ABSTRACT
For Pseudomonas aeruginosa, levels of cyclic di-GMP (c-di-GMP) govern the transition from the planktonic state to biofilm formation. Type IV pili (T4P) are crucial determinants of biofilm structure and dynamics, but it is unknown how levels of c-di-GMP affect pilus dynamics. Here, we scrutinized how c-di-GMP affects molecular motor properties and adhesive behavior of T4P. By means of retraction, T4P generated forces of ∼30 pN. Deletion mutants in the proteins with known roles in biofilm formation, swarming motility, and exopolysaccharide (EPS) production (specifically, the diguanylate cyclases sadC and roeA or the c-di-GMP phosphodiesterase bifA) showed only modest effects on velocity or force of T4P retraction. At high levels of c-di-GMP, the production of exopolysaccharides, particularly of Pel, is upregulated. We found that Pel production strongly enhances T4P-mediated surface adhesion of P. aeruginosa, suggesting that T4P-matrix interactions may be involved in biofilm formation by P. aeruginosa. Finally, our data support the previously proposed model of slingshot-like “twitching” motility of P. aeruginosa.
IMPORTANCE Type IV pili (T4P) play various important roles in the transition of bacteria from the planktonic state to the biofilm state, including surface attachment and surface sensing. Here, we investigate adhesion, dynamics, and force generation of T4P after bacteria engage a surface. Our studies showed that two critical components of biofilm formation by Pseudomonas aeruginosa, T4P and exopolysaccharides, contribute to enhanced T4P-mediated force generation by attached bacteria. These data indicate a crucial role for the coordinated impact of multiple biofilm-promoting factors during the early stages of attachment to a surface. Our data are also consistent with a previous model explaining why pilus-mediated motility in P. aeruginosa results in characteristic “twitching” behavior.
KEYWORDS: Pseudomonas aeruginosa, pili, biofilm, cyclic di-GMP, exopolysaccharide, molecular motor
INTRODUCTION
Type IV pili (T4P) are crucial factors in bacterial biofilm formation, as they mediate initial surface attachment and govern the structure of biofilms (1). For Pseudomonas aeruginosa, a role for T4P as surface sensors is emerging (2); in particular, active T4P retraction has been proposed to be required (3, 4). It is currently unclear, however, how T4P dynamics affect the earliest stages of biofilm formation.
Bacterial T4P are extracellular polymeric cell appendages required for initial attachment to biotic and abiotic surfaces, host cell signaling, microcolony formation, and natural transformation. The polymer incorporates major pilins (PilA) and a variety of minor pilins (5). The pilus polymer is anchored within the cell envelope by a membrane-spanning complex (6). Importantly, the length of the T4P is dynamic; elongation of T4P requires a cytoplasmic ATPase formed by PilB, and retraction requires its antagonist PilT (7). For Neisseria gonorrhoeae and Myxococcus xanthus, retraction of T4P generates a high mechanical force exceeding 100 pN (8, 9). Considering that T4P-related surface motility (also known as twitching motility) requires force generation (10, 11) and that P. aeruginosa is capable of twitching motility (12), it is reasonable to assume that the T4P of P. aeruginosa generate mechanical force by retraction. However, the motor properties in the species have not been addressed.
For P. aeruginosa, the current understanding of transition from the planktonic lifestyle to life within biofilms involves surface sensing and subsequent upregulation of second messengers (2). When grown in liquid culture, the T4P level is low compared to that of its growth on solid surfaces (13–15). The T4P participate in an uncharacterized surface engagement process that upregulates cyclic AMP (cAMP) in response to surface contact through the Pil-Chp chemosensory system (3, 4, 16, 17). As a consequence, the T4P-associated protein PilY1 is upregulated, leading to a subsequent increase in the level of the second messenger cyclic di-GMP (c-di-GMP) (3, 18). C-di-GMP, in turn, mediates the transition to biofilm formation, including downregulation of flagellar machinery and the production of exopolysaccharides (EPS) (19–23). The levels of c-di-GMP depend on diguanylate cyclases and phosphodiesterases. Different diguanylate cyclases govern different functions; for example, RoeA promotes the production of EPS, whereas SadC controls flagellar motility (21). Deletion of either sadC or roeA reduces the c-di-GMP level approximately 2-fold. The phosphodiesterase BifA modulates Pel production and flagellar motility (23), and deleting the gene that codes for BifA increases the c-di-GMP level approximately 5- to 10-fold. Thus, while there is evidence that T4P retraction induces upregulation of c-di-GMP, the effect of c-di-GMP levels on T4P dynamics remains elusive.
Various roles of EPS in biofilm initiation have been proposed. For P. aeruginosa strain PA14, used in this study, Pel is the dominant EPS, while P. aeruginosa strain PAO1 produces Psl (24–27). EPS is required for stable attachment (27). Interestingly, EPS secretion favors microcolony formation through a positive feedback loop; P. aeruginosa PAO1 cells secrete trails of EPS, and other cells tend to follow these tracks (28), which suggests that EPS enhances surface adhesion (29).
T4P are involved in biofilm initiation through mediating surface attachment, surface motility, and ultimately surface sensing. Here, we address the question of how T4P dynamics evolve once bacteria have committed themselves to a surface. Since motor properties are best analyzed at the single-molecule level, we used mutants with different levels of c-di-GMP and distinct defects in early biofilm formation that resulted from the deletion of two diguanylate cyclases (SadC and RoeA) and a phosphodiesterase (BifA) with known roles in early biofilm formation (21–23). Using laser tweezers to examine these mutant strains, we found no strong dependence of c-di-GMP on T4P velocity and force generation. Finally, our data are consistent with those from a model wherein T4P, together with Pel (an exopolysaccharide controlled by the diguanylate cyclase RoeA), enhance surface adhesion.
