Abstract
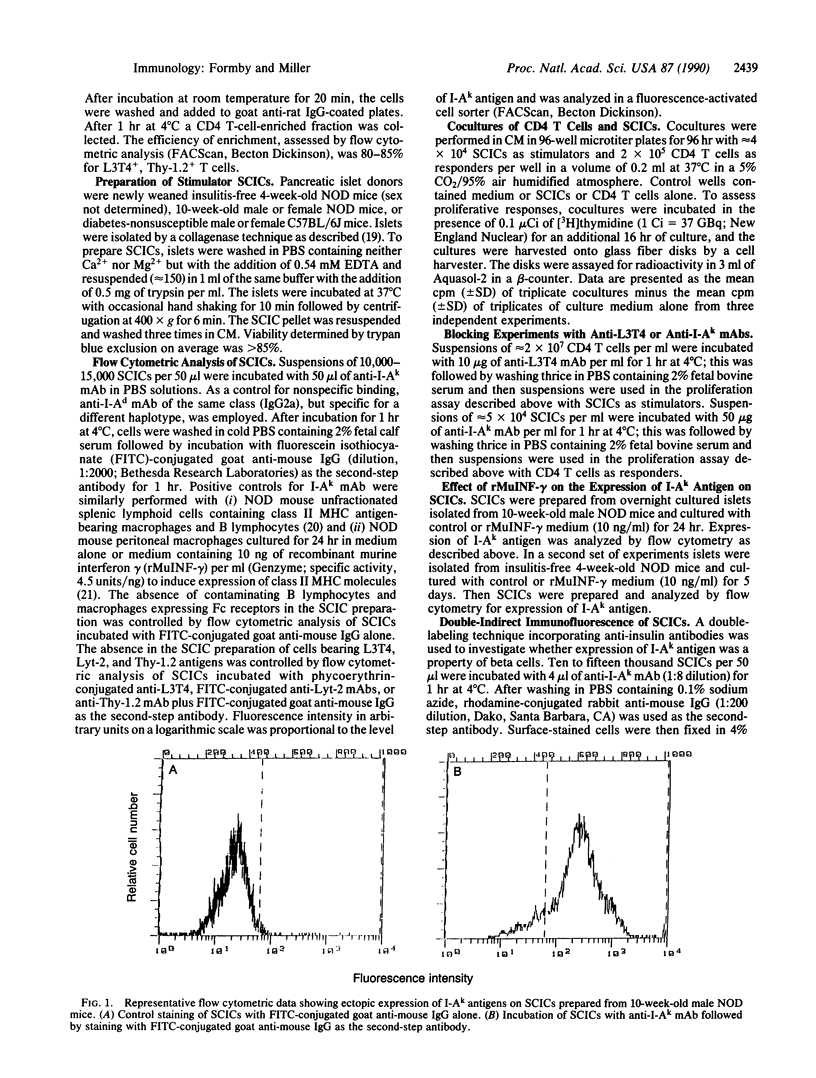
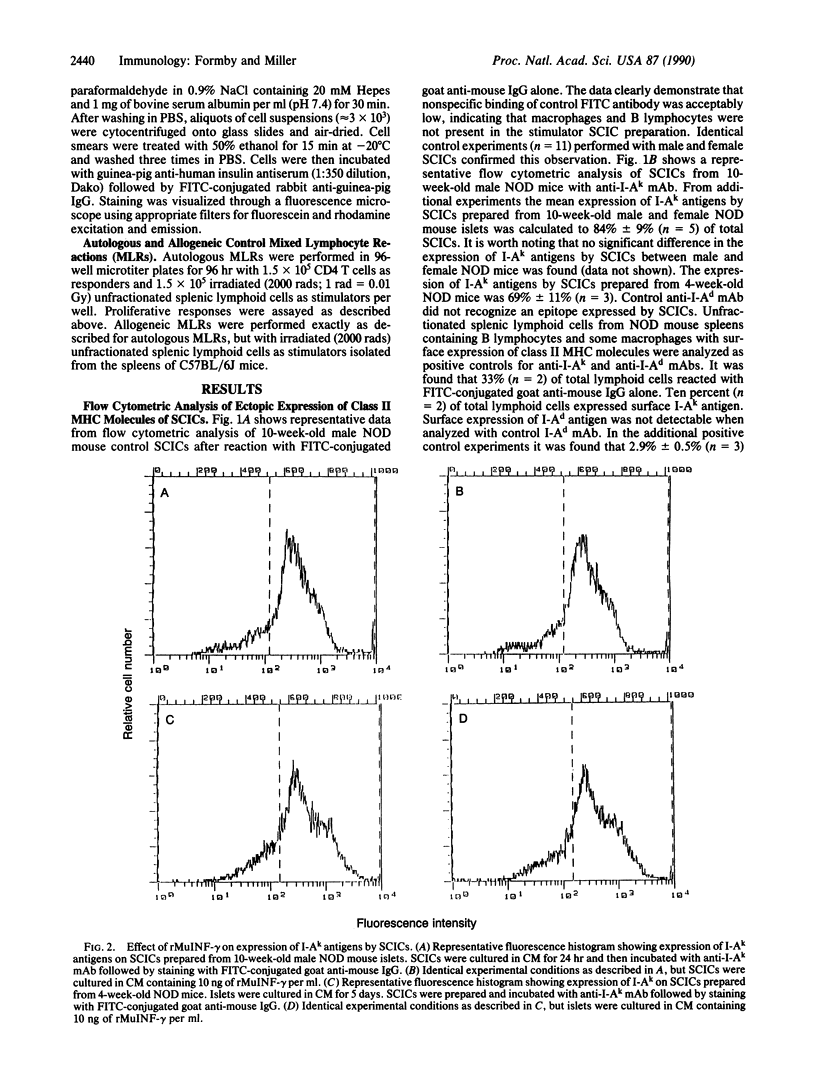
We investigated by flow cytometric analysis the expression of class II major histocompatibility complex (MHC) molecules by viable single-cell islet cells (SCICs) prepared from male and female 4- and 10-week-old nonobese diabetic (NOD) mouse islets. With anti-I-Ak monoclonal antibody (specific for I-Ak,f,r,s beta and produced by clone 11-5-2), and fluorescein isothiocyanate-conjugated goat anti-mouse IgG as second-step antibody, we found that SCICs from both sexes aberrantly expressed class II MHC molecules, which was not altered after SCICs were cultured for 24 hr or 120 hr in the presence of 10 ng of recombinant murine interferon gamma per ml. Double-indirect immunofluorescence of male SCICs indicated that the expression of class II MHC molecules was a property of beta cells. Control experiments documented that macrophages and mononuclear cells did not contaminate the SCIC preparations. Coculture experiments with responder splenic CD4 T cells isolated from diabetic NOD mice and stimulator male SCICs indicated a recognition event evidenced by a 12-fold increase in proliferative response. Monoclonal antibodies to class II MHC and CD4 antigens blocked the proliferative response. Results from control autologous and allogeneic mixed lymphocyte reactions suggest that the responder CD4 T cells are autoreactive self-class II MHC restricted. We tentatively conclude that the ability of SCICs from both sexes of NOD mice to express class II MHC molecules as early as 4 weeks of age may represent a mechanism for targeting immune reactions to beta cells and initiate lymphocytic insulitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballardini G., Mirakian R., Bianchi F. B., Pisi E., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984 Nov 3;2(8410):1009–1013. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Bendelac A., Richard M. F., Carnaud C., Bach J. F. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9719–9723. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Todd I., Mirakian R., Belfiore A., Pujol-Borrell R. Organ-specific autoimmunity: a 1986 overview. Immunol Rev. 1986 Dec;94:137–169. doi: 10.1111/j.1600-065X.1986.tb01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. M., Walker R., Bone A. J., Baird J. D., Cooke A. Pre-diabetes in the spontaneously diabetic BB/E rat: lymphocyte subpopulations in the pancreatic infiltrate and expression of rat MHC class II molecules in endocrine cells. Diabetologia. 1985 Jul;28(7):464–466. doi: 10.1007/BF00280892. [DOI] [PubMed] [Google Scholar]
- Formby B., Miller N., Garret R., Peterson C. M. Effects of low-dose cyclosporine prophylaxis in nonobese diabetic mice. J Pharmacol Exp Ther. 1987 Jun;241(3):1106–1111. [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986 Nov;35(11):1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Fujino-Kurihara H., Miyazaki A., Yamada K., Nakajima H., Miyagawa J., Kono N., Tarui S. Expression of class II major histocompatibility complex antigens on pancreatic B cells in the NOD mouse. Diabetologia. 1987 Feb;30(2):104–108. doi: 10.1007/BF00274580. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Sugihara S., Fujino-Kurihara H., Miyagawa J., Miyazaki A., Yoshioka T., Yamada K., Nakajima H., Asakawa H., Kono N. Induction of insulitis by adoptive transfer with L3T4+Lyt2- T-lymphocytes in T-lymphocyte-depleted NOD mice. Diabetes. 1988 Feb;37(2):204–208. doi: 10.2337/diab.37.2.204. [DOI] [PubMed] [Google Scholar]
- Ikegami H., Makino S., Harada M., Eisenbarth G. S., Hattori M. The cataract Shionogi mouse, a sister strain of the non-obese diabetic mouse: similar class II but different class I gene products. Diabetologia. 1988 Apr;31(4):254–258. doi: 10.1007/BF00290594. [DOI] [PubMed] [Google Scholar]
