Abstract
Giant pheochromocytomas are rare silent entities that do not present with the classical symptoms commonly seen in catecholamine-secreting tumors. In many cases they are accidentally discovered. The algorithm to diagnose a pheochromocytoma consists of biochemical evaluation and imaging of a retroperitoneal mass. The female patient in this case report presented with a palpable abdominal mass and was cured with surgical resection. She suffered no recurrence or complications on follow-up. The left retroperitoneal mass measured 27 × 18 × 12 cm and weighed 3,315 grams. Biochemical, radiological, and pathological examinations confirmed the diagnosis of a pheochromocytoma. In this paper, we report on our experience treating this patient and provide a summary of all giant pheochromocytomas greater than 10 cm reported to date in English language medical journals. Our patient's giant cystic pheochromocytoma was the fourth heaviest and fifth largest maximal diameter identified using our literature search criteria. Additionally, this tumor had the largest maximal diameter of all histologically confirmed benign/low metastatic risk pheochromocytomas. Giant cystic pheochromocytomas are rare entities requiring clinical suspicion coupled with strategic diagnostic evaluation to confirm the diagnosis.
1. Introduction
A pheochromocytoma (PCC) is a rare catecholamine-secreting tumor that originates from the chromaffin cells of the adrenal medulla. First described by Frankel [1] in 1886, the estimated worldwide incidence of these tumors is 2 to 8 per million persons per year [2]. Classical symptoms at presentation include severe hypertension with associated headaches, sweating, and palpitations; however, 20–30% of patients remain asymptomatic. Asymptomatic PCCs are typically detected as an incidental adrenal mass on routine screening. Biochemical evidence of elevated plasma free metanephrines provides the highest sensitivity for PCC diagnosis [3]. Most of these lesions are benign/low metastatic potential but histopathological characteristics defined by the pheochromocytoma of the adrenal gland scaled score (PASS) can identify tumors with potentially more aggressive biological behavior such as the presence of chromaffin tissue at extra adrenal sites. Giant PCCs are generally classified as those with maximal diameter greater than 10 cm. They are commonly asymptomatic and are diagnosed incidentally on imaging. Surgical resection is the standard treatment option and is usually curative, preventing future potentially lethal complications of these lesions. We present the case of a 50-year-old female patient with a left side adrenal PCC which was treated successfully with open surgical resection in the surgical unit of Eric Williams Medical Sciences Complex, Trinidad and Tobago.
2. Case Presentation
A 50-year-old East Indian woman with a 6-month history of lower back pain, fatigue, and unintentional weight loss was referred to our surgical outpatient clinic upon detection of a large abdominal mass during an abdominal ultrasound. The mass was located in the left upper quadrant and thought to be possibly splenic or renal in origin. Physical examination revealed a large nontender lump arising from the left upper quadrant and crossing the midline. Initial laboratory investigations were unremarkable except for microcytic anemia with a mean corpuscular hemoglobin concentration (MCHC) of 7.3 g/dL. The patient had no history of hypertension, headache, palpitations, or excessive sweating and no family history of cancer.
Computed tomography (CT) revealed a 23.2 × 17.6 × 13.6 cm mass with predominant areas of necrosis, punctate areas of calcification, and irregular contours superior to the left kidney (Figures 1 and 2). The mass displaced the pancreas anteriorly and compressed the left kidney inferiorly. The nonvisualization of a normal left adrenal gland strongly suggested an adrenocortical carcinoma. The liver, spleen, right adrenal gland, and right kidney were normal. Despite mesenteric and para-aortic lymphadenopathy there was no evidence of distant metastasis. Preoperative biopsy was not performed, due to the risk of tumor seeding along the biopsy path.
Figure 1.