RESULTS
P. aeruginosa T4P retraction generates force.
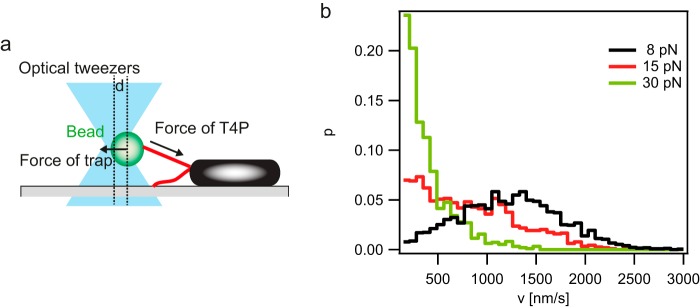
Type IV pili can generate considerable mechanical force by retraction (10). For example, the force generated by a single T4P of Neisseria gonorrhoeae or Myxococcus xanthus amounts to more than 100 pN under normal laboratory conditions (8, 9). Since T4P are responsible for the twitching motility of P. aeruginosa, it was conceivable that the T4P of P. aeruginosa likewise generate force. Here, we confirmed this assumption. To this end, the P. aeruginosa PA14 ΔflgK strain was inoculated on glass coverslides, and polystyrene beads were placed close to the bacterial cell poles using laser tweezers. When T4P bind to the bead and retract, they deflect the bead from the center of the trap (Fig. 1a). We used the force clamp mode to measure the velocity of T4P retraction at various forces. In this mode, the force is kept constant through feedback between the deflection of the bead (d) and the optical table (30). We found that at a force (F) of 8 pN, the average (±standard error) velocity (v) was as follows: v(8 pN) = 1,187 nm/s ± 13 nm/s (Fig. 1b). The distribution of velocities shifted lower at F of 15 pN, and T4P retraction stalled at F of approximately 30 pN (Fig. 1b).
FIG 1.
Velocity of P. aeruginosa PA14 T4P retraction. (a) Sketch of the experimental setup. (b) Distribution of T4P retraction speeds at clamped forces (F) of 8 pN, 15 pN, and 30 pN (n, >950 retraction intervals of 100 ms for each condition). In these experiments, the P. aeruginosa PA14 ΔflgK mutant was used to eliminate any contribution of the flagellum to the adhesion or movement of the cell.
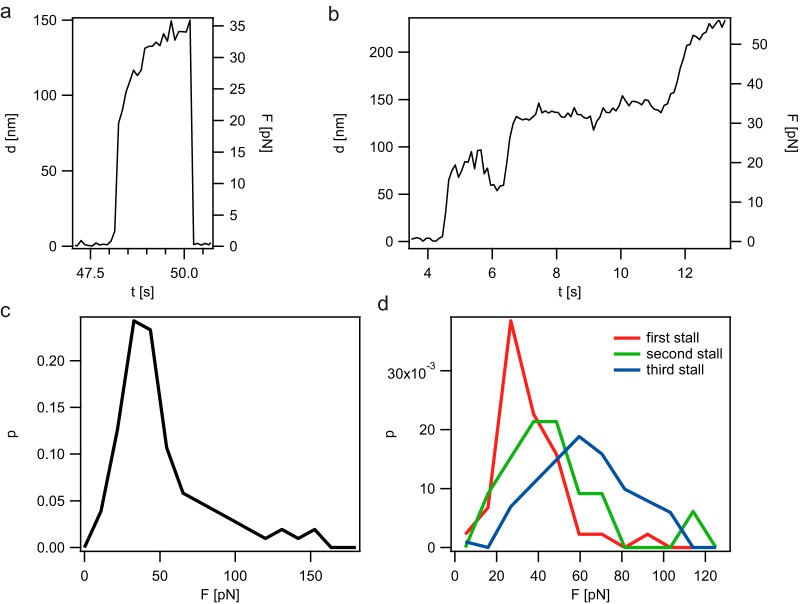
The position clamp mode of the laser trap was used for characterizing the stalling forces. In this mode, the force acting on the T4P increases linearly with the deflection of the bead (d). Some T4P retraction events showed a single stalling event and subsequent rupture between T4P and the bead (Fig. 2a). In contrast to earlier experiments that characterized T4P retraction in N. gonorrhoeae (11), detachment of T4P from the bead was an unlikely event at forces less than the stalling force. As a consequence, transient pausing was often observed (Fig. 2b); T4P retraction stalled but resumed after a pause. We assume that the resumption of T4P retraction was caused by binding and retraction of a second or multiple T4P. The stalling force was defined as the force at which the velocity (v) was less than 250 nm/s for at least 500 ms; its distribution showed a maximum at F of 37 pN and a pronounced tail (Fig. 2c). Thus, we plotted the stalling forces of the first, second, and third stalling events independently (Fig. 2d). The force distribution of the first stalling event does not show the tail found when all stalling events were pooled. This observation strongly suggests that we observed the stalling force distribution of single T4P retractions. Thus, the average (±standard error) stalling force (Fstall) of a single T4P retraction was as follows: Fstall = 31 pN ± 1 pN. We conclude that T4P retraction of P. aeruginosa generates mechanical force on the order of ∼30 pN under the laboratory conditions used in this study.
