Abstract
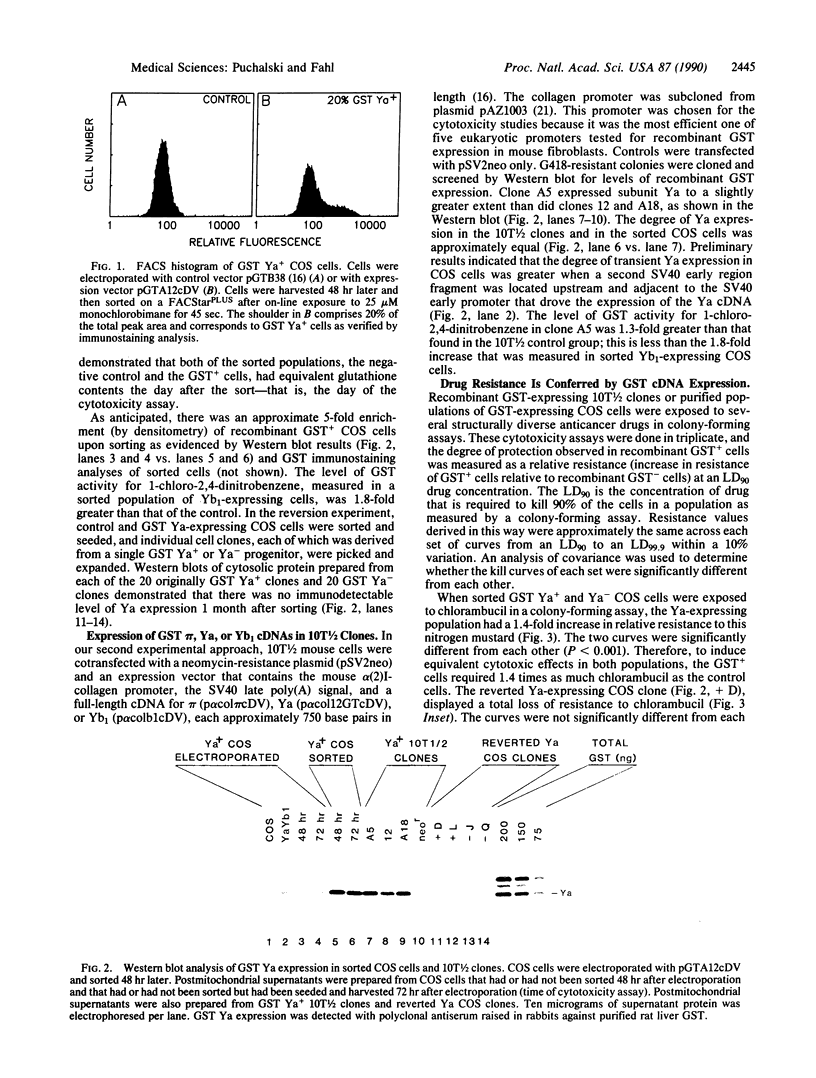
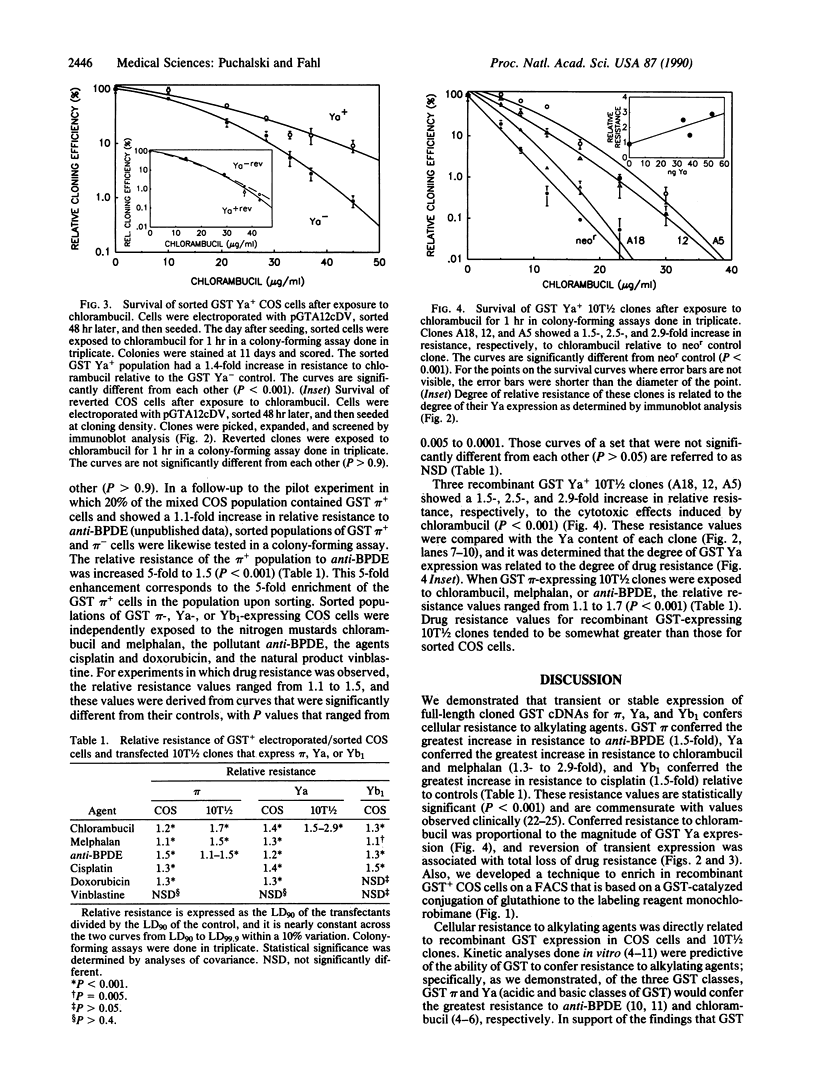
Increased levels of glutathione S-transferase (GST; RX:glutathione R-transferase; EC 2.5.1.18) mRNA, protein, and activity in tumor biopsy samples and in drug-resistant cultured cells are associated with resistance to anticancer drugs. We report that each of three full-length cloned GST cDNAs, that for pi (acidic), Ya (basic), and Yb1 (neutral), can confer drug resistance when expressed in cultured mammalian cells. In one approach, stably transfected mouse C3H/10T1/2 cells that express GST pi, Ya, or Yb1 were cloned and analyzed for drug resistance in colony-forming assays. Transiently transfected COS cells that were sorted on a fluorescence-activated cell sorter were used in the second approach to avoid interclonal variation in factors other than the recombinant GST and to show that reversion of transient GST expression correlated with loss of drug resistance. A sorting technique, developed to separate the 20% of the electroporated COS cell population that transiently expressed GST pi, Ya, or Yb1 from the nonexpressing population, was based on a GST-catalyzed intracellular conjugation of glutathione to the fluorescent labeling reagent monochlorobimane. GST Ya conferred the greatest increase in resistance to chlorambucil and melphalan (1.3- to 2.9-fold), Yb1 conferred the greatest increase in resistance to cisplatin (1.5-fold), and pi conferred the greatest increase in resistance to a racemic mixture of 7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a] pyrene and 7 alpha,8 beta-dihydroxy-9 beta,10 beta-epoxy-7,8,9,10-tetrahydrobenzo [a]pyrene and doxorubicin (1.5- and 1.3-fold) relative to controls. These resistance values to alkylating agents are commensurate with values observed clinically. Cytotoxicity curves representing recombinant GST+ populations were significantly different from their controls with P values ranging from 0.005 to 0.0001. No resistance to vinblastine was detected. Conferred drug resistance was proportional to the magnitude of GST