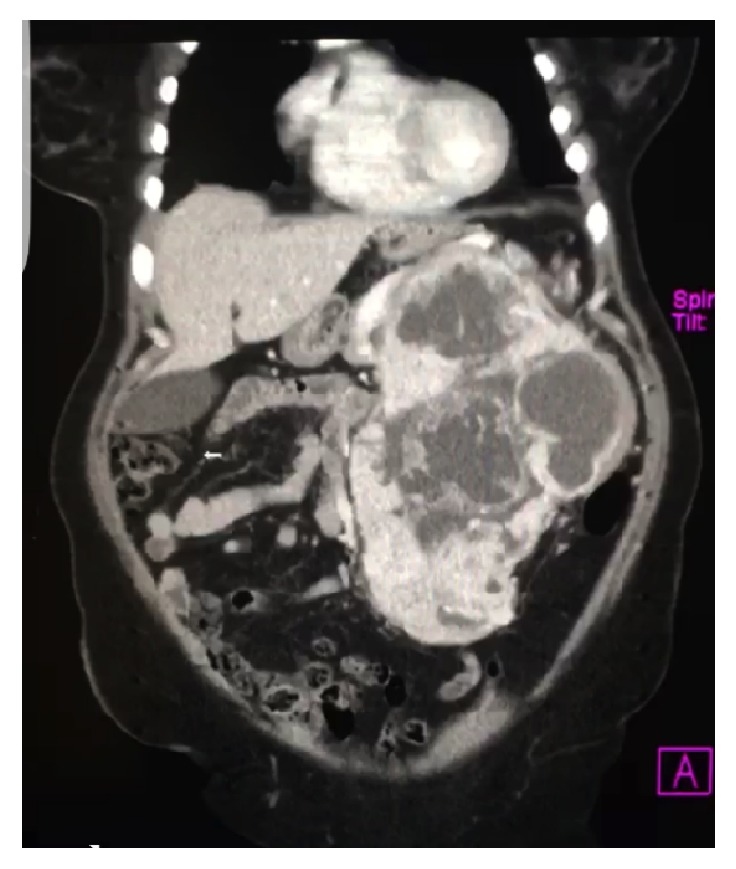
CT scan showing a 23 cm, thick-walled, multicystic mass occupying most of the left upper quadrant of the abdomen.
Figure 2.
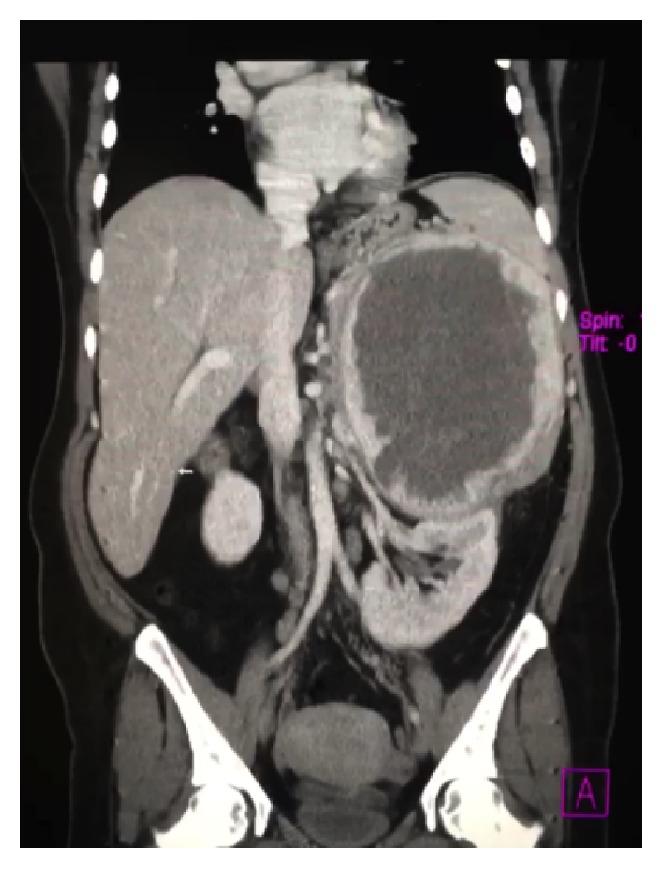
Coronal CT image demonstrating the mass displacing the left kidney inferiorly. Evidence of locally invasive disease was not present.
The tumor markers carcinoembryonic antigen, carbohydrate antigen (CA) 15-3, CA 19-9, and CA 125 were normal. The differential diagnosis included adrenocortical carcinoma, pheochromocytoma, myelolipoma, metastasis from an unidentified primary tumor, sarcoma, and lymphoma. Biochemical investigations were performed to exclude a functional adrenal mass, and the diagnosis of pheochromocytoma was made upon observation of elevated plasma free metanephrines, urine catecholamines, and their metabolites (Table 1).
Table 1.