FIG 2.
Stalling forces of P. aeruginosa PA14 T4P retraction. (a and b) Typical examples for T4P retraction events in position clamp mode. Deflections (d) of the bead from the center of the laser trap (compare with Fig. 1a) and the corresponding forces (F) are shown. (c) Distribution of stalling forces (force at which the velocity was less than 250 nm/s for at least 500 ms) (n = 103). (d) Distribution of stalling forces. First, second, and third stalling events of single pilus retractions are plotted (n > 21 for each condition). In these experiments, the P. aeruginosa PA14 ΔflgK mutant was used to eliminate any contribution of the flagellum to the adhesion or movement of the cell.
Impact of oxygen availability on T4P function in P. aeruginosa PA14.
N. gonorrhoeae T4P switch their retraction speed from 2 μm/s under aerobic conditions to 1 μm/s under anaerobic conditions, while M. xanthus is not motile under anaerobic conditions (31, 32). We assessed whether T4P retraction was active in P. aeruginosa under conditions that mimic low-oxygen environments. The T4P retraction assay was performed under aerobic conditions and in the presence of an oxygen scavenger and carbonyl cyanide m-chlorophenylhydrazone (CCCP), which disrupts the function of the electron transport chain. While 25% of the sampled bacteria actively retracted their pili under aerobic conditions, no T4P retraction activity was observed when the oxygen scavenger or CCCP was added (see Fig. S1 in the supplemental material), indicating that P. aeruginosa shows no T4P retraction in microoxic or anoxic growth conditions and/or when electron transport chain function is impaired. In a control experiment, we found that the addition of CCCP did not alter the viability of P. aeruginosa over the experimental period (data not shown).
The force-dependent velocity of T4P retraction is only weakly dependent on the presence of the diguanylate cyclases SadC and RoeA and on the phosphodiesterase BifA.
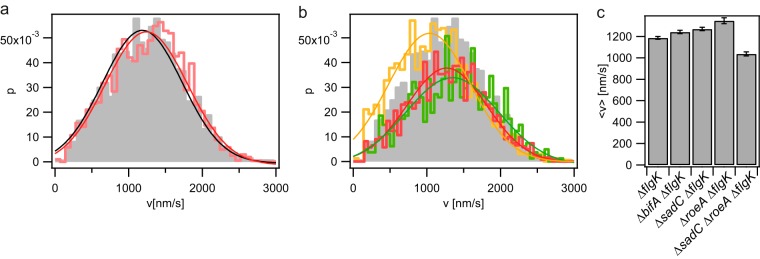
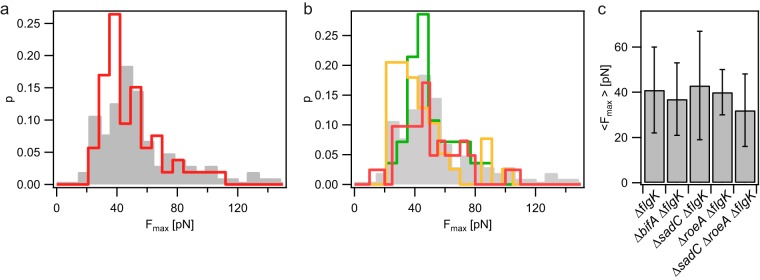
We addressed the question of whether the level of c-di-GMP affects velocity or force generation by T4P. To this end, we measured the velocity of T4P retraction at F of 8 pN in mutants with deletions of diguanylate cyclases and a phosphodiesterase that play important roles in the early stages of biofilm formation by P. aeruginosa. The measured force distributions were in good agreement with Gaussian distributions. Neither deletion of sadC nor deletion of bifA resulted in a significant effect on the distribution of T4P retraction velocities (Fig. 3). Deletion of the roeA gene resulted in a very modest but significant increase in velocity from that for the wild type (vwt), as follows: vwt(8 pN) = 1,187 nm/s ± 13 nm/s to vroeA(8 pN) = 1,346 nm/s ± 28 nm/s, where vroeA is the velocity for the roeA mutant. The double deletion of sadC and roeA reduced the average speed of T4P retraction slightly but significantly: vsadCroeA(8 pN) = 1,037 nm/s ± 19 nm/s, where vsadCroeA is the velocity for the sadC roeA double mutant. Similar to the distribution of stalling forces in the wild-type background, the stalling force distributions of the deletion mutants were non-Gaussian. Deletion of sadC, roeA, or bifA did not significantly affect the stalling force of T4P retraction, and the sadC roeA strain showed a slightly but significantly reduced stalling force (Fig. 4). In summary, deletion of the diguanylate cyclase genes sadC and roeA or the phosphodiesterase gene bifA showed only minor and nonsystematic effects on the motor properties of T4P, indicating that the motor properties are not systematically dependent on the levels of c-di-GMP.
FIG 3.
Speed of T4P retraction for diguanylate cyclase and c-di-GMP phosphodiesterase deletion strains. (a) Distribution of T4P retraction speeds for the ΔflgK parent strain (gray) and the ΔbifA ΔflgK mutant (red). (b) Distribution of T4P retraction speeds for the ΔflgK parent strain (gray) and the ΔsadC ΔflgK (red), ΔroeA ΔflgK (green), and ΔsadC ΔroeA ΔflgK (orange) mutants. (c) Average T4P retraction speeds of the indicated strains. Error bars, standard errors of the mean of a Gaussian fit (full lines in panels a and b) (n > 850 for each condition).