- Jansson R., Karlsson A., Forsum U. Intrathyroidal HLA-DR expression and T lymphocyte phenotypes in Graves' thyrotoxicosis, Hashimoto's thyroiditis and nodular colloid goitre. Clin Exp Immunol. 1984 Nov;58(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Christianson G. J., Serreze D. V., Ting A. T., Worthen S. M. MHC antigen induction by interferon gamma on cultured mouse pancreatic beta cells and macrophages. Genetic analysis of strain differences and discovery of an "occult" class I-like antigen in NOD/Lt mice. J Exp Med. 1989 Oct 1;170(4):1243–1262. doi: 10.1084/jem.170.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., McDevitt H. O. Genetics and expression of mouse Ia antigens. Annu Rev Immunol. 1985;3:367–396. doi: 10.1146/annurev.iy.03.040185.002055. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Nepom B. S., Palmer J., Kim S. J., Hansen J. A., Holbeck S. L., Nepom G. T. Specific genomic markers for the HLA-DQ subregion discriminate between DR4+ insulin-dependent diabetes mellitus and DR4+ seropositive juvenile rheumatoid arthritis. J Exp Med. 1986 Jul 1;164(1):345–350. doi: 10.1084/jem.164.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Hanafusa T., Chiovato L., Bottazzo G. F. Lectin-induced expression of DR antigen on human cultured follicular thyroid cells. Nature. 1983 Jul 7;304(5921):71–73. doi: 10.1038/304071a0. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Signore A., Pozzilli P., Gale E. A., Andreani D., Beverley P. C. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia. 1989 May;32(5):282–289. doi: 10.1007/BF00265543. [DOI] [PubMed] [Google Scholar]
- Sinha A. A., Brautbar C., Szafer F., Friedmann A., Tzfoni E., Todd J. A., Steinman L., McDevitt H. O. A newly characterized HLA DQ beta allele associated with pemphigus vulgaris. Science. 1988 Feb 26;239(4843):1026–1029. doi: 10.1126/science.2894075. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]