Biochemical investigations confirming the pheochromocytoma diagnosis.
| Patient values | Normal values | |
|---|---|---|
| Test (fractionated plasma) | ||
| Metanephrine | 259 pg/mL | <58 pg/mL |
| Normetanephrine | 4603 pg/mL | <149 pg/mL |
| Total metanephrine | 4862 pg/mL | <206 pg/mL |
| Epinephrine | 16 pg/mL | <84 pg/mL |
| Norepinephrine | 509 pg/mL | <420 pg/mL |
| Dopamine | <30 pg/mL | <60 pg/mL |
| Total catecholamines | 525 pg/mL | <504 pg/mL |
| Test (24-hour urine values) | ||
| Total catecholamines | 131 ug/24 hr | <100 ug/24 hr |
| Norepinephrine | 120 ug/24 hr | <80 ug/24 hr |
| Epinephrine | 11 ug/24 hr | <20 ug/24 hr |
| Dopamine | 466 ug/24 hr | <500 ug/24 hr |
| Vanillylmandelate (VMA) | 88.3 ug/24 hr | 3.8–6.7 mg/24 hr |
| Creatinine | 1.47 ug/24 hr | 0.63–2.50 g/24 hr |
Preoperatively, the patient was transfused with packed red blood cells to stable hemoglobin of 10 g/dL. Adequate catecholamine blockade was achieved, after medical consultation using the alpha adrenergic blocker, terazosin (Hytrin). An open left adrenalectomy was performed through a Chevron incision extending to the left flank. The mass was completely resected en bloc with the spleen, distal pancreas, left kidney, and a 2 cm area of the left hemidiaphragm (Figure 3). Intraoperatively, there were significant fluctuations in the patient's blood pressure, which was well managed by the surgical team. There were no other significant surgical complications and the patient made an uneventful recovery prior to discharge on postoperative day 11. Three months later, at the time of this report, the patient remains stable and disease-free. Given that there is recurrence in approximately 10% of these cases, long-term follow-up with CT scans and hematologic monitoring is warranted.
Figure 3.
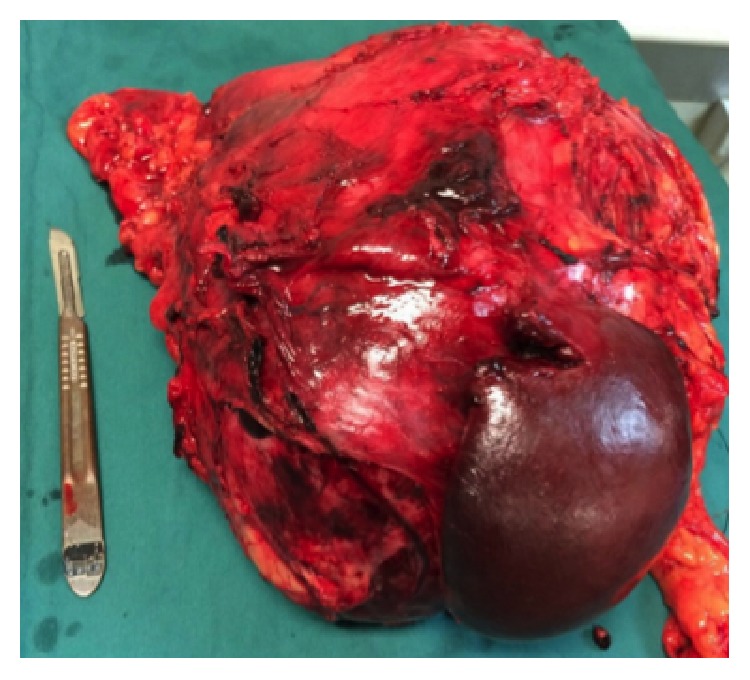
En bloc resection of the left adrenal mass, pancreatic tail, spleen, and left kidney.
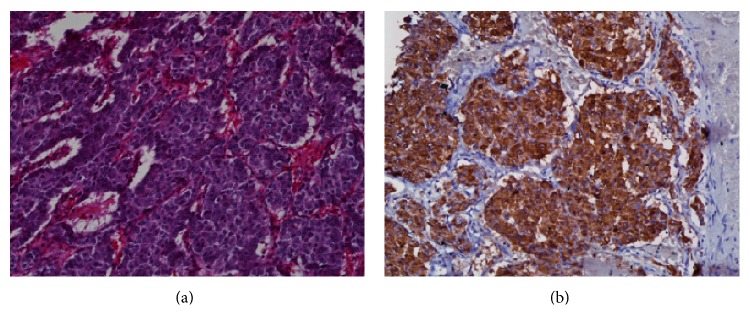
Pathological examination of the surgical specimen revealed a circumscribed mass, surrounded by a variably thick fibrous capsule/pseudocapsule with relatively scantly attached fat, measuring 27 × 18 × 12 cm and weighing 3,315 g (Figure 3). Residual normal-appearing adrenal gland parenchyma was present, while neither infiltration of the surrounding fat, vascular invasion, nor confluent tumor necrosis was identified. Mitoses were rare (<1 per high-power field). Histological sections revealed typical morphological features of PCC predominantly characterized by nests of plump tumor cells with abundant basophilic granular cytoplasm surrounded by sustentacular cells (Figure 4(a)). No unfavorable features such as diffuse growth pattern, increased cellularity, or increased pleomorphism were observed. The spleen, distal pancreas, and kidney did not show any pathologic abnormalities. Immunohistochemical staining revealed that the tumor cells were diffusely positive for chromogranin A (Figure 4(b)), while there was no significant highlighting of sustentacular cells by S-100. Of note, the lack of prominent staining of sustentacular cells by S-100 has been suggested to be predictive of nonfamilial sporadic PCC [38].