FIG 4.
Maximum force of T4P retraction for diguanylate cyclase and c-di-GMP phosphodiesterase deletion strains. (a) Distribution of maximum forces for the ΔflgK parent strain (gray) and the ΔbifA ΔflgK mutant (red). (b) Distribution of maximum force for the ΔflgK parent strain (gray) and the ΔsadC ΔflgK (red), ΔroeA ΔflgK (green), and ΔsadC ΔroeA ΔflgK (orange) mutants. (c) Average maximum forces. Error bars, standard deviations. The P value of 0.008, obtained from a Kolmogorov-Smirnov test between the ΔflgK parent strain and the ΔsadC ΔroeA ΔflgK mutant, indicates a significant difference (n > 28 for each condition).
Coating the surface with the exopolysaccharide PelA enhances T4P-mediated surface interaction.
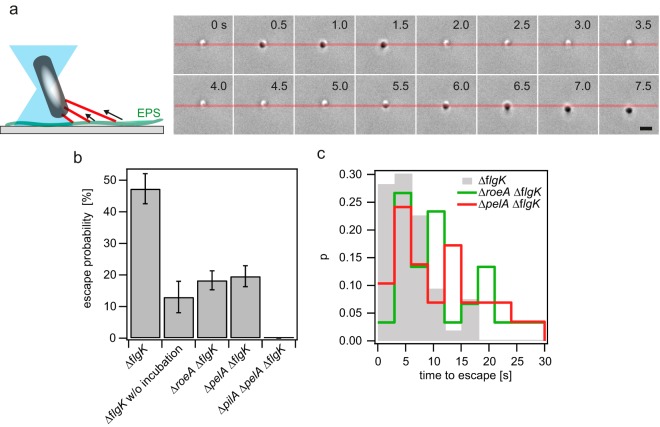
In response to high c-di-GMP levels, P. aeruginosa PA14 upregulates production of the Pel exopolysaccharide (EPS) (23, 33, 34). Here, we studied whether the presence of EPS impacts T4P function. Glass coverslides were incubated with a high concentration of P. aeruginosa cells for 1 h, allowing these microbes the opportunity to produce surface-associated EPS, as reported (28). Subsequently, most bacteria were rinsed off the surface. From the remaining bacteria, single bacteria were trapped in the laser trap. P. aeruginosa assumes an upright position in the laser trap, reminiscent of other rod-shaped bacterial species (35, 36). When T4P retraction is active, the bacteria actively move away from the center of the laser trap (Fig. 5a). While we were unable to determine the force generated by the rod-like bacteria, the probability that the bacteria escape from the trap was determined. In these experiments, increased escape from the trap was associated with increased force generation. An escape event was defined as an event where the distance between the center of the trap and the bacterium was greater than 300 nm.
FIG 5.
Type IV pili interact with PelA deposited on a surface. (a) Sketch of setup (left) and typical sequence of trapped P. aeruginosa (right). Bacteria were incubated on a glass surface for 70 to 80 min. An escape was defined by displacement of the cell body by at least 300 nm from the center of the laser trap (e.g., event starting at 7 s). Bar, 2 μm. (b) Escape probabilities within 30 s of the ΔflgK, ΔroeA ΔflgK, ΔpelA ΔflgK, and ΔpelA ΔpilA ΔflgK mutants. Error bars, standard errors. (c) Distribution of times elapsed before escape for the ΔflgK, ΔroeA ΔflgK, and ΔpelA ΔflgK mutants.
The probability (P) (±standard deviation) of escaping from the trap within 30 s was 47% ± 5% for P. aeruginosa PA14 after preincubation with the wild-type EPS-producing strain of this microbe (Fig. 5b). The escape probability was not sampled for longer periods of time, because the bacteria tended to lose activity after 30 s of trapping. When the glass coverslide was not preincubated with bacteria (and thus lacked any deposited EPS), the escape probability was significantly reduced to 13% ± 5% (Fig. 5b).
The experiments discussed above are consistent with a model wherein, during preincubation, bacteria deposit EPS onto the coverslide; T4P can exert a force that is higher on the EPS-coated surface than on a plain glass surface. To test whether EPS secretion was indeed responsible for the different observed adhesion forces, we repeated the experiment with preincubation of the coverslide using a ΔpelA mutant strain. PelA is essential for production of the Pel exopolysaccharide (24, 25). We found that the escape probability of the pelA mutant was severely reduced to 20% ± 3% (Fig. 5b). Furthermore, for the cases where the bacterium escaped, the distribution of escape times was shifted toward considerably higher values (Fig. 5c, red line), indicating that T4P retraction confers a stronger interaction to Pel than to the glass surface.
The diguanylate cyclase RoeA upregulates Pel production (21). Correspondingly, deletion of roeA reduces the escape probability to 18% ± 3% (Fig. 5b) and shifts the escape time to higher values on par with what was observed for the pelA mutant (Fig. 5c). Finally, we confirmed that T4P were responsible for bacterial escape from the laser trap. No escape was observed when the gene encoding the major pilin subunit, pilA, was deleted (Fig. 5b). Together, our data indicate that the presence of EPS enhances the adhesion mediated by T4P.
