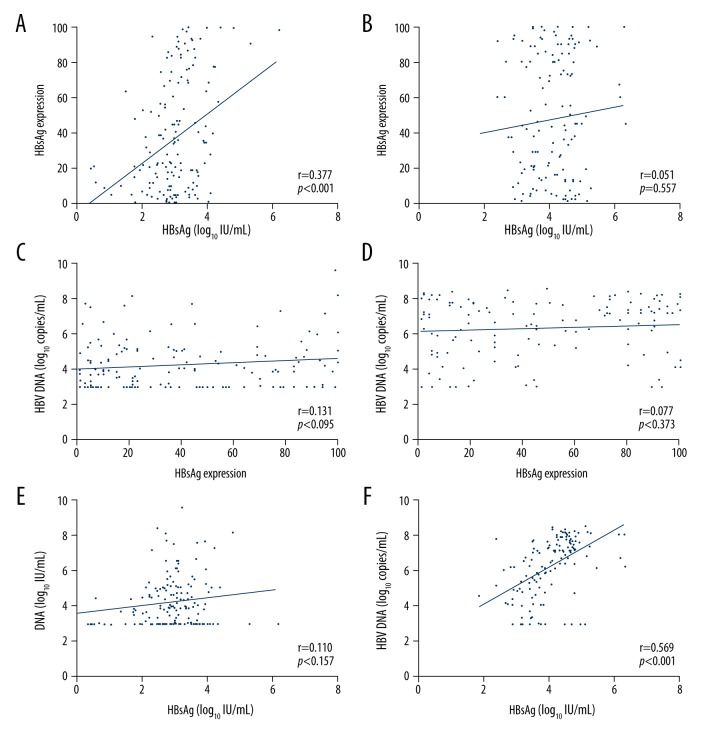
The correlations between serum HBsAg titers and HBsAg expression in hepatocytes varied depending on HBeAg status (HBeAg-positive: r=0.051, p=0.557; HBeAg-negative: r=0.377, p<0.001; Figure 2A, 2B). Regardless of HBeAg status of patients (positive of negative), HBV-DNA levels showed no significant correlation with HBsAg expression in hepatocytes (all p>0.05; Figure 2C, 2D). However, in HBeAg-positive patients, HBV DNA level did correlate with serum HBsAg titers (r=0.569, p<0.001; Figure 2F). In HBeAg-negative patients, HBV DNA levels did not correlate with serum HBsAg titers (r=0.110, p=0.157; (Figure 2E).
Figure 2.
Correlation between HBsAg titers in hepatocytes and serum, and serum HBV DNA according to HBeAg status. The relationship between serum HBsAg levels and HBsAg expression in hepatocytes by HBeAg status (A: negative, B: positive). The relationship between the serum HBV DNA levels and HBsAg expression in hepatocytes by HBeAg status (C: negative, D: positive). The relationship between serum HBV DNA levels and serum HBsAg levels by HBeAg status (E: negative, F: positive).
The relationship between HBsAg expression in hepatocytes and serum HBsAg level
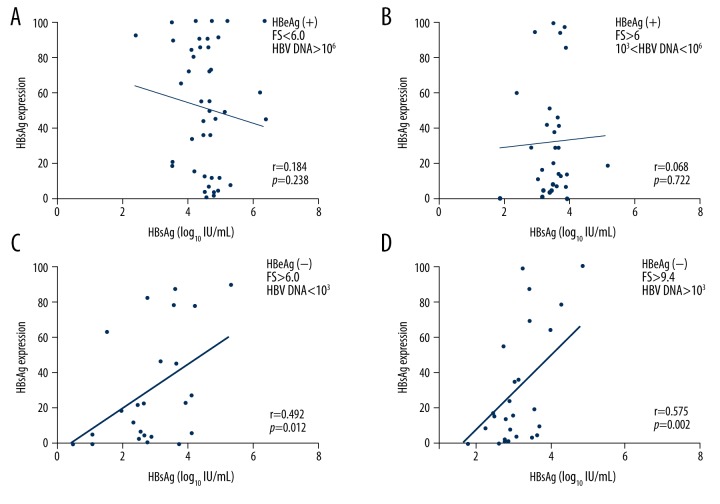
The degree of association between serum HBsAg level and HBsAg expression in hepatocytes during chronic HBV infection increased along with phase progression (immune tolerance: r=−0.184, p=0.238; immune clearance: r=0.068, p=0.722; inactive: r=0.496, p=0.012; and recovery: r=0.575, p=0.002; Figure 3).