Figure 4.
Microscopic images of pheochromocytoma. (a) Routine hematoxylin and eosin (H&E) staining at low magnification (20x) highlights the typical “Zellballen” growth pattern characterized by nests of tumor cells surrounded by delicate fibrovascular stroma. (b) Immunohistochemistry for the neuroendocrine marker chromogranin A shows strong, diffuse staining in the tumor cells.
To determine the clinicopathological features of giant PCCs, an extensive literature search of the PubMed/MEDLINE and Embase databases was conducted. Search terms included “giant pheochromocytoma”, “cystic giant pheochromocytoma”, “English language” and “case reports”. One case in which the tumor lacked dimensions but was found to be one of the heaviest PCCs recorded was included. Full texts were accessed to confirm eligibility for inclusion. A summary of the 36 final cases are presented in Table 2. Relative to the others, our patient's tumor was the fourth heaviest with the fifth greatest maximal diameter and the largest histologically confirmed pheochromocytoma with a low risk of malignancy/benign classification. Unlike patients with classical symptoms, those with giant PCCs may be asymptomatic as occurred in 31% (=11) of the cases. Twenty-two % (=8) and 17% (=6) of the cases presented with hypertension and back/abdominal pain, respectively. Only one case had evidence of metastasis at the time of diagnosis, characterized by invasion of the right lobe of the liver [5]. Twenty-two percent (=8) of the cases presented at similar locations, commonly in the left abdomen. The mean age at diagnosis was 49.46 years (range 12–85 years) which was the age of our patient.
Table 2.
A summary of reported giant pheochromocytomas with maximal diameter greater than 10 cm, arranged by largest to smallest maximum diameter. The weight was not recorded in many of the papers.
| Author/year | Sex/age | Country | Size (cm)/weight (g) | Location | Presentation | Histopathological evaluation | Metastasis |
|---|---|---|---|---|---|---|---|
| Grissom et al./1979 [4] | F/54 | USA | 45 × 25/3000+ | Left abdomen | Asymptomatic | Unknown | None |
| Costa et al./2008 [5] | M/46 | Brazil | 30/unknown | Right adrenal | Abdominal pain | Malignant | Liver |
| Basso et al./1996 [6] | M/47 | Italy | 29 × 21 × 12/4050 | Left abdomen | Asymptomatic | Malignant | None |
| Karumanchery et al./2012 [7] | F/85 | England | 28 × 16 × 13/2300 | Left abdomen | Lower back pain | Unknown | None |
| Current case | F/50 | Trinidad & Tobago | 27 × 18 × 12/3315 | Left abdomen | Lower back pain | Low risk of malignancy | None |
| Gupta et al./2016 [8] | F/65 | India | 25 × 17 × 15/2750 | Left abdomen | Asymptomatic | Benign | None |
| Okuda et al./2013 [9] | F/43 | Japan | 24 × 23 × 16/5900 | Abdomen | Vulva edema | Unknown | None |
| Suga et al./2000 [10] | M/48 | Japan | 21 × 13 × 21/3900 | Left abdomen | Asymptomatic | Unknown | None |
| Terk et al./1993 [11] | M/35 | USA | 21 × 20 × 11/2870 | Organ of Zuckerkandl | Disproportionate abdominal girth, hypertension | Unknown | None |
| Soufi et al./2012 [12] | F/17 | India | 21 × 15 | Right upper abdomen | Asymptomatic | Malignant | None |
| Arcos et al./2009 [13] | F/36 | Canada | 21 × 17 × 11 | Left abdomen | Lower back pain | Malignant | Lymph node |
| Melegh et al./2002 [14] | M/55 | Hungary | 20/unknown | Left renal hilus | Asymptomatic | Unknown | None |
| Korgali et al./2014 [15] | M/63 | Turkey | 20 × 17 × 9/1736 | Left adrenal gland | Chest pain, sweating, nausea | Malignant | Rib |
| Ambati et al./2014 [16] | F/77 | Canada | 19 × 18 × 12/2460 | Right retroperitoneum | Dyspnea | Benign | None |