DISCUSSION
Force generation by P. aeruginosa T4P.
By direct force measurements, we confirmed that T4P in P. aeruginosa are force-generating molecular motors. The speed of T4P retraction was previously determined by direct visualization of T4P retraction to be 0.5 μm/s at 29°C (12). Here, we measured 2-fold-higher velocities at 37°C (a physiologically relevant temperature) in the presence of low external force. With the different temperatures taken into account, the results are in reasonable agreement. Interestingly, the forces generated by T4P retraction in P. aeruginosa are considerably lower than those generated in N. gonorrhoeae and M. xanthus, where forces exceeding 100 pN were observed (9, 11). Replacing the gene encoding major pilin subunit pilE in N. gonorrhoeae by the major pilin of P. aeruginosa, PAK, supported high force generation in the range of 100 pN (37), suggesting that the difference is unrelated to the structure of the major pilin. We note that our experiments were conducted in standard lysogeny broth (LB) medium. We cannot exclude that other nutritional conditions support higher force generation in P. aeruginosa.
The twitching motility phenotypes of P. aeruginosa and N. gonorrhoeae show remarkable differences (38). The motility of P. aeruginosa has been described as slingshot-like, with periods of low velocity (0.3 μm/s) lasting for several seconds followed by short translations at velocities of 1 μm/s (39). In contrast, N. gonorrhoeae performs a random walk with directional persistence at a speed comparable to that of single T4P retraction (40). Since the motor forces (100 pN) are considerably higher than the detachment forces (on the order of 10 pN), the movement can be explained by a tug-of-war between multiple pili emanating in random directions (11). When the ratio between motor force and detachment force is decreased, trapping is observed for N. gonorrhoeae (41). As shown in this report, for P. aeruginosa, the motor forces are considerably lower, and therefore the prerequisite for a tug-of-war-like movement (motor forces much larger than detachment force) is most likely not fulfilled. In fact, detachment of T4P from the beads used for probing the motor properties was an unlikely process, indicating that the detachment forces were higher than the motor forces. Therefore, it was impossible to assess the force-dependent detachment force systematically.
The data presented here lead to a better understanding of the molecular mechanisms generating slingshot-like motility of P. aeruginosa. During the periods of slow movement, multiple T4P are likely attached and pulling in different directions, generating a force balance and resulting in only slow movement. Eventually, a T4P ruptures and perturbs the balance. As a result, the dominating T4P retracts at its typical speed of ∼1 μm/s, generating a rapid “twitching” movement.
The role of c-di-GMP in motor properties of T4P.
The level of c-di-GMP is an important determinant of the transition from the planktonic lifestyle to the biofilm state. For Escherichia coli, a direct connection between c-di-GMP levels and the speed of flagellar rotation has been identified through the role of a molecular brake that binds to the flagellar rotor in a c-di-GMP-dependent way (42). For P. aeruginosa, it is known that reversal frequencies of flagellar rotation are affected by the c-di-GMP level, but the molecular mechanism has not been identified (22, 23, 43). In terms of T4P, earlier reports showed that the level of piliation changes during biofilm formation through cAMP-dependent upregulation of the pilus machinery (3), but how the c-di-GMP level impacts T4P function had not been explored. Here, we modulated the levels of c-di-GMP by deleting genes that play roles in early biofilm formation and investigated their effect on T4P motor properties. We found no major changes in velocity or force generation when we decreased the production level of c-di-GMP by deleting diguanylate cyclase SadC or RoeA. The velocity was slightly increased (12%) by deleting roeA and decreased (by 14%) when roeA and sadC were deleted. The stalling force was decreased by 22% in a ΔroeA ΔsadC strain. While these effects were statistically significant, we consider them modest and unlikely to have a large impact on the function of T4P. Likewise, when the degradation of c-di-GMP was impaired by mutation of the BifA phosphodiesterase, the motor properties of T4P were unaffected. Mutations in sadC and bifA caused a defect in twitching motility (44). While the motor properties are largely unchanged, as shown in this study, the density of T4P may be affected, causing the defect in twitching motility. However, approximately 30 other proteins in P. aeruginosa are involved in the synthesis and/or degradation of c-di-GMP, including some which impact twitching motility (44). It is formally possible that one or more of these proteins impact motor function in a manner analogous to the molecular brake described above (42).
Putative role of EPS in T4P-mediated adhesion.
T4P mediate attachment of bacteria to abiotic surfaces (17, 41, 45, 46) and to host cells (17, 47). Moreover, T4P-T4P–mediated attractive forces control the shape, fusion, and sorting in microcolonies formed by N. gonorrhoeae (1, 48–51). Here, we provide evidence that the exopolysaccharide Pel enhances the surface adhesion mediated by T4P. The deposition of exopolysaccharides can influence the interaction between P. aeruginosa and surfaces in at least two ways. First, it has been reported that Pel is involved in surface attachment of P. aeruginosa PAO1 by generating short-ranged attractive forces (52). Thus, the presence of Pel might directly enhance bacterial interaction with a surface. Second, T4P may bind to EPS, thus enhancing the strength of attachment between the T4P and the surface. Consistent with this idea, for Myxococcus xanthus, direct interaction between T4P and its secreted slime material has been observed (53).
MATERIALS AND METHODS
Bacterial strains.