Figure 3.
The correlation between serum HBsAg levels and HBsAg expression in hepatocytes along the phases of HBV infection. Immune-tolerant phase: HBeAg(+), FS <6, and HBV DNA >106 copies/mL (A). Immune-clearance phase: HBeAg(+), FS > 6, and HBV DNA between 103 and 106 copies/mL (B). Inactive phase: HBeAg(−), FS >6, and HBV DNA <103 copies/mL (C). Recovery phase: HBeAg(−), FS >9.4, and HBV DNA >103 copies/mL (D).
Discussion
Data from this study showed that HBsAg and serum HBV DNA levels were correlated in HBeAg-positive patients; however, no such association was observed in HBeAg-negative patients. The severity of inflammatory activity in the liver tissue, as well as evidence of hepatic fibrosis, increased when serum HBV DNA levels were in decline in HBeAg-positive patients, while these same symptoms were observed in HBeAg-negative patients when serum HBV DNA levels were on the rise. These results agreed with those previous reports [22–24].
Several studies reported three possible pathways for HBsAg secretion into the serum (Figure 4). Pathway 1: transcription from integration from the S gene (after the integration of the pre-S gene into the host genome, HBsAg is synthesized and truncated within the endoplasmic reticulum and Golgi apparatus, followed by secretion into serum); Pathway 2: transcription from HBV cccDNA (pre-S1 mRNA and pre-S2 smRNA lead to synthesis of S proteins of different sizes, followed by HBsAg production from S protein in the endoplasmic reticulum and Golgi apparatus before entering the serum); or Pathway 3: transcription from HBV genomic DNA (the core protein kinase and HBV polymerase package pregenomic RNA to form a mature nucleocapsid through reverse transcription). Mature Dane particles are then formed by the nucleocapsid and S protein in the endoplasmic reticulum and secreted into the serum through the Golgi apparatus [5,25]. Generally, HBsAg synthesis and secretion occurs via the first pathway, less often by the second pathway, and rarely by the third pathway.
Figure 4.
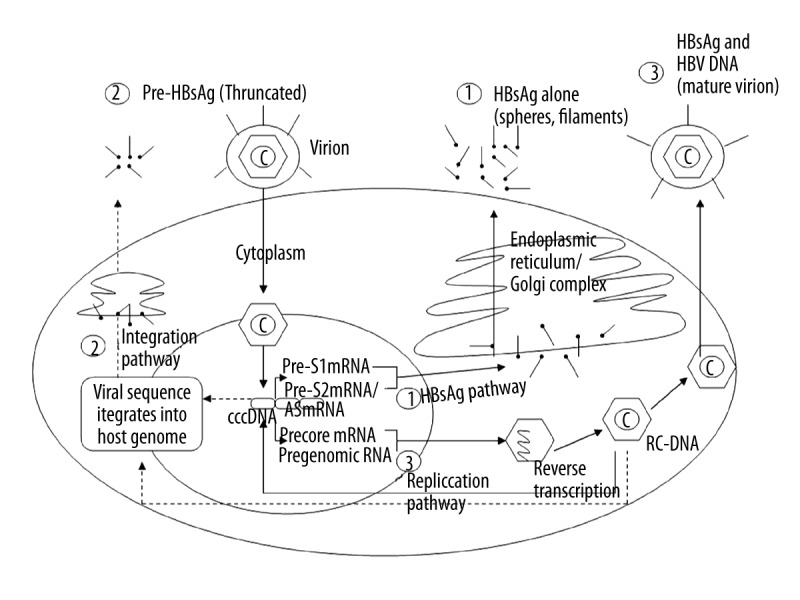
The pathways associated with HBsAg synthesis and secretion in chronic HBV patients.