| Pan et al./2008 [17] | M/46 | USA | 18 × 14 × 13/1450 | Left abdomen | Episodic hypertension and headache | Unknown | Unknown |
| Uysal et al./2015 [18] | M/37 | Turkey | 18 × 8 × 13 | Left abdomen | Hypertension | Malignant | Multiorgan∗ |
| Sharma/2006 [19] | M/55 | India | 17 × 12/850 | Right adrenal gland | Asymptomatic | Benign | None |
| Daughtry et al./1977 [20] | M/53 | USA | 17/1150 | Unknown | Mild hypertension | Unknown | Unknown |
| Gupta et al./2016 [8] | M/40 | India | 16.4 × 14 /1836 | Left thyroid gland | Severe hypertension | Unknown | Unknown |
| Costa et al./2008 [5] | F/43 | Brazil | 16 | Right upper abdomen | Abdominal pain | Malignant | Unknown |
| Jain and Agarwal /2002 [21] | F/26 | India | 16 × 11 | Left abdomen | Asymptomatic | Malignant | Unknown |
| Wu et al./2000 [22] | F/49 | USA | 15 × 12 × 12 | Right upper abdomen | Asymptomatic | Unknown | Unknown |
| Santarone et al./2008 [23] | F/81 | Italy | 13 | Right upper abdomen | Hypertension, palpitation, sweating | Unknown | Unknown |
| Sundahl et al./2016 [24] | F/54 | Sweden | 12.5 × 10 × 3/204 | Right adrenal gland | Asymptomatic | Benign | None |
| Zhu et al./2014 [25] | F/67 | Japan | 12.1 × 10.8 | Left adrenal gland | Dizziness, vomiting, and stomachache | Unknown | None |
| Ikegami et al./2009 [26] | M/47 | Japan | 12 × 10 | Abdomen | Back pain | Unknown | Unknown |
| Li et al./2012 [27] | M/56 | Canada | 12 × 11 × 11 | Right upper abdomen | Progressive weight loss and nausea | Benign | None |
| Kakoki et al./2015 [28] | M/70 | Japan | 12 × 11 × 8/530 | Left adrenal gland | Ileus after hypertension medication | Benign | None |
| Schnakenburg et al./1976 [29] | M/12 | Ukraine | 12 × 10 × 9/1100 | Right abdomen | Hemihypertrophy | Malignant | Lung, brain |
| Filippou et al./2003 [30] | M/70 | Greece | 12 × 8 × 10 | Left abdomen | Asymptomatic | Malignant | None |
| Sarveswaran et al./2015 [31] | F/59 | India | 11.2 × 9.6 × 9.8 | Right suprarenal region | Upper right abdominal discomfort | Unknown | Unknown |
| Chan et al./2000 [32] | M/63 | China | 11 × 6.6 × 11 | Right suprarenal region | Asymptomatic | Malignant | Bone, lung |
| Awada et al./2003 [33] | F/26 | USA | 11 × 10 × 9 | Right adrenal gland | Dyspnea, paresthesia, chest pain, palpitation | Unknown | Unknown |
| Goldberg et al./2011 [34] | F/27 | Canada | 10.5 × 10.6 | Right adrenal gland region | Headaches, episodic palpitations, pallor | Unknown | Unknown |
| Antedomenico and Wascher/2005 [35] | F/39 | USA | 10.5/782 | Left upper abdomen | Abdominal pain | Unknown | Unknown |
| Wang et al./2015 [36] | F/36 | China | 10.3 × 9.3 | Left upper abdomen | Abdominal pain | Unknown | Unknown |
| Basiri and Radfar/2010 [37] | M/53 | Iran | Unknown/3150 | Left abdomen | Abdominal pain | Benign | None |
M, male; F, female. ∗Liver, lymph nodes, right adrenal gland, lungs, and bones.
3. Discussion
Pheochromocytomas are neoplasms that arise from the chromaffin cells of the sympathoadrenal system [39]. Eighty-five percent of these lesions arise in the adrenal medulla [40]. Sporadic cases of PCC usually present in the fourth to fifth decades of life. Various hereditary conditions such as multiple endocrine neoplasia 2A and 2B, Von Hippel-Lindau syndrome, and neurofibromatosis 1 are associated with increased risk for PCC [41]. There are no known environmental, dietary, or lifestyle risk factors that impact the risk of developing PCC. The classic tetrad of symptoms consists of palpitations, headaches, sweating, and hypertension. However, approximately 49–57% of PCC patients are asymptomatic with an adrenal mass being detected incidentally during unrelated imaging.