All strains used in this study were isogenic with the flagellum-defective mutant P. aeruginosa PA14 ΔflgK (Table 1). This mutant was used to eliminate any contribution of the flagellum to adhesion or the other assays performed here. As such, the ΔflgK mutant served as the positive control in all studies. All the strains were grown on lysogeny broth (LB) at 37°C. All the mutants used in this study contained a deletion of the coding region of the indicated gene, and they carried no antibiotic marker.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotypea | Source or reference |
|---|---|---|
| SMC5845 | ΔflgK | 52 |
| SMC6591 | ΔsadC ΔflgK | This study |
| SMC6592 | ΔroeA ΔflgK | This study |
| SMC6593 | ΔsadC ΔroeA ΔflgK | This study |
| SMC6594 | ΔbifA ΔflgK | This study |
| SMC7296 | ΔpelA ΔflgK | This study |
| SMC7297 | ΔpelA ΔpilA ΔflgK | This study |
All strains are mutants of P. aeruginosa PA14. Mutants bear a deletion of the coding region of the indicated gene(s), and these strains do not carry an antibiotic marker.
Construction of ΔflgK mutants.
In-frame deletion mutants of P. aeruginosa were constructed via allelic exchange as previously described (54). DNA fragments were amplified from P. aeruginosa PA14 genomic DNA by PCR to generate upstream and downstream fragments of the flgK gene with nucleotide tails complementary to plasmid pMQ30. The primer pairs flgK KO P1 (tgtaaaacgacggccagtgccaagcttgcatgcctgTCATGAACAGCCAGACCACC) and flgK KO P2 (TCTCTCGGTTAAGGCGCTCACATGGGTCAGTTCCTCCTTG) (upstream fragments) and flgK KO P3 (CAAGGAGGAACTGACCCATGTGAGCGCCTTAACCGAGAGA) and flgK KO P4 (ccatgattacgaattcgagctcggtacccggggatccACGGTATTGAGCAAGCTGCG) (downstream fragments) were used to generate the PCR fragments. The lowercase letters mark sequences complementary to the cloning vector pMQ30.
PCR products were cloned into pMQ30 by in vivo homologous recombination in Saccharomyces cerevisiae INVSc1 (Invitrogen) as previously described (54). The pMQ30-flgK deletion construct was transformed by electroporation into E. coli strain S17 and introduced into P. aeruginosa ΔsadC (strain SMC 4465), ΔbifA (SMC 3351), ΔroeA (SMC 3812), ΔsadC ΔroeA (SMC 3809), ΔpilA (SMC 3782), and ΔpelA (SMC 2893) mutants by conjugation. The ΔpilA ΔflgK ΔpelA mutant was built by introducing the pMQ30-pelA knockout construct (SMC 6955) into P. aeruginosa ΔpilA ΔflgK by conjugation. Integrants were isolated on gentamicin (20 μg/ml) and nalidixic acid (20 μg/ml), followed by sucrose counterselection. Resolved integrants were confirmed by PCR and sequencing.
T4P retraction assay.
Prior to laser tweezer experiments, bacteria were cultivated on agar plates (BD Bacto Agar; Fisher Scientific) containing LB (lysogeny broth; Roth) at 37°C for at least 8 h and then diluted in LB medium to an optical density (OD) of 0.002. Fifty microliters of the vortexed cell suspension was applied to polystyrene-coated coverslides. The diluted cells were incubated on the coverslides for an additional 30 min at 37°C. Subsequently, the medium was exchanged by a microsphere suspension of 2-μm carboxylated polystyrene beads (Invitrogen) before the sample was sealed. The observation time was limited to 20 min to avoid potential oxygen depletion.
Single pilus retraction events were measured with optical tweezers in position clamp and force clamp modes (30). In short, in position clamp mode, the T4P pulls the bead out of the center of the laser trap, and the optical restoring force increases linearly with the deflection. This mode is useful for determining the stall force but less convenient for determining speed, since the elastic properties of the T4P are nonlinear. In force clamp mode, software-based feedback clamps the deflection of the bead from the center of the trap at a constant position, thus keeping the force acting on the T4P constant. Pilus retraction events were analyzed in Matlab as previously described (31), but the parameters were adjusted to account for lower retraction velocities and stronger force dependence. In particular, a stalling event was detected when the velocity was lower than 250 nm/s for at least 500 ms. Prior to analysis, the data were down-sampled from 20 kHz to 10 Hz.
Oxygen depletion studies.
The effects of depletion of oxygen and proton motive force on T4P dynamics were assessed using the oxygen scavenger system protocatechuic acid (PCA)–protocatechuate-3,4-dioxygenase (PCD) and CCCP (carbonyl cyanide m-chlorophenylhydrazone), respectively, as described previously (32). To ensure that CCCP treatment did not affect the survival of P. aeruginosa, we treated cells with 50 μM CCCP for 15 min and subsequently plated them on agar plates. The number of CFU was comparable to the number of untreated cells.
Escape assay.
Prior to laser tweezer experiments, bacteria were cultivated overnight on agar plates containing LB at 37°C. Subsequently, bacteria were suspended in LB medium to an OD of 0.2 and incubated on coverslides for 70 to 80 min to ensure the secretion of exopolysaccharides onto the surface of the cover slides. Subsequently, the cells sitting on the cover slides were washed repeatedly (10 to 20 times) using a pipette and fresh medium to ensure cell densities feasible for the experimental procedure.
