Abstract
The Flaviviridae is a family of small enveloped viruses with RNA genomes of 9000–13 000 bases. Most infect mammals and birds. Many flaviviruses are host-specific and pathogenic, such as hepatitis C virus in the genus Hepacivirus. The majority of known members in the genus Flavivirus are arthropod borne, and many are important human and veterinary pathogens (e.g. yellow fever virus, dengue virus). This is a summary of the current International Committee on Taxonomy of Viruses (ICTV) report on the taxonomy of the Flaviviridae, which is available at www.ictv.global/report/flaviviridae.
Keywords: Flaviviridae, taxonomy, ICTV Report
Virion
Virions are typically spherical in shape with a lipid envelope (Table 1, Fig. 1). Virions have a single, small, basic capsid (C) protein and two (genera Flavivirus, Hepacivirus and Pegivirus) or three (genus Pestivirus) envelope proteins.
Table 1. Characteristics of the family Flaviviridae.
| Typical member: | yellow fever virus-D17 (X03700), species Yellow fever virus, genus Flavivirus |
|---|---|
| Virion | Enveloped, 40–60 nm virions with a single core protein (except for genus Pegivirus) and 2 or 3 envelope glycoproteins |
| Genome | Approximately 9.0–13 kb of positive-sense, non-segmented RNA |
| Replication | Cytoplasmic, in membrane vesicles derived from the endoplasmic reticulum (ER); assembled virions bud into the lumen of the ER and are secreted through the vesicle transport pathway |
| Translation | Directly from genomic RNA containing a type I cap (genus Flavivirus) or an internal ribosome entry site (other genera) |
| Host range | Mammals (all genera); most members of genus Flavivirus are arthropod borne |
| Taxonomy | Currently four genera containing more than 60 species |
Fig. 1.
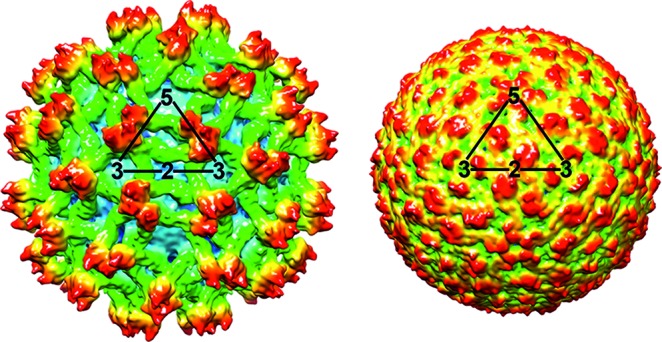
Three-dimensional cryo-electron reconstruction of immature (left) and mature (right) particles of an isolate of dengue virus (courtesy of Richard Kuhn and Michael Rossmann). Shown is a surface rendering of immature dengue virus at 12.5 Å resolution (left) and mature dengue virus at 10 Å resolution (right). The viruses are depicted to scale, but not coloured to scale. Triangles outline one icosahedral unit, with the 2-, 3- and 5-fold axes of symmetry.
Genome
Virus genomes are positive-stranded, non-segmented RNA of approximately 9.2–11, 12.3–13, 8.9–10.5 and 8.9–11.3 kb for members of the genera Flavivirus, Pestivirus, Hepacivirus and Pegivirus, respectively (Fig. 2). They contain a single, long ORF flanked by 5′- and 3′-terminal non-coding regions, which form specific secondary structures required for genome replication and translation. Translational initiation of genomic RNA is cap dependent in the case of members of the genus Flavivirus, whereas internal ribosome entry site elements are present in members of the other genera.
Fig. 2.
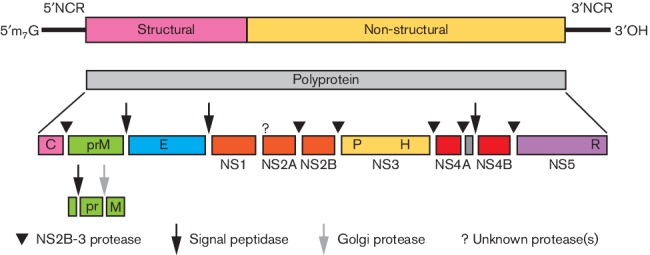
Genome organization and polyprotein processing of members of the genus Flavivirus. Boxes below the genome indicate viral proteins generated by proteolytic processing. NCR, non-coding region.
Replication
Viral proteins are synthesized as part of a polyprotein that is co- and post-translationally cleaved by viral and cellular proteases. The structural proteins are contained in the N-proximal portion of this polyprotein and the non-structural proteins in the remainder. Replication of members of the family Flaviviridae occurs through the synthesis of an antigenome as the template for genome RNA production. Genome RNA also acts as a translational template for the synthesis of viral proteins. Replication complexes are sequestered with a complex topology in membranous structures within the endoplasmic reticulum. Replication enzymes include a serine protease, an RNA helicase and an RNA-dependent RNA polymerase. These proteins are homologous among all members of genus Flavivirus, contain conserved motifs and are encoded at similar locations in the genome. Virion assembly, including acquisition of a glycoprotein-containing lipid envelope, occurs by budding through intracellular membranes. Particles are transported in cytoplasmic vesicles through the secretory pathway and released by exocytosis.
Taxonomy
Flavivirus
This genus consists primarily of >50 species of arthropod-borne viruses, with distinct groups infecting mosquitoes or ticks [1]. Mammals and birds are the usual primary hosts, in which infections range from asymptomatic to severe or fatal haemorrhagic fever or neurological disease. Important human pathogens include yellow fever virus, dengue virus, Japanese encephalitis virus, West Nile virus and tick-borne encephalitis virus. Other members cause economically important diseases in domestic or wild animals. Additional viruses infecting only arthropods or only mammals (e.g. Tamana bat virus) have been described recently.
Pestivirus
These viruses infect pigs and ruminants, including cattle, sheep, goats and wild ruminants [2], and are transmitted through contact with infected secretions (respiratory droplets, urine or faeces). Infections may be subclinical or cause enteric, haemorrhagic or wasting diseases, including those by the economically important bovine viral diarrhoea virus and classical swine fever virus.
Hepacivirus
This genus includes hepatitis C virus, a major human pathogen causing progressive liver disease [3], and several other viruses of unknown pathogenicity that infect horses, rodents, bats, cows and primates [4]. Infections are typically persistent and target the liver.
Pegivirus
Members are widely distributed in a range of mammalian species, in which they cause persistent infections [5]. To date, they have not been clearly associated with disease.
Resources
Full ICTV Online (10th) Report: www.ictv.global/report/flaviviridae.
Hepatitis C virus classification: http://talk.ictvonline.org/links/hcv/hcv-classification.html.
Funding information
Production of this summary, the online chapter and associated resources was funded by a grant from the Wellcome Trust (WT108418AIA).
Acknowledgements
Members of the ICTV Report Consortium are Elliot J. Lefkowitz, Andrew J. Davison, Stuart G. Siddell, Peter Simmonds, Michael J. Adams, Donald B. Smith, Richard J. Orton and Nick J. Knowles.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the Flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 2.Tautz N, Tews BA, Meyers G. The molecular biology of pestiviruses. Adv Virus Res. 2015;93:47–160. doi: 10.1016/bs.aivir.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 4.Scheel TK, Simmonds P, Kapoor A. Surveying the global virome: identification and characterization of HCV-related animal hepaciviruses. Antiviral Res. 2015;115:83–93. doi: 10.1016/j.antiviral.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol. 2011;92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