The malignant potential of a PCC cannot be determined preoperatively unless there is evidence of local invasion or metastases at the time of diagnosis. Previously, it was believed that size was a predictor of malignant potential; however, with numerous case reports of giant benign PCCs, size can no longer be used as a definitive indicator of aggressive disease [42]. Histologically, the PASS is referenced to distinguish tumors of high from those with a low risk of malignancy [43]. This scoring system assesses vascular invasion, capsular invasion, extension into the periadrenal adipose tissue, the presence of focal or confluent necrosis, high cellularity, tumor cell spindling, cellular monotony, >3 mitoses per high-power field, atypical mitotic figures, profound nuclear pleomorphism, and increased tumor cell hyperchromasia [43]. Biologically aggressive tumors have been found to have a PASS ≥ 4 whereas lesions with a low risk of malignancy have a PASS < 4. Our patient harbored a giant PCC with a low risk of malignancy as noted by the PASS of 2.
Approximately 20 to 30% of all PCCs are clinically silent [12]. Factors that contribute to the lack of symptoms include extensive necrosis of the adrenal gland, decreasing the production of catecholamines and the retention of these hormones within the capsular mass after secretion. Consequently, the time to diagnosis is delayed and tumor size tends to be larger once it is detected. A recent report that reviewed 20 cases of PCCs larger than 10 cm reported that 13 presented with abdominal pain with only 5 presenting with any of the classical symptoms of PCCs [16].
Surgical resection is the only curative option for these giant lesions. Laparoscopic adrenalectomy is considered safe and effective for tumors up to 12 cm in its greatest dimensions. However, in the realm of these giant PCCs, open en bloc resection is required. Intraoperative manipulation of these tumors is frequently associated with profound hypertension. However, early isolation of the tumor's venous drainage decreases the risk of intraoperative hypertensive crises [36]. This must be coupled with catecholamine blockade and intravenous fluids to diminish the risk of postoperative hypotension. It is therefore critical to have good coordination between the anesthesiologist and surgeon before and during surgery. Intensive care monitoring is crucial for at least 24 hours postoperatively as it is common for patients to experience fluctuations in blood pressure and heart rate as well as hypoglycemia. Pheochromocytomas have an excellent prognosis, with 5-year survival exceeding 95% in benign tumors and a recurrence rate of less than 10% [44]. Statistical data are not available for malignant PCCs due to their low incidence.
4. Conclusion
In this report, we presented the case of a 50-year-old female with a giant PCC that was the fourth heaviest and had the fifth largest maximal diameter of all reported PCCs. Additionally, using our search criteria, the tumor had the largest maximal diameter of reported, histologically confirmed PCCs with a low risk of malignancy. Our patient, 3 months after resection of the tumor, remains stable and disease-free. Giant PCCs do not present with the classical symptoms associated with smaller PCCs and are usually associated with a lower risk of malignancy. Previously, larger size was believed to be an indicator of malignancy in these adrenal lesions; however, upon review of the 10 largest known PCCs, this belief was unsubstantiated.
Acknowledgments
Wayne A. Warner was supported by Washington University School of Medicine, St. Louis (Grant no. GSAS/CGFP, Fund 94028C).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bravo E. L., Gifford R. W., Jr. Pheochromocytoma: diagnosis, localization and management. The New England Journal of Medicine. 1984;311(20):1298–1303. doi: 10.1056/nejm198411153112007. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre M., Foulkes W. D. Pheochromocytoma and paraganglioma syndromes: genetics and management update. Current Oncology. 2014;21(1):e8–e17. doi: 10.3747/co.21.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen-Martin M. A., Hammer G. D. Pheochromocytoma: an update on risk groups, diagnosis, and management. Hospital Physician. 2006;42(2):17–24. [Google Scholar]