In this study, HBsAg expression and serum HBsAg titers in HBeAg-positive patients were found to be much higher than those observed in HBeAg-negative patients. There are several possible explanations for this. First, it is generally accepted that HBeAg-positive patients mostly remain in the immune-tolerant and immune-clearance phases, and exhibit high levels of HBsAg, HBeAg, and serum HBV DNA. HBsAg synthesis and secretion occurs primarily through Pathway 1 in HBeAg-positive patients, while HBeAg-negative patients mostly remain in the low-replication and reactivation phases, during which HBsAg synthesis and secretion primarily occur through Pathway 2. As a result, compared to HBeAg-positive patients, HBsAg synthesis and secretion are significantly lower in HBeAg-negative patients. Second, a negative-feedback mechanism affecting HBsAg synthesis through HBV-related cccDNA in the liver has been reported [25]. HBeAg-positive patients in the immune-tolerant and immune-clearance phases exhibit negative-feedback factors capable of inhibiting the secretion of relevant compositions, such as HBsAg, HBeAg, and HBV DNA, resulting in significant HBsAg accumulation in liver tissue. Third, as HBeAg-positive patients became HBeAg-negative, liver hepatitis, necrosis, and fibrosis are aggravated, leading to a decrease in liver parenchymal cells where HBV replication occurs. Last, HBsAg expression in liver tissues can be reduced due to immune-mediated non-cytolytic mechanisms, which can also result in decreased HBsAg expression in HBeAg-negative patients.
Our data showed that there was no correlation between HBsAg expression in the liver and serum HBsAg levels in HBeAg-positive patients, while in HBeAg-negative patients, a positive correlation was observed. This could be explained by the difference in primary pathways by which HBsAg was synthesized and secreted. Our data indicated that HBsAg synthesis and secretion may mainly occur via Pathway 1 in HBeAg-positive patients, while HBeAg-negative patients mostly remain in the low-replication and reactivation phases. In recent years, mutations A1762T and G1764A in the basal core promoter (BCP) region have been identified [26,27]. HBV variants with these mutations remained and replicated under immune pressure and left an integrated S gene in liver cells. As a result, Pathway 2 then plays a greater role than Pathway 1 in HBsAg synthesis and secretion in HBeAg-positive patients [28,29]. In HBeAg-negative patients, HBsAg, HBeAg, and serum HBV DNA levels were low, and thus the titers of negative-feedback factors were also correspondingly low. Therefore, a correlation between HBsAg levels in the liver and serum appeared in these patients. Thus, reduction in negative-feedback factors and accumulation of mutations in the host immune system may significantly affect HBV production and disease activity in HBeAg-negative phases.
In this study, HBV patients were divided into four groups according to the dynamic phases of their disease (immune tolerance, immune clearance, inactive, and recovery) and the relationship between HBsAg level in serum and HBsAg expression in hepatocytes was studied. The results suggested correlation between serum HBsAg level and HBsAg expression in hepatocytes in chronic hepatitis B patients, and that this correlation increased along with phase progression (Figure 3). Our results suggested that in patients with chronic hepatitis B, HBsAg synthesis and secretion through Pathway 2 increased along with phase progression of HBV infection, which was distinguishable by virological and serological markers.
Conclusions
In patients with chronic HBV infection, HBsAg synthesis and secretion might be influenced by many factors, including the levels of HBV-DNA, HBsAg, or HBeAg in serum, mutations, as well as host immunologic function. HBsAg synthesis and secretion through Pathway 2 increased along with phase progression of HBV infection. Data from this study suggested that HBsAg synthesis and secretion may occur via different mechanisms during different phases of chronic HBV infection. However, elucidation of specific mechanisms requires further study in the future.
Footnotes
Ethics committee approval
Ethics committee approval was received for this study from the ethics committee of Anhui Medical University.
Conflict of interest
No conflict of interest was declared by the authors.