The characterization of bacterium-surface interaction followed a previously established protocol for N. gonorrhoeae (48, 49). While for N. gonorrhoeae it was possible to select for spherical monococci and thus measure the escape force (49), P. aeruginosa bacteria are rod shaped and thus align vertically within the laser trap. As their T4P reside at the cell pole, retraction causes tilting of the bacterial body with respect to the major axis of the laser trap; therefore, quantification of escape forces was not possible. Instead, we characterized the frequencies at which P. aeruginosa escaped for the laser trap. In short, bacteria moving close to the surface were trapped in the optical trap and placed 2 to 3 μm above the surface. A single bacterium was sampled for 30 s, since photodamage became obvious at longer periods of time. Time-lapse movies were recorded at 0.1 Hz. An escape event was defined as a displacement (d) of >300 nm from the center of the laser trap.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Lena Dewenter and Tom Cronenberg for experimental support and Gerard Wong, Ramin Golestanian, and members of the O'Toole and Maier labs for helpful discussions.
This work was supported by the Human Frontiers in Science Project through grant RGP0061 and by National Institutes of Health grant R37 AI83256-06 and the Munck-Pfefferkorn Fund (to G.A.O.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00859-16.
REFERENCES
- 1.Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol 50:61–68. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- 2.O'Toole GA, Wong GC. 2016. Sensational biofilms: surface sensing in bacteria. Curr Opin Microbiol 30:139–146. doi: 10.1016/j.mib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Y, Zhao K, Baker AE, Kuchma SL, Coggan KA, Wolfgang MC, Wong GC, O'Toole GA. 2015. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 6:e02456-14. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. 2015. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giltner CL, Nguyen Y, Burrows LL. 2012. Type IV pilin proteins: versatile molecular modules. Microbiol Mol Biol Rev 76:740–772. doi: 10.1128/MMBR.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YW, Rettberg LA, Treuner-Lange A, Iwasa J, Sogaard-Andersen L, Jensen GJ. 2016. Architecture of the type IVa pilus machine. Science 351:aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leighton TL, Buensuceso RN, Howell PL, Burrows LL. 2015. Biogenesis of Pseudomonas aeruginosa type IV pili and regulation of their function. Environ Microbiol 17:4148–4163. doi: 10.1111/1462-2920.12849. [DOI] [PubMed] [Google Scholar]
- 8.Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. 2002. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A 99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen M, Jakovljevic V, Sogaard-Andersen L, Maier B. 2009. High-force generation is a conserved property of type IV pilus systems. J Bacteriol 191:4633–4638. doi: 10.1128/JB.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merz AJ, So M, Sheetz MP. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 11.Marathe R, Meel C, Schmidt NC, Dewenter L, Kurre R, Greune L, Schmidt MA, Muller MJ, Lipowsky R, Maier B, Klumpp S. 2014. Bacterial twitching motility is coordinated by a two-dimensional tug-of-war with directional memory. Nat Commun 5:3759. doi: 10.1038/ncomms4759. [DOI] [PubMed] [Google Scholar]
- 12.Skerker JM, Berg HC. 2001. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A 98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly NM, Kluftinger JL, Pasloske BL, Paranchych W, Hancock RE. 1989. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun 57:3841–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speert DP, Loh BA, Cabral DA, Salit IE. 1986. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun 53:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley DE. 1972. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun 47:142–149. doi: 10.1016/S0006-291X(72)80021-4. [DOI] [PubMed] [Google Scholar]
- 16.Inclan YF, Persat A, Greninger A, Von Dollen J, Johnson J, Krogan N, Gitai Z, Engel JN. 2016. A scaffold protein connects type IV pili with the Chp chemosensory system to mediate activation of virulence signaling in Pseudomonas aeruginosa. Mol Microbiol 101:590–605. doi: 10.1111/mmi.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaussart A, Baker AE, Kuchma SL, El-Kirat-Chatel S, O'Toole GA, Dufrene YF. 2014. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV pili. ACS Nano 8:10723–10733. doi: 10.1021/nn5044383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siryaporn A, Kuchma SL, O'Toole GA, Gitai Z. 2014. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci U S A 111:16860–16865. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchma SL, Griffin EF, O'Toole GA. 2012. Minor pilins of the type IV pilus system participate in the negative regulation of swarming motility. J Bacteriol 194:5388–5403. doi: 10.1128/JB.00899-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuchma SL, Ballok AE, Merritt JH, Hammond JH, Lu W, Rabinowitz JD, O'Toole GA. 2010. Cyclic-di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa: the pilY1 gene and its impact on surface-associated behaviors. J Bacteriol 192:2950–2964. doi: 10.1128/JB.01642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merritt JH, Ha DG, Cowles KN, Lu W, Morales DK, Rabinowitz J, Gitai Z, O'Toole GA. 2010. Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. mBio 1:e00183-10. doi: 10.1128/mBio.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt JH, Brothers KM, Kuchma SL, O'Toole GA. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J Bacteriol 189:8154–8164. doi: 10.1128/JB.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, O'Toole GA. 2007. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman L, Kolter R. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol 186:4457–4465. doi: 10.1128/JB.186.14.4457-4465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman L, Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690. [DOI] [PubMed] [Google Scholar]