- 4.Grissom J. R., Yamase H. T., Prosser P. R. Giant pheochromocytoma with sarcoidosis. Southern Medical Journal. 1979;72(12):1605–1607. doi: 10.1097/00007611-197912000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Costa S. R. P., Cabral N. M., Abhrão A. T., da Costa R. B., da Silva L. M., Lupinacci R. A. Giant cystic malignant pheochromocytoma invading right hepatic lobe: report on two cases. Sao Paulo Medical Journal. 2008;126(4):229–231. doi: 10.1590/s1516-31802008000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basso L., Lepre L., Melillo M., Fora F., Mingazzini P. L., Tocchi A. Giant phaeochromocytoma: case report. Irish Journal of Medical Science. 1996;165(1):57–59. doi: 10.1007/bf02942808. [DOI] [PubMed] [Google Scholar]
- 7.Karumanchery R., Nair J. R., Hakeem A., Hardy R. An unusual case of back pain: a large Pheochromocytoma in an 85 year old woman. International Journal of Surgery Case Reports. 2012;3(1):16–18. doi: 10.1016/j.ijscr.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Bains L., Agarwal M. K., Gupta R. Giant cystic pheochromocytoma: a silent entity. Urology Annals. 2016;8(3):384–386. doi: 10.4103/0974-7796.184886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda K., Nishizawa T., Oshima T., Misawa K. A case of cystic pheochromocytoma weighing 5,900 g. Nihon Rinsho Geka Gakkai Zasshi. 2013;74(4):1075–1080. doi: 10.3919/jjsa.74.1075. [DOI] [Google Scholar]
- 10.Suga K., Motoyama K., Hara A., Kume N., Ariga M., Matsunaga N. Tc-99m MIBG imaging in a huge clinically silent pheochromocytoma with cystic degeneration and massive hemorrhage. Clinical Nuclear Medicine. 2000;25(10):796–800. doi: 10.1097/00003072-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Terk M. R., de Verdier H., Colletti P. M. Giant extra-adrenal pheochromocytoma: magnetic resonance imaging with gadolinium-DTPA enhancement. Magnetic Resonance Imaging. 1993;11(1):47–50. doi: 10.1016/0730-725x(93)90410-f. [DOI] [PubMed] [Google Scholar]
- 12.Soufi M., Lahlou M. K., Benamr S., et al. Giant malignant cystic pheochromocytoma: a case report. Indian Journal of Surgery. 2012;74(6):504–506. doi: 10.1007/s12262-012-0719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcos C. T., Luque V. R., Luque J. A., García P. M., Jiménez A. B., Muñoz M. M. Malignant giant pheochromocytoma: a case report and review of the literature. Journal of the Canadian Urological Association. 2009;3(6):E89–E91. doi: 10.5489/cuaj.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melegh Z., Rényi-Vámos F., Tanyay Z., Köves I., Orosz Z. Giant cystic pheochromocytoma located in the renal hilus. Pathology Research and Practice. 2002;198(2):103–106. doi: 10.1078/0344-0338-00194. [DOI] [PubMed] [Google Scholar]
- 15.Korgali E., Dundar G., Gokce G., et al. Giant malignant pheochromocytoma with palpable rib metastases. Case Reports in Urology. 2014;2014:4. doi: 10.1155/2014/354687.354687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambati D., Jana K., Domes T. Largest pheochromocytoma reported in Canada: a case study and literature review. Canadian Urological Association Journal. 2014;8(5-6):E374–E377. doi: 10.5489/cuaj.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Z., Repertinger S., Deng C., Sharma P. A giant cystic pheochromocytoma of the adrenal gland. Endocrine Pathology. 2008;19(2):133–138. doi: 10.1007/s12022-008-9016-4. [DOI] [PubMed] [Google Scholar]
- 18.Uysal E., Kirdak T., Gürer A. O., İkidağ M. A. Giant multicystic malignant pheochromocytoma. Turkish Journal of Surgery. 2015 doi: 10.5152/ucd.2015.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P. P. Pheochromocytoma: a composite resection. Indian Journal of Surgery. 2006;68(3):163–164. [Google Scholar]
- 20.Daughtry J. D., Susan L. P., Straffon R. A., Stewart B. H. A case of a giant pheochromocytoma. Journal of Urology. 1977;118(5):840–842. doi: 10.1016/s0022-5347(17)58215-4. [DOI] [PubMed] [Google Scholar]
- 21.Jain S. K., Agarwal N. Asymptomatic giant pheochromocytoma. Journal of Association of Physicians of India. 2002;50(6):842–844. [PubMed] [Google Scholar]
- 22.Wu J., Ahya S. N., Reploeg M. D., et al. Pheochromocytoma presenting as a giant cystic tumor of the liver. Surgery. 2000;128(3):482–484. doi: 10.1067/msy.2000. [DOI] [PubMed] [Google Scholar]
- 23.Santarone M., Borghi C., Miglierina E., Senatore S., Corrado G. Giant cystic pheochromocytoma. Journal of Cardiovascular Medicine. 2008;9(9):971–972. doi: 10.2459/JCM.0b013e328303698b. [DOI] [PubMed] [Google Scholar]
- 24.Sundahl N., Van Slycke S., Brusselaers N. A rare case of clinically and biochemically silent giant right pheochromocytoma: case report and review of literature. Acta Chirurgica Belgica. 2016;116(4):239–242. doi: 10.1080/00015458.2016.1139838. [DOI] [PubMed] [Google Scholar]