Ya expression, and reversion of transient expression in GST Ya+ COS cell clones to a GST Ya- phenotype was associated with total loss of drug resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahokas J. T., Davies C., Ravenscroft P. J., Emmerson B. T. Inhibition of soluble glutathione S-transferase by diuretic drugs. Biochem Pharmacol. 1984 Jun 15;33(12):1929–1932. doi: 10.1016/0006-2952(84)90549-5. [DOI] [PubMed] [Google Scholar]
- Buller A. L., Clapper M. L., Tew K. D. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C. Conversion of melphalan to 4-(glutathionyl)phenylalanine. A novel mechanism for conjugation by glutathione-S-transferases. Drug Metab Dispos. 1987 Mar-Apr;15(2):195–199. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hulbert P. B., Yakubu S. I. Monobromobimane: a substrate for the fluorimetric assay of glutathione transferase. J Pharm Pharmacol. 1983 Jun;35(6):384–386. doi: 10.1111/j.2042-7158.1983.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Jernström B., Martinez M., Meyer D. J., Ketterer B. Glutathione conjugation of the carcinogenic and mutagenic electrophile (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-oxy-7,8,9,10-tetra hydrobenzo[a]pyrene catalyzed by purified rat liver glutathione transferases. Carcinogenesis. 1985 Jan;6(1):85–89. doi: 10.1093/carcin/6.1.85. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Meyer D. J. Glutathione transferases: a possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutat Res. 1989 Sep;214(1):33–40. doi: 10.1016/0027-5107(89)90195-4. [DOI] [PubMed] [Google Scholar]
- Khillan J. S., Schmidt A., Overbeek P. A., de Crombrugghe B., Westphal H. Developmental and tissue-specific expression directed by the alpha 2 type I collagen promoter in transgenic mice. Proc Natl Acad Sci U S A. 1986 Feb;83(3):725–729. doi: 10.1073/pnas.83.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard R. E., Jr, Oken M. M., Barnes J. M., Humphrey R. L., Glick J. H., Silverstein M. N. High-dose cyclophosphamide. An effective treatment for advanced refractory multiple myeloma. Cancer. 