- 26.Matsukawa M, Greenberg EP. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol 186:4449–4456. doi: 10.1128/JB.186.14.4449-4456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol 186:4466–4475. doi: 10.1128/JB.186.14.4466-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao K, Tseng BS, Beckerman B, Jin F, Gibiansky ML, Harrison JJ, Luijten E, Parsek MR, Wong GC. 2013. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 497:388–391. doi: 10.1038/nature12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelimson A, Zhao K, Lee CK, Kranz WT, Wong GC, Golestanian R. 2016. Multicellular self-organization of P. aeruginosa due to interactions with secreted trails. Phys Rev Lett 117:178102. doi: 10.1103/PhysRevLett.117.178102. [DOI] [PubMed] [Google Scholar]
- 30.Clausen M, Koomey M, Maier B. 2009. Dynamics of type IV pili is controlled by switching between multiple states. Biophys J 96:1169–1177. doi: 10.1016/j.bpj.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurre R, Maier B. 2012. Oxygen depletion triggers switching between discrete speed modes of gonococcal type IV pili. Biophys J 102:2556–2563. doi: 10.1016/j.bpj.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurre R, Kouzel N, Ramakrishnan K, Oldewurtel ER, Maier B. 2013. Speed switching of gonococcal surface motility correlates with proton motive force. PLoS One 8:e67718. doi: 10.1371/journal.pone.0067718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol 65:1474–1484. doi: 10.1111/j.1365-2958.2007.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min TL, Mears PJ, Chubiz LM, Rao CV, Golding I, Chemla YR. 2009. High-resolution, long-term characterization of bacterial motility using optical tweezers. Nat Methods 6:831–835. doi: 10.1038/nmeth.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horner F, Woerdemann M, Muller S, Maier B, Denz C. 2010. Full 3D translational and rotational optical control of multiple rod-shaped bacteria. J Biophotonics 3:468–475. doi: 10.1002/jbio.201000033. [DOI] [PubMed] [Google Scholar]
- 37.Winther-Larsen HC, Wolfgang MC, van Putten JP, Roos N, Aas FE, Egge-Jacobsen WM, Maier B, Koomey M. 2007. Pseudomonas aeruginosa type IV pilus expression in Neisseria gonorrhoeae: effects of pilin subunit composition on function and organelle dynamics. J Bacteriol 189:6676–6685. doi: 10.1128/JB.00407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier B, Wong GC. 2015. How bacteria use type IV pili machinery on surfaces. Trends Microbiol 23:775–788. doi: 10.1016/j.tim.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Jin F, Conrad JC, Gibiansky ML, Wong GC. 2011. Bacteria use type-IV pili to slingshot on surfaces. Proc Natl Acad Sci U S A 108:12617–12622. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holz C, Opitz D, Greune L, Kurre R, Koomey M, Schmidt MA, Maier B. 2010. Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys Rev Lett 104:178104. doi: 10.1103/PhysRevLett.104.178104. [DOI] [PubMed] [Google Scholar]
- 41.Zaburdaev V, Biais N, Schmiedeberg M, Eriksson J, Jonsson AB, Sheetz MP, Weitz DA. 2014. Uncovering the mechanism of trapping and cell orientation during Neisseria gonorrhoeae twitching motility. Biophys J 107:1523–1531. doi: 10.1016/j.bpj.2014.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Guttenplan SB, Kearns DB. 2013. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev 37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ha DG, Richman ME, O'Toole GA. 2014. Deletion mutant library for investigation of functional outputs of cyclic diguanylate metabolism in Pseudomonas aeruginosa PA14. Appl Environ Microbiol 80:3384–3393. doi: 10.1128/AEM.00299-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu S, Giuliani M, Harvey H, Burrows LL, Wickham RA, Dutcher JR. 2015. Nanoscale pulling of type IV pili reveals their flexibility and adhesion to surfaces over extended lengths of the pili. Biophys J 108:2865–2875. doi: 10.1016/j.bpj.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holz C, Opitz D, Mehlich J, Ravoo BJ, Maier B. 2009. Bacterial motility and clustering guided by microcontact printing. Nano Lett 9:4553–4557. doi: 10.1021/nl903153c. [DOI] [PubMed] [Google Scholar]
- 47.Merz AJ, So M. 1997. Attachment of piliated, Opa- and Opc-gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun 65:4341–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dewenter L, Volkmann TE, Maier B. 2015. Oxygen governs gonococcal microcolony stability by enhancing the interaction force between type IV pili. Integr Biol (Camb) 7:1161–1170. doi: 10.1039/C5IB00018A. [DOI] [PubMed] [Google Scholar]
- 49.Oldewurtel ER, Kouzel N, Dewenter L, Henseler K, Maier B. 2015. Differential interaction forces govern bacterial sorting in early biofilms. Elife 4:e10811. doi: 10.7554/eLife.10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higashi DL, Lee SW, Snyder A, Weyand NJ, Bakke A, So M. 2007. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect Immun 75:4743–4753. doi: 10.1128/IAI.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber CA, Lin YT, Biais N, Zaburdaev V. 2015. Formation and dissolution of bacterial colonies. Phys Rev E Stat Nonlin Soft Matter Phys 92:032704. doi: 10.1103/PhysRevE.92.032704. [DOI] [PubMed] [Google Scholar]
- 52.Cooley BJ, Thatcher TW, Hashmi SM, L'Her G, Le HH, Hurwitz DA, Provenzano D, Touhami A, Gordon VD. 2013. The extracellular polysaccharide Pel makes the attachment of P. aeruginosa to surfaces symmetric and short-ranged. Soft Matter 9:3871–3876. doi: 10.1039/c3sm27638d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc Natl Acad Sci U S A 100:5443–5448. doi: 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.