- 25.Zhu D., Yu J., Li X., Jiang X., Zhuang C. Takotsubo-like cardiomyopathy in a giant pheochromocytoma. International Journal of Cardiology. 2014;176(3):e113–e116. doi: 10.1016/j.ijcard.2014.07.240. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami Y., Tsukada Y., Abe M., Abe Y., Tase C. Delayed shock after minor blunt trauma due to myocarditis caused by occult giant pheochromocytoma. The Journal of Trauma: Injury, Infection, and Critical Care. 2009;67(3):E65–E68. doi: 10.1097/ta.0b013e31802b9587. [DOI] [PubMed] [Google Scholar]
- 27.Li C., Chen Y., Wang W., Teng L. A case of clinically silent giant right pheochromocytoma and review of literature. Canadian Urological Association Journal. 2012;6(6):E267–E269. doi: 10.5489/cuaj.11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakoki K., Miyata Y., Shida Y., et al. Pheochromocytoma multisystem crisis treated with emergency surgery: a case report and literature review Case Reports. BMC Research Notes. 2015;8(1, article 758) doi: 10.1186/s13104-015-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnakenburg K. V., Müller M., Dörner K., et al. Congenital hemihypertrophy and malignant giant pheochromocytoma—a previously undescribed coincidence. European Journal of Pediatrics. 1976;122(4):263–273. doi: 10.1007/bf00481506. [DOI] [PubMed] [Google Scholar]
- 30.Filippou D. C., Rizos S., Nissiotis A., Papadopoulos V. A rare case of clinically silent giant pheochromocytoma. The Internet Journal of Oncology. 2003;2(1) [Google Scholar]
- 31.Sarveswaran V., Kumar S., Kumar A., Vamseedharan M. A giant cystic pheochromocytoma mimicking liver abscess an unusual presentation—a case report. Clinical Case Reports. 2015;3(1):64–68. doi: 10.1002/ccr3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan F. K. W., Choi K. L., Tiu S. C., Shek C. C., Au Yong T. K. A case of giant malignant phaeochromocytoma. Hong Kong Medical Journal. 2000;6(3):325–328. [PubMed] [Google Scholar]
- 33.Awada S. H., Grisham A., Woods S. E. Large dopamine-secreting pheochromocytoma: case report. Southern Medical Journal. 2003;96(9):914–917. doi: 10.1097/01.smj.0000077069.95831.62. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg A., Pautler S. E., Harle C., et al. Giant cystic pheochromocytoma containing high concentrations of catecholamines and metanephrines. The Journal of Clinical Endocrinology & Metabolism. 2011;96(8):2308–2309. doi: 10.1210/jc.2011-0465. [DOI] [PubMed] [Google Scholar]
- 35.Antedomenico E., Wascher R. A. A case of mistaken identity: giant cystic pheochromocytoma. Current Surgery. 2005;62(2):193–198. doi: 10.1016/j.cursur.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang H.-L., Sun B.-Z., Xu Z.-J., Lei W.-F., Wang X.-S. Undiagnosed giant cystic pheochromocytoma: a case report. Oncology Letters. 2015;10(3):1444–1446. doi: 10.3892/ol.2015.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basiri A., Radfar M. H. Giant cystic pheochromocytoma. Urology Journal. 2010;7(1):p. 16. [PubMed] [Google Scholar]
- 38.Lloyd R. V., Blaivas M., Wilson B. S. Distribution of chromogranin and S100 protein in normal and abnormal adrenal medullary tissues. Archives of Pathology & Laboratory Medicine. 1985;109(7):633–635. [PubMed] [Google Scholar]
- 39.Pederson L. C., Lee J. E. Pheochromocytoma. Current Treatment Options in Oncology. 2003;4(4):329–337. doi: 10.1007/s11864-003-0008-9. [DOI] [PubMed] [Google Scholar]
- 40.Dahia P. L. M. Evolving concepts in pheochromocytoma and paraganglioma. Current Opinion in Oncology. 2006;18(1):1–8. doi: 10.1097/01.cco.0000198017.45982.06. [DOI] [PubMed] [Google Scholar]
- 41.Fishbein L., Nathanson K. L. Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genetics. 2012;205(1-2):1–11. doi: 10.1016/j.cancergen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J., Song C., Park M., et al. Predictive characteristics of malignant pheochromocytoma. Korean Journal of Urology. 2011;52(4):241–246. doi: 10.4111/kju.2011.52.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenhofer G., Bornstein S. R., Brouwers F. M., et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocrine-Related Cancer. 2004;11(3):423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 44.Reisch N., Peczkowska M., Januszewicz A., Neumann H. P. H. Pheochromocytoma: presentation, diagnosis and treatment. Journal of Hypertension. 2006;24(12):2331–2339. doi: 10.1097/01.hjh.0000251887.01885.54. [DOI] [PubMed] [Google Scholar]