1984 Apr 1;53(7):1456–1460. doi: 10.1002/1097-0142(19840401)53:7<1456::aid-cncr2820530705>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Burgess J. A., Ho D., Newell C. L., Fahl W. E. Integration of a mutant c-Ha-ras oncogene into C3H/10T1/2 cells and its relationship to tumorigenic transformation. Carcinogenesis. 1985 Sep;6(9):1295–1301. doi: 10.1093/carcin/6.9.1295. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Puchalski R. B., Burgess J. A., Pickett C. B., Fahl W. E. Promoter-glutathione S-transferase Ya cDNA hybrid genes. Expression and conferred resistance to an alkylating molecule in mammalian cells. J Biol Chem. 1987 Mar 15;262(8):3739–3745. [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Cowan K. H. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989 Jul;36(1):22–28. [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. High-dose cisplatin therapy in ovarian cancer. Semin Oncol. 1985 Dec;12(4 Suppl 6):21–30. [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Robertson I. G., Guthenberg C., Mannervik B., Jernström B. Differences in stereoselectivity and catalytic efficiency of three human glutathione transferases in the conjugation of glutathione with 7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Cancer Res. 1986 May;46(5):2220–2224. [PubMed] [Google Scholar]
- Smith M. T., Evans C. G., Doane-Setzer P., Castro V. M., Tahir M. K., Mannervik B. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989 May 15;49(10):2621–2625. [PubMed] [Google Scholar]
- Tsui L. C., Breitman M. L. Replication of pSV2-gpt in COS-1 cells: stability of plasmid DNA in the presence and absence of biochemical selection. Somat Cell Mol Genet. 1985 Mar;11(2):167–176. doi: 10.1007/BF01534705. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Macpherson J. S., Smyth J. F. Evidence for the metabolism of mitozantrone by microsomal glutathione transferases and 3-methylcholanthrene-inducible glucuronosyl transferases. Biochem Pharmacol. 1986 May 1;35(9):1577–1581. doi: 10.1016/0006-2952(86)90127-9. [DOI] [PubMed] [Google Scholar]