Source of support: The study was supported by Anhui Provincial Natural Science Foundation (1608085MH162) and National Science Foundation of China (81273142)
References
- 1.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.You CR, Lee SW, Jang JW, et al. Update on hepatitis B virus infection. World J Gastroenterol. 2014;20:13293–305. doi: 10.3748/wjg.v20.i37.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu S, Zhou Q, Zhao XM, et al. Comparison of the antiviral effects of different nucleos(t)ide analogues in chinese patients with chronic hepatitis B: A head-to-head study. Saudi J Gastroenterol. 2014;20:350–55. doi: 10.4103/1319-3767.145320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan HL, Wong VW, Wong GL, et al. Early hepatitis B virus DNA suppression can predict virologic response to peginterferon and lamivudine treatment. Clin Gastroenterol Hepatol. 2008;6:1022–26. doi: 10.1016/j.cgh.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: A review. Hepatology. 2011;53:2121–29. doi: 10.1002/hep.24364. [DOI] [PubMed] [Google Scholar]
- 6.ter Borg MJ, van Zonneveld M, Zeuzem S, et al. Patterns of viral decline during PEG-interferon alpha-2b therapy in HBeAg-positive chronic hepatitis B: Relation to treatment response. Hepatology. 2006;44:721–27. doi: 10.1002/hep.21302. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–44. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 8.Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–58. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Chen EQ, Wang TT, Bai L, et al. Quantitative hepatitis B surface antigen titres in Chinese chronic hepatitis B patients over 4 years of entecavir treatment. Antivir Ther. 2013;18:955–65. doi: 10.3851/IMP2579. [DOI] [PubMed] [Google Scholar]
- 10.Hadziyannis SJ, Vassilopoulos D. Immunopathogenesis of hepatitis B e antigen negative chronic hepatitis B infection. Antiviral Res. 2001;52:91–98. doi: 10.1016/s0166-3542(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25:1498–506. doi: 10.1111/j.1440-1746.2010.06282.x. [DOI] [PubMed] [Google Scholar]
- 12.Sonneveld MJ, Zoutendijk R, Janssen HL. Hepatitis B surface antigen monitoring and management of chronic hepatitis B. J Viral Hepat. 2011;18:449–57. doi: 10.1111/j.1365-2893.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 13.Grandjacques C, Pradat P, Stuyver L, et al. Rapid detection of genotypes and mutations in the pre-core promoter and the pre-core region of hepatitis B virus genome: Correlation with viral persistence and disease severity. J Hepatol. 2000;33:430–39. doi: 10.1016/s0168-8278(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–62. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 15.Fan YF, Lu CC, Chang YC, et al. Identification of a pre-S2 mutant in hepatocytes expressing a novel marginal pattern of surface antigen in advanced diseases of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2000;15:519–28. doi: 10.1046/j.1440-1746.2000.02187.x. [DOI] [PubMed] [Google Scholar]
- 16.Su IJ, Wang HC, Wu HC, et al. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2008;23:1169–74. doi: 10.1111/j.1440-1746.2008.05348.x. [DOI] [PubMed] [Google Scholar]
- 17.Rijckborst V, Hansen BE, Cakaloglu Y, et al. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–61. doi: 10.1002/hep.23722. [DOI] [PubMed] [Google Scholar]
- 18.Vigano M, Paggi S, Lampertico P, et al. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: A cohort study with internal validation. Aliment Pharmacol Ther. 2011;34:353–62. doi: 10.1111/j.1365-2036.2011.04722.x. [DOI] [PubMed] [Google Scholar]
- 19.Mangia A, Chung YH, Hoofnagle JH, et al. Pathogenesis of chronic liver disease in patients with chronic hepatitis B virus infection without serum HBeAg. Dig Dis Sci. 1996;41:2447–52. doi: 10.1007/BF02100141. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100–7. doi: 10.1111/j.1478-3231.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 21.Hou JL, Wei L. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;12:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Croagh CM, Bell SJ, Slavin J, et al. Increasing hepatitis B viral load is associated with risk of significant liver fibrosis in HBeAg-negative but not HBeAg-positive chronic hepatitis B. Liver Int. 2010;30:1115–22. doi: 10.1111/j.1478-3231.2010.02267.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naive, e antigen-positive patients. J Hepatol. 2013;58:1089–95. doi: 10.1016/j.jhep.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Sanai FM, Helmy A, Bzeizi KI, et al. Discriminant value of serum HBV DNA levels as predictors of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2011;18:e217–25. doi: 10.1111/j.1365-2893.2011.01437.x. [DOI] [PubMed] [Google Scholar]
- 25.Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: New trick of old dog. J Gastroenterol. 2013;48:13–21. doi: 10.1007/s00535-012-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan YW, Zhang YH, Jiang WJ, et al. Relationship between metastasis or recurrence of hepatocellular carcinoma and hepatitis B virus DNA or double mutation at 1762/1764 in the basic core promoter. Zhonghua Gan Zang Bing Za Zhi. 2013;21:679–83. doi: 10.3760/cma.j.issn.1007-3418.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Hu Y, Shi W, et al. Associations between hepatitis B virus x gene mutations and hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2015;23:599–603. doi: 10.3760/cma.j.issn.1007-3418.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Manesis EK, Papatheodoridis GV, Tiniakos DG, et al. Hepatitis B surface antigen: relation to hepatitis B replication parameters in HBeAg-negative chronic hepatitis B. J Hepatol. 2011;55:61–68. doi: 10.1016/j.jhep.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Saitta C, Tripodi G, Barbera A, et al. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int. 2015;35:2311–17. doi: 10.1111/liv.12807. [DOI] [PubMed] [Google Scholar]